Abstract
The smoothnose wedgefish Rhynchobatus laevis, a shark-like ray classified as Critically Endangered by the International Union for Conservation of Nature, has received limited research attention. To address the knowledge gaps in its spatial behaviour, this study utilised satellite monitoring of a fortuitously captured female over a 51-day period in the Arabian Gulf. Based on the resulting movement track, the individual covered a minimum distance of 712 km, traversing the exclusive economic zones of three different countries. Its track began in the United Arab Emirates, from where it headed north, entering Iranian waters, where it spent most of its time, before continuing westward across the Gulf and eventually reaching Qatari waters. Novel insights into its behaviour revealed an observation of rapid ascents over the study period. Although both diurnal and nocturnal ascents were observed, they occurred predominantly during nocturnal periods. Contrary to the prevailing belief that wedgefish are bottom-associated, the study animal spent a considerable amount of time in the water column. The use of various depths in both nearshore and offshore waters highlights elevated susceptibility to multiple types of fishing gear. This underscores the need for an international cooperative approach to the management and conservation of shark-like rays in the Arabian Gulf.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shark, skate, and ray (Elasmobranchii) populations are experiencing serious global declines due to a combination of fishing pressure, habitat loss, and degradation (Dulvy et al. 2014, 2017; Yan et al. 2021; Sherman et al. 2023). Among these threats, overexploitation is of particular concern. The major share of global elasmobranch landings can be attributed to industrial fishing fleets (Worm et al. 2013), whereas within the context of developing countries, substantial contributions arise from artisanal fishing practices (Walker 1998).
Shark-like rays (Rhinidae, Rhinobatidae, Glaucostegidae, Trygonorrhinidae, and Pristidae) have received limited attention in previous studies. Hence, management and conservation efforts are hampered by a lack of biological and ecological data as well as taxonomic uncertainties (Henderson et al. 2016; D’Alberto et al. 2019), which is especially true for the Arabian region (Moore 2011; Jabado 2018; Jabado et al. 2018; Henderson 2020). Among shark-like rays, wedgefishes (Rhinidae) are considered particularly vulnerable (Dulvy et al. 2014; Kyne et al. 2020; Choy et al. 2022; Seidu et al. 2022).
The Arabian Gulf and adjacent waters are assumed to be home to at least three species of wedgefish, namely, Rhynchobatus djiddensis (Forsskål, 1775), Rhynchobatus australiae Whitley, 1939, and Rhynchobatus laevis (Bloch and Schneider, 1801), all of which have been declared Critically Endangered by the International Union for the Conservation of Nature (Kyne and Jabado 2019; Kyne et al. 2019a, b). To date, there has been a notable absence of studies specifically addressing the movement patterns of wedgefish in this region. Surprisingly, this knowledge gap persists on a wider scale, with only a limited number of studies conducted on these critically endangered species (Farrugia et al. 2011; White et al. 2014; Anderson et al. 2021; Jordaan et al. 2021; Gong 2022). Following the catastrophic and largely unnoticed decline of sawfish around the Arabian Peninsula, evidence indicates that wedgefish, based on their shared biological traits, are likely to experience a similar fate (Moore 2017). However, effective limits on fishing, based on scientific research and strictly enforced governance, can result in the recovery of shark and ray populations in the long term (Pacoureau et al. 2023). Currently, most studies conducted on wedgefish in the Arabian region are fishery-dependent and based solely on landing-site surveys (Moore 2011; Jabado et al. 2018; Kyne et al. 2020; Purushottama et al. 2020). Thus, even fragmented data investigating species biology and ecology can generate information of high value for fishery management.
The smoothnose wedgefish, Rhynchobatus laevis, is widely distributed in the Indo-West Pacific region; however, it is primarily found within the Indian Ocean (Last et al. 2016). Its extinction risk was elevated from Vulnerable to Critically Endangered in 2018, mainly due to high fishing pressure, with a reported population decline of > 80% over the last 45 years (Kyne and Jabado 2019). Further exacerbating the decline is the high demand for wedgefish fins in the Asian market (Keong 1996; Hopkins 2011; Kyne et al. 2020), yielding almost double the price of shark fins (Cripps et al. 2015) and promoting both targeted and illicit fishing practices.
The current study was an opportunistic investigation that occurred upon the fortuitous capture of a R. laevis individual during a routine scientific survey within the Arabian Gulf. The animal was tagged with a pop-up archival satellite transmitter, allowing novel insights into the spatiotemporal movements and behaviour of this species to be gathered. Hence, this study reports the findings of the first satellite-tagged female R. laevis in the Arabian Gulf region. Specific goals were to (i) determine the depth and temperature ranges used by the tracked individual, (ii) detect if any identifiable behavioural trends persist over longer time periods, and (iii) describe horizontal and vertical movements of the tracked individual.
Material and methods
The individual under consideration was captured during a routine longline survey in the wider Khor Faridah Region, Abu Dhabi, United Arab Emirates (U.A.E.) (Fig. 1). The fishing gear consisted of a bottom-set longline (800 m), with branchlines attached every 10 m. Each branchline was equipped with a Mustad 14/0 circular hook, baited with locally sourced cuttlefish (Sepia pharaonis Ehrenberg, 1831), and left to soak for two hours.
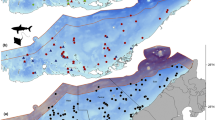
Estimated movement track of R. laevis based on the most likely position resulting from a MiniPAT pop-up satellite archival tag for 51 days within the Arabian Gulf. The movement track provided a visual representation of the R. laevis spatial path and time spent throughout the deployment period and its 95% confidence interval. Exclusive economic zones of each country are highlighted with black lines. The black polygon covers an area that is disputed
The study individual was caught on the 14th of October 2022 at 13:21. It was brought onboard and transferred to a holding pool to facilitate data collection. The total length (LT) was recorded to the nearest centimetre, and sex was determined based on the absence of claspers. A fin clip for molecular-assisted species identification was placed in 95% ethanol. The individual was tagged with a MiniPAT pop-up satellite archival tag (PSAT) (WILDLIFE Computers, Redmond, 8310 154th Ave NE, USA) anchored at the base of the first dorsal fin. The PSAT was set to initiate the release sequence after 120 days. It was programmed to generate daily light levels and sea surface temperature (SST), geolocation data and log depth and temperature every 3 s throughout the deployment duration. Additionally, auto-detect mortality was defined as being at a constant depth for a minimum of 10 days (2-m depth variance).
The individual was identified to the species level in the field based on morphological characteristics following Last et al. (2016), with a particular focus on colour pattern and rostral shape. Laboratory-based DNA isolation was performed using a NZYT gDNA isolation kit (NZYTech, 1649–038 Lisboa, Portugal). The obtained DNA was amplified via polymerase chain reaction (PCR) using ANSIM and ILEM primers (Naylor et al. 2012) targeting the NADH2 gene. The PCR product was purified using the ExoSAP-IT reagent and prepared for sequencing using the BigDye Terminator v3.1 Sequencing Kit and ethanol/EDTA purification. Thereafter, the PCR product was sequenced (Sanger sequencing) using a 3500 Genetic Analyser (Thermo Fisher Scientific, Waltham, MA, USA). The generated forward and reverse sequences were used to create a consensus sequence using Geneious software (version 2023.1.1) (GenBank accession no. OR338595). The Basic Local Alignment Search Tool (BLAST) was used to compare the consensus sequence with reference sequences in GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
The PSAT was physically recovered after it was detached from the animal, permitting analysis of the full archived dataset. All data analysis was performed using R statistical software v. R-4.3.1 (R Core Team 2023). The movement track was calculated using the integrated GPE3 model (state–space model) from the Wildlife Computers portal (https://my.wildlifecomputers.com/data/). The GPE3 model uses twilight, SST, and dive depth data in combination with reference data (SST: NOAA OI SST V2 High Resolution; Bathymetry: ETOPO1-Bedrock) to generate the most likely animal location. The location data provided by the model were filtered, and only those with a score of > 90% were considered.
Movement track data were analysed using the R package tidyverse (Wickham et al. 2019) and ggnewscale (Campitelli 2020) for spatial data analysis and visualisation (Pebesma 2018). Bathymetry data were sourced from the General Bathymetric Chart of the Oceans (GEBCO) portal (https://download.gebco.net/), land masses were plotted using data from the rnaturalearth package (Massicotte and South 2023), and EEZ boundaries were downloaded from the Marine Regions portal (https://marineregions.org).
Vertical movement activity was assessed using 1-m depth bins and 0.5 °C temperature bins. These data were separated by photoperiod, namely ‘diurnal’ or ‘nocturnal’, and delineated by sunset and sunrise times. In addition to describing the general vertical behavioural trends, rapid ascent behaviours were assessed in more detail. Rapid ascent phases were defined as periods of positive depth change at rates equal to or greater than 5 m/min.
Results
The highest match returned by BLAST was Rhynchobatus laevis (accession no. NC_047241.1), with 99.25% identity and 100.0% query coverage. The study animal was a female individual with a LT of 147 cm, captured at a depth of 3.8 m in the Khor Faridah region, Abu Dhabi. The attached PSAT was retained for 51 days, popping off on the 4th of December 2022 in Qatari waters, and was 69 days shorter than the programmed release date. The PSAT was physically recovered on the 6th of December 2022.
Over the course of 51 days, the animal was estimated to have travelled a minimum distance of 712 km, passing through the exclusive economic zones (EEZ) of the three countries, i.e. United Arab Emirates, Iran, and Qatar. It was tagged on the coast of Abu Dhabi (Fig. 1), and within 5 days of its northward movement, the wedgefish entered Iranian waters. There, it spent an extended period (approximately 28 days) around Farur Island before moving westward across the Gulf into Qatari waters. The study animal covered a mean horizontal distance of 88.9 km (S.D. ± 35.1) per calendar week, with the maximum distance travelled in week six of deployment duration (Table 1).
While the PSAT was attached to the animal, it predominately occupied a depth range of 1–35 m, with occasional excursions to depths up to 39.5 m during the last week of deployment. Mean depth differed significantly between diurnal and nocturnal periods (unpaired two-sample t-test, n = 1,471,787, p < 0.01); however, the difference was relatively minor with a mean depth of 21.4 m (± 5.9) during diurnal periods and 20.2 m (± 6.7) during nocturnal periods (Fig. 2). The depth profile over time showed that the study animal spent a substantial amount of time at a depth of 20–25 m, with a discernible trend of increasing depth during the course of the deployment. However, when viewed in more detail, the depth profile over time presented considerable changes over short intervals on a diel basis (Fig. 3). These depth changes displayed a strong cyclical pattern until the 4th of November (Fig. 3), when the study animal spent time near Farur Island (Fig. 1). At this time and thereafter, the vertical movement activity was still frequent but more sporadic.
These vertical movements did not correspond to water temperature, as no consistent vertical temperature gradient was detected by the PSAT (Fig. 3). However, a gradual cooling trend was observed throughout the study (Fig. 3). Interestingly, on one occasion during the study between the 5th and 7th of November 2022, there was a notable difference in temperature between the shallower and deeper depths occupied by the monitored animal, and the more sporadic vertical movements commenced around that time (Fig. 3). This also broadly coincides with the full-moon lunar phase (Fig. 3).
When viewed in more detail, the animal’s vertical movement followed a clear diel trend, alternating between approximately 12 h of remaining at depth during diurnal periods followed by approximately 12 h of active ascents and descents during nocturnal periods (Fig. 4). Typically, these nocturnal movements consisted of an ascent towards surface waters followed by approximately 15 min of near-surface or mid-water depth oscillations and then a return to the original depth (Fig. 4). This behaviour was repeated multiple times during the nocturnal period. However, it should be noted that this behaviour also occurred occasionally during diurnal periods, although significantly less so (Mann–Whitney U test, n = 103, p < 0.05) (Fig. 5).
Diel rapid ascents of R. laevis over a 51-day deployment period. Mean rapid ascent counts are displayed with the corresponding error bars representing each standard deviation. The rapid ascents are characterised by a 5-m depth change within 1 min and displayed based on the corresponding time of the day, indicated by background shading
Discussion
Until recently, the taxonomy of the Rhinidae was poorly resolved, and all individuals within the Gulf were commonly believed to be the whitespotted wedgefish Rhynchobatus djiddensis. However, it is now believed that up to three species may occur here, i.e. R. djiddensis, R. australiae, and R. laevis (Last et al. 2016). Morphological and molecular assessment of the current individual confirmed it to be a female R. laevis, and based on the known size at maturity (190 cm LT) (Purushottama et al. 2020), it was most likely sexually immature.
Differences in horizontal movement patterns have been observed in wedgefish based on maturity. Mature individuals have been reported to depart from monitored research areas and return to the same location annually (Jordaan et al. 2021). However, the extent of their movement outside the boundaries of such areas has not been documented (White et al. 2014; Gong 2022; Murray et al. 2023). Conversely, it has been observed that juveniles exhibit a more pronounced affinity for certain areas, but once they leave, they tend not to return on an annual basis, and there is limited information available about their continuing movement patterns (Farrugia et al. 2011; Jordaan et al. 2021). However, the wedgefish in the current study exhibited predominantly mobile behaviour, with the exception of a 4-week period around Farur Island (Fig. 1). When mobile it primarily utilised open waters and did not remain nearshore. This continuity of movement has not been reported in wedgefish previously, but this is most likely due to the limited spatial coverage of previous studies (Farrugia et al. 2011; White et al. 2014; Jordaan et al. 2021). The current data demonstrate that these animals are capable of extensive mobility punctuated by periods of transient site association.
Horizontal movements in bottom-associated elasmobranchs are often associated with annual seasonal migrations, which are typically driven by foraging and mating behaviours, or external environmental factors (Dudgeon et al. 2013; Schlaff et al. 2014; Espinoza et al. 2016; Lea et al. 2018; Lubitz et al. 2022; Peterson and Grubbs 2023). It seems highly unlikely that the horizontal movements observed in the present study were associated with mating behaviour given the apparent immature condition of the animal. However, the areas surrounding Farur Island and utilised by the animal thereafter are notable for the presence of active mesoscale eddies (Rahnemania et al. 2019). These are known to increase prey accessibility and to be energetically beneficial (Gaube et al. 2018; Schmid et al. 2020) and may have played a role in driving this animal’s movements. Moreover, it is evident from the seawater temperature profile (Fig. 3) that it moved from an area of higher water temperature to one of lower temperature during the monitored period. This may indicate that temperature also influenced this movement, although the observed timeframe is too short to make any definitive conclusion in this regard.
The wedgefish in this study exhibited extensive vertical movements, and such movements have been reported in several other bottom-associated elasmobranch species (Neat et al. 2015; Humphries et al. 2017; Griffiths et al. 2020; Kneebone et al. 2020; Lavender et al. 2021; Poos et al. 2023). However, in these studies, it is unclear if the vertical movements are due to horizontal variations in bottom depth or actual movements away from the seabed (Lavender et al. 2021). This is also true of sections of the track followed by the current study animal, as the 95% confidence interval of the horizontal position encompassed a broad depth range (Fig. 1). Nonetheless, other sections of the track indicate extensive horizontal movement higher in the water column as the seabed depth was considerably deeper than the maximum depth occupied by the animal. Specifically, the fact that the animal never descended below 39.5 m and traversed areas with a depth of over 80 m indicates that it was capable of spending extended periods of multiple days away from the seabed. Indeed, a bowmouth guitarfish individual was sighted by Forget and Muir (2021) at approximately 25 m depth between the African continent and the Seychelles, with a bottom depth of ~ 4000 m.
Vertical movement by elasmobranchs has been linked to a variety of drivers, including thermoregulation, bioenergetic efficiency, foraging behaviour, tidal conditions, and lunar or seasonal cycles (Sims et al. 2006; Andrews et al. 2009; Carlson et al. 2014; Arostegui et al. 2020; Griffiths et al. 2020; Lavender et al. 2021; Andrzejaczek et al. 2022). In this study, it is worth noting that the PSAT did not record a persistent thermocline, precluding temperature as a primary driver of diel vertical movements. However, a notable change in the overall vertical behaviour occurred around November 5th, coinciding with a decrease in water temperature. This suggests that temperature has at least some influence over movement behaviour.
The vertical movements in this study were strongly linked to photoperiod, with significantly higher activity observed during nocturnal periods. This aligns with the findings of previous elasmobranch studies (Cartamil et al. 2003; Whitney et al. 2007; Lavender et al. 2021; Wheeler et al. 2022). Large-scale nocturnal vertical migrations are common in marine environments, mainly revolving around the movement of zooplankton into surface waters (Fréon and Misund 1999; Mincks et al. 2000; D’Elia et al. 2016), suggesting that the vertical movements observed here may have been driven by prey availability. It is notable that the vertical movements in the current study were less pronounced during the full moon, which is also when mass vertical migrants avoid near-surface waters (Alldredge and King 1980; Shima et al. 2022).
The Arabian Gulf is surrounded by eight countries, each of which manages marine resources within its territorial waters (Morgan 2006). Even within countries, regulations can vary at the local level (ANON 2019; FAO 2023). Although industrial demersal trawling is limited to Iran, Iraq, Kuwait, and Saudi Arabia (FAO 2003, 2015, 2019, 2023), fish traps, gill nets, seine nets, and baited longlines are used extensively throughout the region (FAO 2023), and wedgefish are susceptible to capture in all of these gears. Moreover, given the extensive horizontal and vertical movements exhibited by the wedgefish in the present study, they are likely to come into contact with all such fisheries. Therefore, gear-specific conservation strategies targeting wedgefish should be aligned across jurisdictions. Unfortunately, the region has a poor record of implementing long-term cooperative and strategic plans to protect shared fish stocks (Grandcourt 2012).
In conclusion, the study animal traversed the EEZs of three countries within a period of 51 days, exposing it to the majority, if not all, of the fishing gears present in the Arabian Gulf. Understanding the complex movement patterns of marine fish with extensive home ranges is of utmost importance for successful conservation efforts (Speed et al. 2010; Hays et al. 2019; Jorgensen et al. 2022). Collaborative resource management of shared marine fish resources has been shown to support effective conservation outcomes (Pomeroy et al. 2007; Mulazzani et al. 2013). Satellite telemetry in particular has been instrumental in the development and implementation of elasmobranch management measures elsewhere (Graham et al. 2016; Doherty et al. 2017; Lea et al. 2018); however, such studies in the Arabian Gulf are limited to this work and that of Robinson et al. (2017). Notwithstanding the reliance on a solitary individual, the present data suggest an extensive activity space for these critically endangered animals. Therefore, it is imperative that conservation efforts are coordinated across regional and national boundaries. In fact, given the high risk of extinction faced by most elasmobranchs in the Arabian Gulf, a unified management approach is highly desirable.
Data availability
All data are available from the corresponding author upon request.
References
Alldredge AL, King JM (1980) Effects of moonlight on the vertical migration patterns of demersal zooplankton. J Exp Mar Biol Eco 44:133–156. https://doi.org/10.1016/0022-0981(80)90150-1
Anderson AB, Fiuza TM, Araujo GS, Canterle AM, Canto LM, Freitas RH, Gadig OB, Floeter SR (2021) A safe haven for potential reproductive aggregations of the critically endangered Brazilian guitarfish (Pseudobatos horkelii). J Fish Biol 99:2030–2034. https://doi.org/10.1111/jfb.14880
Andrews KS, Williams GD, Farrer D, Tolimieri N, Harvey CJ, Bargmann G, Levin PS (2009) Diel activity patterns of sixgill sharks, Hexanchus griseus: the ups and downs of an apex predator. Anim Behav 78:525–536. https://doi.org/10.1016/j.anbehav.2009.05.027
Andrzejaczek S, Lucas TCD, Goodman MC et al (2022) Diving into the vertical dimension of elasmobranch movement ecology. Sci Adv 8:eabo1754. https://doi.org/10.1126/sciadv.abo1754
ANON (2019) Ministry of Climate Change bans use of gargoor fishing nets in Abu Dhabi.URL https://wam.ae/en/details/1395302744329. Accessed 10 September 2023
Arostegui MC, Gaube P, Berumen ML, DiGiulian A, Jones BH, Røstad A, Braun CD (2020) Vertical movements of a pelagic thresher shark (Alopias pelagicus): insights into the species’ physiological limitations and trophic ecology in the Red Sea. Endanger Species Res 43:387–394. https://doi.org/10.3354/esr01079
Campitelli E (2020) Ggnewscale: multiple fill and colour scales in’ggplot2’. R package version 0.4, 5. https://CRAN.R-project.org/package=ggnewscale
Carlson AE, Hoffmayer ER, Tribuzio CA, Sulikowski JA (2014) The use of satellite tags to redefine movement patterns of Spiny Dogfish (Squalus acanthias) along the U.S. east coast: implications for fisheries management. PLoS One 9:e103384. https://doi.org/10.1371/journal.pone.0103384
Cartamil DP, Vaudo JJ, Lowe CG, Wetherbee BM, Holland KN (2003) Diel movement patterns of the Hawaiian stingray, Dasyatis lata: implications for ecological interactions between sympatric elasmobranch species. Mar Biol 142:841–847. https://doi.org/10.1007/s00227-003-1014-y
Choy CPP, Jabado RW, Clark-Shen N, Huang D, Choo MY, Rao M (2022) Unraveling the trade in wedgefishes and giant guitarfishes in Singapore. Mar Policy 136:104914. https://doi.org/10.1016/j.marpol.2021.104914
Cripps G, Harris A, Humber F, Harding S, Thomas T (2015) A preliminary value chain analysis of shark fisheries in Madagascar. Programme for the implementation of a Regional Fisheries Strategy for the Eastern and Southern Africa—Indian Ocean Region vol SF/2015/34. Indian Ocean Commission, Ebene, Mauritius, 82
D’Alberto BM, Carlson JK, Pardo SA, Simpfendorfer CA (2019) Population productivity of shovelnose rays: inferring the potential for recovery. PLoS One 14:e0225183. https://doi.org/10.1371/journal.pone.0225183
D’Elia M, Warren JD, Rodriguez-Pinto I, Sutton TT, Cook A, Boswell KM (2016) Diel variation in the vertical distribution of deep-water scattering layers in the Gulf of Mexico. Deep Sea Res Part i: Oceanogr Res Papers 115:91–102. https://doi.org/10.1016/j.dsr.2016.05.014
Doherty PD, Baxter JM, Godley BJ, Graham RT, Hall G, Hall J, Hawkes LA, Henderson SM, Johnson L, Speedie C, Witt MJ (2017) Testing the boundaries: seasonal residency and inter-annual site fidelity of basking sharks in a proposed marine protected area. Biol Conserv 209:68–75. https://doi.org/10.1016/j.biocon.2017.01.018
Dudgeon CL, Lanyon JM, Semmens JM (2013) Seasonality and site fidelity of the zebra shark, Stegostoma fasciatum, in southeast Queensland, Australia. Anim Behav 85:471–481. https://doi.org/10.1016/j.anbehav.2012.12.013
Dulvy NK, Simpfendorfer CA, Davidson LNK, Fordham SV, Bräutigam A, Sant G, Welch DJ (2017) Challenges and priorities in shark and ray conservation. Curr Biol 27:R565–R572. https://doi.org/10.1016/j.cub.2017.04.038
Dulvy NK, Fowler SL, Musick JA et al (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 3. https://doi.org/10.7554/elife.00590
Espinoza M, Heupel MR, Tobin AJ, Simpfendorfer CA (2016) Evidence of partial migration in a large coastal predator: opportunistic foraging and reproduction as key drivers? PLoS One 11(2):e0147608. https://doi.org/10.1371/journal.pone.0147608
FAO (2023) Report of the Regional Commission for Fisheries (RECOFI) Data Workshop Series. FAO. https://doi.org/10.4060/cc7409en
FAO (2003) Fishery and aquaculture country profiles. Kuwait, 2003. Country Profile Fact Sheets. Fisheries and Aquaculture Division. Rome. Updated Dec 6, 2017.https://www.fao.org/fishery/en/facp/kwt?lang=en Assessed 08 October 2023
FAO (2015) Fishery and aquaculture country profiles. Iran (Islamic Rep. of), 2015. Country Profile Fact Sheets. Fisheries and Aquaculture Division. Rome. Updated Apr 1, 2016 https://www.fao.org/fishery/en/facp/irn#prodsectorintroinlandlandingsites. Assessed 08 October 2023
FAO (2019) Fishery and aquaculture country profiles. Iraq, 2019. Country Profile Fact Sheets. Fisheries and Aquaculture Division. Rome. Updated Jan 16, 2013 https://www.fao.org/fishery/en/facp/irq?lang=en. Assessed 08 October 2023
Farrugia TJ, Espinoza M, Lowe CG (2011) Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary. Mar Freshw Res 62:648–657. https://doi.org/10.1071/MF10173
Forget F, Muir J (2021) The critically endangered bowmouth guitarfish (Rhina ancylostoma) in the open ocean with an associated tuna school. Mar Biodivers 51(4):69. https://doi.org/10.1007/s12526-021-01195-8
Fréon P, Misund OA (1999) Dynamics of pelagic fish distribution and behaviour: effects on fisheries and stock assessment, vol 348. Fishing News Books, Oxford
Gaube P, Braun CD, Lawson GL, McGillicuddy DJ, Penna AD, Skomal GB, Fischer C, Thorrold SR (2018) Mesoscale eddies influence the movements of mature female white sharks in the Gulf Stream and Sargasso Sea. Sci Rep 8:7363. https://doi.org/10.1038/s41598-018-25565-8
Gong A (2022) Movement patterns of the shovelnose guitarfish (Pseudobatos productus) and California bat ray (Myliobatis californica) in the Southern California Bight. Dissertation, University of San Diego. https://doi.org/10.22371/02.2022.002
Graham F, Rynne P, Estevanez M, Luo J, Ault JS, Hammerschlag N (2016) Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Divers Distrib 22:534–546. https://doi.org/10.1111/ddi.12425
Grandcourt E (2012) Reef Fish and Fisheries in The Gulf. In: Riegl BM, Purkis SJ (eds) Coral Reefs of the Gulf, Coral Reefs of the World. Springer, Netherlands, Dordrecht, pp 127–161
Griffiths CA, Wright SR, Silva JF, Ellis JR, Righton DA, Phillips SRM (2020) Horizontal and vertical movements of starry smooth-hound Mustelus asterias in the northeast Atlantic. PLoS One 15(10):e0239480. https://doi.org/10.1371/journal.pone.0239480
Hays GC, Bailey H, Bograd SJ et al (2019) Translating marine animal tracking data into conservation policy and management. Trends Ecol Evol 34:459–473. https://doi.org/10.1016/j.tree.2019.01.009
Henderson AC (2020) A review of potential taxonomic barriers to the effective management of Gulf elasmobranch fisheries. Aquat Ecosyst Health Manag 23:210–219. https://doi.org/10.1080/14634988.2020.1800327
Henderson AC, Reeve AJ, Jabado RW, Naylor GJP (2016) Taxonomic assessment of sharks, rays and guitarfishes (Chondrichthyes: Elasmobranchii) from south-eastern Arabia, using the NADH dehydrogenase subunit 2 (NADH2) gene. Zool J Linn Soc 176:399–442. https://doi.org/10.1111/zoj.12309
Hopkins C (2011) External actors, high value resources and threatened species: shark fin commodity chains of Northern Madagascar, interception for conservation. Dissertation, Department of Life Sciences, Silwood Park, Imperial College London. https://doi.org/10.13140/RG.2.2.27800.19207
Humphries NE, Simpson SJ, Sims DW (2017) Diel vertical migration and central place foraging in benthic predators. Mar Ecol Prog Ser 58:163–180. https://doi.org/10.3354/meps12324
Jabado RW (2018) The fate of the most threatened order of elasmobranchs: shark-like batoids (Rhinopristiformes) in the Arabian Sea and adjacent waters. Fish Res 204:448–457. https://doi.org/10.1016/j.fishres.2018.03.022
Jabado RW, Kyne PM, Pollom RA et al (2018) Troubled waters: threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters. Fish Fish 19:1043–1062. https://doi.org/10.1111/faf.12311
Jordaan G, Mann B, Daly R, Dunlop S, Cowley P (2021) Movement patterns and growth rate of the whitespotted wedgefish Rhynchobatus djiddensis in southern Africa based on tag-recapture data. Afr J Mar Sci 43:201–213. https://doi.org/10.2989/1814232X.2021.1906318
Jorgensen SJ, Micheli F, White TD, Van Houtan KS, Alfaro-Shigueto J, Andrzejaczek S, Arnoldi NS, Baum JK, Block B, Britten GL, Butner C (2022) Emergent research and priorities for shark and ray conservation. Endanger Species Res 47:171–203. https://doi.org/10.3354/esr01169
Keong CH (1996) Shark fisheries and trade in sharks and shark products in Southeast Asia, TRAFFIC Southeast Asia. In: Last PR, Stevens JD (eds) Sharks and Rays of Australia. CSIRO, Australia, Melbourne, pp 1–129
Kneebone J, Sulikowski J, Knotek R, McElroy WD, Gervelis B, Curtis T, Jurek J, Mandelman J (2020) Using conventional and pop-up satellite transmitting tags to assess the horizontal movements and habitat use of thorny skate (Amblyraja radiata) in the Gulf of Maine. ICES J Mar Sci 77:2790–2803. https://doi.org/10.1093/icesjms/fsaa149
Kyne PM, Jabado RW, Rigby CL, Dharmadi GMA, Pollock CM, Herman KB, Cheok J, Ebert DA, Simpfendorfer CA, Dulvy NK (2020) The thin edge of the wedge: extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat Conserv: Mar Freshw 30:1337–1361. https://doi.org/10.1002/aqc.3331
Kyne PM and Jabado RW (2019) Rhynchobatus laevis. The IUCN Red List of Threatened Species 2019: e.T41854A124422344. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T41854A124422344.en.
Kyne PM, Gledhill K, Jabado RW (2019a) Rhynchobatus djiddensis. The IUCN Red List of Threatened Species 2019: e.T39394A121035795. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T39394A121035795.en.
Kyne PM, Rigby CL, Dharmadi and Jabado RW (2019b) Rhynchobatus australiae. The IUCN Red List of Threatened Species 2019: e.T41853A68643043. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T41853A68643043.en.
Last P, White WT, de Carvalho MR, Séret B, Stehmann MFW, Naylor GJP, McEachran JD (2016) Rays of the World, Illustrated, edition. Comstock Publishing Associates, Ithaca NY
Lavender E, Aleynik D, Dodd J, Illian J, James M, Wright PJ, Smout S, Thorburn J (2021) Environmental cycles and individual variation in the vertical movements of a benthic elasmobranch. Mar Biol 168:164. https://doi.org/10.1007/s00227-021-03973-1
Lea JSE, Wetherbee BM, Sousa LL, Aming C, Burnie N, Humphries NE, Queiroz N, Harvey GM, Sims DW, Shivji MS (2018) Ontogenetic partial migration is associated with environmental drivers and influences fisheries interactions in a marine predator. ICES J Mar Sci 75:1383–1392. https://doi.org/10.1093/icesjms/fsx238
Lubitz N, Bradley M, Sheaves M, Hammerschlag N, Daly R, Barnett A (2022) The role of context in elucidating drivers of animal movement. Ecol Evol 12(7):e9128. https://doi.org/10.1002/ece3.9128
Massicotte P and South A (2023) A Rnaturalearth: World Map Data from Natural Earth. R package version 0.3, 2. https://docs.ropensci.org/rnaturalearth/https://github.com/ropensci/rnaturalearth.
Mincks SL, Bollens SM, Madin LP, Horgan E, Butler M, Kremer PM, Craddock JE (2000) Distribution, abundance, and feeding ecology of decapods in the Arabian Sea, with implications for vertical flux. Deep Sea Res Part II: Topical Stud Oceanogr 47:1475–1516. https://doi.org/10.1016/S0967-0645(99)00151-4
Moore ABM (2011) Elasmobranchs of the Persian (Arabian) Gulf: Ecology, human aspects and research priorities for their improved management. Rev Fish Biol Fish 22:35–61. https://doi.org/10.1007/s11160-011-9222-x
Moore ABM (2017) Are guitarfishes the next sawfishes? Extinction risk and an urgent call for conservation action. Endanger Species Res 34:75–88. https://doi.org/10.3354/esr00830
Morgan G (2006) Subregional review: northwest Indian Ocean. In: De Young C Subregional review: Northwest Indian Ocean edition. Review of the state of world marine capture fisheries management: Indian Ocean. FAO Fisheries Technical Paper 488 pp 51–66
Mulazzani L, Curtin R, Andrés M, Malorgio G (2013) Multilevel governance and management of shared stocks with integrated markets: the European anchovy case. Mar Policy 38:407–416. https://doi.org/10.1016/j.marpol.2012.06.020
Murray T, Elston C, Bennett R, Childs A-R, Cowley P (2023) Movement patterns and underestimation of the maximum age of a vulnerable endemic guitarfish species inferred from mark-recapture studies. Afr J Mar Sci 45:149–154. https://doi.org/10.2989/1814232X.2023.2224829
Naylor GJP, Caira JN, Jensen K, Rosana KAM, White WT, Last PR (2012) A DNA sequence–based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull Am Mus Nat Hist 1–262. https://doi.org/10.1206/754.1
Neat F, Pinto C, Burrett I, Cowie L, Travis J, Thorburn J, Gibb F, Wright P (2015) Site fidelity, survival and conservation options for the threatened flapper skate (Dipturus cf. intermedia). Aquat Conserv: Mar Freshw 25. https://doi.org/10.1002/aqc.2472
Pacoureau N, Carlson JK, Kindsvater HK et al (2023) Conservation successes and challenges for wide-ranging sharks and rays. Proc Natl Acad Sci U S A 120(5):e2216891120. https://doi.org/10.1073/pnas.2216891120
Pebesma EJ (2018) Simple features for R: standardized support for spatial vector data. R J 10(1):439. https://doi.org/10.32614/RJ-2018-009
Peterson CT, Grubbs RD (2023) Temporal community structure and seasonal climatic migration of coastal sharks and large teleost fishes in the northeast Gulf of Mexico. J Fish Aquat Sci 80:1335–1350. https://doi.org/10.1139/cjfas-2022-0124
Pomeroy R, Parks J, Pollnac R, Campson T, Genio E, Marlessy C, Holle E, Pido M, Nissapa A, Boromthanarat S, Thu Hue N (2007) Fish wars: conflict and collaboration in fisheries management in Southeast Asia Mar. Policy 31:645–656. https://doi.org/10.1016/j.marpol.2007.03.012
Poos JJ, Staeudle T, Greenway E, Batsleer J (2023) Spatial distribution, migration, and population structure of North Sea rays. Wageningen University & Research, Wageningen. https://doi.org/10.18174/632935
Purushottama GB, Raje T, Akhilesh KV, Kizhakudan SJ, Zacharia PU (2020) Reproductive biology and diet composition of Rhynchobatus laevis (Bloch and Schneider, 1801) (Rhinopristiformes: Rhinidae) from the northern Indian Ocean. Indian J Fish 67(4):13–23. https://doi.org/10.21077/ijf.2020.67.4.95636-02
R Core Team (2023) R: a language and environment for statistical computing. Vienna, Austria. Available at: https://www.R-project.org/. Accessed Mar 2023
Rahnemania A, Bidokhti AA, Ezam M, Lari K, Ghader S (2019) A numerical study of the frontal system between the inflow and outflow waters in the Persian Gulf. J Appl Fluid Mech 12. https://doi.org/10.29252/jafm.12.05.29770
Robinson DP, Jaidah MY, Bach SS, Rohner CA, Jabado RW, Ormond R, Pierce SJ (2017) Some like it hot: repeat migration and residency of whale sharks within an extreme natural environment. PLoS One 12(9):e0185360. https://doi.org/10.1371/journal.pone.0185360
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fish 24:1089–1103. https://doi.org/10.1007/s11160-014-9364-8
Schmid MS, Cowen RK, Robinson K, Luo JY, Briseño-Avena C, Sponaugle S (2020) Prey and predator overlap at the edge of a mesoscale eddy: fine-scale, in-situ distributions to inform our understanding of oceanographic processes. Sci Rep 10:921. https://doi.org/10.1038/s41598-020-57879-x
Seidu I, Cabada-Blanco F, Brobbey LK, Asiedu B, Barnes P, Seidu M, Dulvy NK (2022) “Every fish in the sea is meat and so are guitarfishes”: socio-economic drivers of a guitarfish fishery in Ghana. Mar Policy 143:105159. https://doi.org/10.1016/j.marpol.2022.105159
Sherman CS, Simpfendorfer CA, Pacoureau N et al (2023) Half a century of rising extinction risk of coral reef sharks and rays. Nat Commun 14(1):15. https://doi.org/10.1038/s41467-022-35091-x
Shima JS, Osenberg CW, Alonzo SH, Noonburg EG, Swearer SE (2022) How moonlight shapes environments, life histories, and ecological interactions on coral reefs. Emerg Top Life Sci 6:45–56. https://doi.org/10.1042/ETLS20210237
Sims DW, Wearmouth VJ, Southall EJ, Hill JM, Moore P, Rawlinson K, Hutchinson N, Budd GC, Righton D, Metcalfe JD, Nash JP, Morritt D (2006) Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J Anim Ecol 75:176–190. https://doi.org/10.1111/j.1365-2656.2005.01033.x
Speed CW, Field IC, Meekan MG, Bradshaw CJ (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293. https://doi.org/10.3354/meps08581
Walker TI (1998) Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Mar Freshw Res 49:553–572. https://doi.org/10.1071/mf98017
Wheeler CR, Kneebone J, Heinrich D, Strugnell JM, Mandelman JW, Rummer JL (2022) Diel rhythm and thermal independence of metabolic rate in a benthic shark. J Biol Rhythms 37:484–497. https://doi.org/10.1177/07487304221107843
White J, Simpfendorfer CA, Tobin AJ, Heupel MR (2014) Spatial ecology of shark-like batoids in a large coastal embayment. Environ Biol Fish 97:773–786. https://doi.org/10.1007/s10641-013-0178-7
Whitney NM, Papastamatiou YP, Holland KN, Lowe CG (2007) Use of an acceleration data logger to measure diel activity patterns in captive whitetip reef sharks. Triaenodon Obesus Aquat Living Resour 20(4):299–305. https://doi.org/10.1051/alr:2008006
Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M (2019) Welcome to the Tidyverse. JOSS 4(43):1686. https://doi.org/10.21105/joss.01686
Worm B, Davis B, Kettemer L, Ward-Paige CA, Chapman D, Heithaus MR, Kessel ST, Gruber SH (2013) Global catches, exploitation rates, and rebuilding options for sharks. Mar Policy 4:194–204. https://doi.org/10.1016/j.marpol.2012.12.034
Yan HF, Kyne PM, Jabado RW, Leeney RH, Davidson LNK, Derrick DH, Finucci B, Freckleton RP, Fordham SV, Dulvy NK (2021) Overfishing and habitat loss drive range contraction of iconic marine fishes to near extinction. Sci Adv 7(7):eabb6026. https://doi.org/10.1126/sciadv.abb6026
Acknowledgements
The authors are grateful for the logistical support provided by the United Arab Emirates University.
Funding
This research was partially funded by the United Arab Emirates University grant G00003663.
Author information
Authors and Affiliations
Contributions
Fieldwork was conducted by S.B., A.H., and S. H. Data analysis was performed by S.B and E.S. All authors contributed to the writing of the manuscript, and all have approved the final, submitted version.
Corresponding author
Ethics declarations
Ethics approval
All research was conducted in accordance with Abu Dhabi and UAE federal laws.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bruns, S., Al Hameli, S., Sulanke, E. et al. A wandering wedgefish illustrates the need for cooperative elasmobranch conservation in the Arabian Gulf. Environ Biol Fish 107, 307–318 (2024). https://doi.org/10.1007/s10641-024-01531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-024-01531-4