Abstract
Foam consists of a mixture of water loaded with surfactant and gas. Injected into the porous medium, foam has many useful properties for soil remediation. The properties of surfactants facilitate the mobilization of pollutants, and the presence of gas greatly reduces the consumption of reagents but also makes it possible to block the passage of water. The foam rheology also leads to specific effects such as the stabilization of the front. This chapter first describes the characteristics of the foam in air and then in the porous medium. Subsequently, a review of the literature on the experiments carried out in the laboratory makes it possible to highlight all the effects of the foam. The following section is devoted to rare foam injection experiments carried out in a real environment. Finally, a section is devoted to the modeling of foam displacement in a porous medium.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Foam is defined as a dispersion of gas bubbles within a surfactant solution. In bulk, gas bubbles are separated by a continuous liquid phase composed of interconnected thin films called lamellae. Foam lamellae are stabilized by amphiphilic surfactant molecules characterized by hydrophobic tails and hydrophilic heads, a configuration which enables the molecules to rapidly adsorb onto the gas–liquid interface. Surfactants reduce the interfacial tension of biphasic systems, thereby promoting gas–liquid emulsification. As the active matter concentration of a surfactant solution is increased, the interfacial tension of the system will decrease, until the point at which surfactant molecules fully saturate the gas–liquid interface. At this concentration, referred to as the critical micelle concentration (CMC), the interfacial tension of the system is minimized and will remain relatively constant despite any further increases in active matter concentration. At concentrations above the CMC—the latter which typically ranges from 0.01 to 0.3 wt%—surfactant molecules will self-associate to form structures called micelles. Any given surfactant’s CMC value will depend on the charge of its hydrophilic head (cationic, anionic, or non-ionic), as well as on the ionic strength of the solution and its temperature. The interfacial tension induced by surfactant stabilizes the lamellae; foam lamellae can be further stabilized through the addition of polymers and/or nanoparticles to the surfactant solution.
An important characteristic of injected foam is its gas fractional flow rate (equivalent to the volume of gas divided by total foam volume), commonly referred to as foam quality (fq). Given that the gas phase is usually the major component of foam, fq typically ranges from 30 to 99%. Foams have large surface areas (~200 m2/L of solution) and can occupy large volumes (~0.1 m3/L of solution), therefore making it useful in many applications (Cantat et al. 2013). Foams are often described in terms of their foamability defined as the capacity of the surfactants to form foam irrespective of the special foam properties. Foam is generated (i.e., lamellae are formed) by mechanical friction in presence of both phases. In bulk, foamability can be measured using normalized tests, such as the Ross-Miles or the Bikerman (Denkov et al. 2020; Longpré-Girard et al. 2019). Foamability depends on surfactant type and concentration and is optimal when the surfactant solution concentration is slightly greater than the CMC. However, foams are thermodynamically metastable systems that undergo constant cycles of bubble generation and destruction. Foam destruction can result from several phenomena: (i) gravitational liquid drainage in the lamellae, (ii) foam coarsening due to lamellae thinning (Ostwald ripening), and (iii) coalescence due to the pressure differences that exist between bubbles of different sizes.
17.2 Foam in Porous Media
In porous medium, the liquid phase is continuous and covers the solid walls, while the gas bubbles, separated by liquid films, are located within the pore space. The presence of a solid phase and a disordered porous network changes the properties of foam from the one measured outside the porous medium (liquid/gas ratio, viscosisty…).
17.2.1 Adsorption of the Surface-Active Agents
Adsorption of surfactants can be important because of the small molecule sizes and depends much on both the nature of the surfactant molecule and the solid material. When the liquid phase containing surfactant molecules is in contact with the solid surface, the rapid adsorption of the surfactant molecules may arise due to several interactions: (i) H-bond and acid–base interactions, (ii) electrostatic interactions between ionic groups, and (iii) chemical dispersion forces. Adsorption has been studied extensively and summary of the mechanisms as well as concatenation of results have been reviewed (Rosen and Et Kunjappu 2012; Schramm 2000). In practice, a surfactant slug should be injected before gas injection to ensure local availability of surfactant molecules at concentrations able to make lamellae.
17.2.2 Foam Generation
Following the pioneering work of Roof (1970), the topic of foam generation has been extensively studied in the literature. The following four mechanisms are responsible for bubble generation in porous media, the first of which, snap-off, has the most important influence on the resulting foam rheology of all the mechanisms.
This phenomenon is illustrated in Fig. 17.1a. When gas flows from a pore body toward a pore throat, the velocity increases locally, and the liquid film thickness increases until the two surfaces join and form a lamella that is stabilized by the presence of surfactant.
In the leave behind configuration (Fig. 17.1b), a lamella is generated parallel to the direction of flow due to the pore geometry.
In Lamellae division a moving gas bubble is separated into two parts when it encounters an obstacle (Fig. 17.1c).
17.3 Pinch-Off
Liontas et al. (2013) described this mechanism by observing foam experiments performed in micromodels and showed that Pinch-off differs from snap-off in that it occurs in presence of several bubbles interacting (Fig. 17.2).
Neighbor-wall pinch-off (a) and neighbor-neighbor pinch-off (b) (from Liontas et al. 2013)
17.3.1 Foam Stability in Porous Media
When flowing in a porous medium, the overall stability of foam is directly dependent on the stability of its individual lamellae, which in turn depends on parameters such as surfactant concentration, gas diffusion, and petrophysical properties. Capillary pressure (Pc) plays a key role, as it causes destabilization of liquids film at high values. Khatib et al. (1988) found that foams collapse when capillary pressure exceeds a critical value, Pc*, called limiting capillary pressure. Given that Pc is in general inversely proportional to soil permeability, foam destruction is more significant in fine-grained materials.
Foam structure changes with time due to gas diffusion between bubbles (Fig. 17.3). This mechanism, called foam coarsening or Ostwald ripening, leads to bubble coalescence. It depends on several parameters, including but not limited to surfactant concentration, gas type, pressure, and temperature (Marchalot et al. 2008; Jones et al. 2018).
17.3.2 Foam Rheology in Porous Media
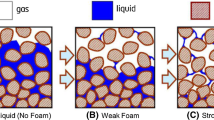
When gas is injected into a porous medium, there are three possible flow behaviors, as illustrated in Fig. 17.4. First, in absence of surfactant in the liquid solution, foam cannot be generated, and gas and liquid flow separately (Fig. 17.4a), because of gravity, the gas flows with upward vertical fingering through the liquid phase. Second, foam may occur with a low lamellae density (density is the number of lamellae per unit volume, see Fig. 17.4b) because of lamellae instability or lack of generation efficiency. The low lamellae density only slightly diminishes the gas flow, and the given foam is called weak foam. Third, the lamellae density is high, resulting in a high resistance to foam flow with flat front displacement into the porous medium (Fig. 17.4c). In this case, the given fluid is called strong foam.
Different regimes of foam flow in porous media (from Sheng 2013)
Starting from the rheology of gas bubbles in capillary tubes where viscosity is due to porous medium resistance to lamellae movement, interface deformation, and surfactant gradient, Hirasaki and Lawson (1985) established the following relationship:
where \({\mu }_{f}\) and \({\mu }_{g}\) are, respectively, foam and gas viscosities, \(\alpha\) is an empirical parameter that depends on the porous medium, surfactant, and flow conditions, \({n}_{f}\) is the density of flowing lamellae, and vg the local gas velocity.
In soils, gas mobility also depends on the size ratio of gas bubbles to pore throats, soil permeability, and gradient as well as the local saturations of the gas and liquid phases (Falls et al. 1988; Maire et al. 2018a, b). For strong foams at steady state, the trapped gas fraction typically ranges from 85 to 99%, and bubbles move irregularly according to the pore geometry, some bubbles being trapped other move more rapidly (Friedmann et al. 1991).
17.3.3 Resistance Factor
A foam’s lamella density is directly associated with the viscosity it exhibits when flowing in porous media: High densities of lamella are associated with highly viscous foam. Resistance factor (RF) is commonly used to express the increase in pressure drop, ΔP, during foam flow by comparing it to the pressure drop induced by a single-phase water flood at the same volumetric flow rate, Q:
Similarly, the mobility reduction factor (MRF) can be used when the gas phase is used for the comparison:
These parameters are useful for describing foam flows in porous media. Typically, strong and weak foams are characterized by MRF values above and below 100, respectively.
17.3.4 Foam Injection in Porous Media
The following three methods can be used to inject foam into a porous medium (Fig. 17.5). The first and third methods listed below result in the highest and lowest injection pressure values, respectively, while the second method is associated with injection pressures that are intermediate between the two (Maire et al. 2018a, b):
-
Pre-generation: Foam is first generated outside the porous medium, then injected into the porous medium. This method is applicable in soils with permeabilities above 10–9 m2 (>150 darcy), and/or in presence of agents that destabilize foams, such as hydrocarbons (Osei-Bonsu et al. 2015, 2017).
-
Co-injection: Simultaneous injection of gas and surfactant solution directly into the porous medium, where foam is formed via the different mechanisms described in the foam generation section.
-
Surfactant alternating gas (SAG): First a surfactant solution slug is injected, followed-up with the injection of a given amount of gas, and the whole operation is repeated many times over. This method of injection allows for better injectability, which is required in soils with permeabilities below 3 × 10–9 m2 (<150 darcy). However, reaching steady state using this method takes a significant amount of time.
17.4 Non-Aqueous Phase Liquid (NAPL) Recovery Mechanisms
At the microscopic scale, foam is a two-phase material that is subjected to many complex mechanisms in porous media, which taken together give foam its macroscopic properties. In an environmental remediation context, foam (i) has improved sweep efficiency in heterogeneous aquifers due to its non-Newtonian properties, (ii) eliminates displacement front instabilities caused by viscous forces, and (iii) lowers the capillary forces that otherwise maintain non-aqueous phase liquid (NAPL) droplets trapped, by decreasing the interfacial tension between the aqueous and organic phases in the presence of surfactants. The main characteristics of foam and a description of how each one contributes to contaminant mass removal are summarized in Fig. 17.6 (Fitzhenry 2021). Surfactant foams are able to recover NAPL present in an aquifer through three mechanisms: solubilization, mobilization, and volatilization.
Foam characteristics and NAPL recovery mechanisms (adapted from Fitzhenry 2021)
17.4.1 Solubilization (Miscible Displacement)
The particular structure of amphipathic surfactant molecules allows them to position themselves at the interface between two immiscible liquids, and to lower the interfacial tension of the system (see Fig. 17.7) as also discussed in Chapter 16.
Structure of foam bubble walls, with gas (green), surfactant solution (blue), and NAPL (red) adapted from Farajzadeh et al. (2012)
In a contaminated aquifer, micelles have the ability to both encapsulate NAPL droplets in their cores (emulsification) and dissolve the molecules of the NAPL constituents directly into the surfactant solution (solubilization). Micellar solubilization thus increases the concentration of the contaminant in the liquid fraction of the foam, making the contaminant more available for removal.
According to the mechanism proposed by Schramm and Novosad (1990), when foam in a porous medium encounters a NAPL, the decrease in interfacial tension between the surfactant solution and the NAPL can lead to the formation of fine micelles around the NAPL droplets which become embedded in lamellae, as shown in Fig. 17.8. Although this allows the droplets to be transported for some distance, the incorporation of emulsified NAPL into lamellae can negatively affect foam stability, causing lamellae to eventually collapse and release the NAPL. Subsequently, an intact lamella may arrive, pick up the NAPL and carry it a little further, and so on. This mechanism of transport is very effective, leading to high ratio of mass of hydrocarbon recovered to mass of surfactant used (10:1; Maire et al. 2015). Moreover, the displacement of initially trapped NAPL droplets in the form of an emulsion results in a reconnected continuous phase of non-wetting fluid, which increases its mobility when subjected to pressure.
Left, visualization of n-hexadecane droplets, stained with Oil Red-O, embedded in lamellae of a foam outside of porous media (Simjoo et al. 2013b). Right, theoretical diagram illustrating the configuration of surfactant monomers (gas in green, surfactant solution in blue, and organic phase in red), adapted from Denkov (2004)
17.4.2 Mobilization (Immiscible Displacement)
Entrapped NAPL saturation (Soe) in an aquifer takes the form of discontinuous and near-immobile NAPL droplets that are trapped in pores by capillary forces. By definition, Pc is the difference between the pressures of the organic (typically the non-wetting phase) and aqueous (wetting) phases (Pc = Po − Pw). In order for NAPL trapped at residual saturation to flow into a neighboring pore, the capillary pressure must exceed the entry capillary pressure of said pore Pce (Joekar-Niasar et al. 2008):
where θ is the contact angle at the interface between the two liquids and the pore surface, and r is the pore radius (m). The efficiency of immiscible displacement of a NAPL by a washing solution is controlled by the capillary number (NCa), which is defined as the ratio of the viscous forces that promote displacement of NAPL droplets, to the capillary forces that maintain NAPL droplets trapped in the pores (Pennell et al. 1996):
where qw and µw correspond to the Darcy flux (m/s) and dynamic viscosity (Pa·s) of the displacing fluid (water), respectively, σow to interfacial tension between the displaced (NAPL) and displacing fluid (N/m), and cos θ to the wettability condition of the medium. In order to promote mobilization of NAPL as a free phase bank in the porous medium, it is necessary to increase NCa significantly by lowering interfacial tension by several orders of magnitude using surfactants.
NAPL recovery by mobilization can be represented by a capillary desaturation curve (CDC), a semi-logarithmic graph that shows the relationship between residual saturation in soils and capillary number (Lake 1989). A typical CDC (Fig. 17.10left) is characterized by a constant residual saturation (Sor) of the non-wetting phase at low capillary numbers, a critical capillary number (NCa*) required to initiate desaturation, and a full desaturation capillary number (NCa**) when Sor becomes zero (Robert et al. 2017).
The mobilization capacity of a NAPL depends on the properties (viscosity, density, wettability, and interfacial tension) of both the NAPL and the displacing fluid, as well as on the properties of the porous medium (permeability and pore connectivity). In fact, the wettability of the system and the particle size distribution of the soil both have an important influence on the shape and position of the CDC. For example, poorly sorted soils result in more inclined CDCs that cover a wide range of capillary numbers, due to large variations in pore size.
In addition, Dwarakanath et al. (2002) observed that the use of anionic surfactants can render an aquifer preferentially water-wet, resulting in a shift of the associated CDC to the left by 1–2 orders of magnitude, as shown in Fig. 17.9. A left-shifted CDC in a water-wetting system means that complete desaturation will be more readily achievable than in a mixed wetting or oil-wetting system, since the same saturation value can be achieved with a lower capillary number. It should be noted that the effect that the adsorption of different types of surfactants onto the porous material has on system wettability is complex. Wettability depends on the way that the solid phase and the surfactant chemically interact, on the degree of saturation of the available adsorption sites, as well as on the way that the surfactant molecules interact with each other (monolayer vs. multilayers).
In the literature, alternatives to the NCa have been proposed, in an effort to improve the assessment of mobilization capacity by accounting for the buoyancy force. The Bond number (NB) is the ratio of buoyancy to capillary forces, while the total trapping number account for both NCa and NB contributions (Pennell et al. 1996).
Despite the destabilizing effect that light hydrocarbons have on foams, the latter still demonstrate better sweep efficiency in heterogeneous media when compared to conventional soil washing techniques that employ liquid solutions (Wang and Chen 2012). This is the result of the foam behavior presented in the first section of this chapter. This phenomenon provides a more uniform sweep of the porous medium. Nevertheless, foam flow in porous media is very complex and difficult to predict at the microscopic scale, which makes the total desaturation difficult to achieve in practice, due in part to bypassing of NAPL due to pore blocking by trapped bubbles.
17.4.3 Volatilization
A foam that is stable enough to flow through the porous medium can be composed of gas up to 98%, which represents the majority of the foam volume. Thus, the presence of gas bubbles in the saturated zone during foam flow makes it possible for some of the contaminant to be transferred into the gas phase by volatilization, depending on the vapor pressure of the contaminant. Mulligan and Eftekhari (2003) demonstrated that a large portion (66%) of pentachlorophenol in a fine sand was removed by volatilization during foam injection tests in columns. Similarly, Yan et al. (2019) conducted foam injection tests in columns of nitrobenzene-contaminated sand and found that 56% of the contaminant was removed by volatilization. Experiments studying the removal of hydrocarbons from creosote-contaminated soils involved mass balances calculations (C10–C40 TPH index) that indicated 80% of the contaminant was removed in the gas phase (Fatin-Rouge 2020). A foam injection experiment conducted by Longpré-Girard et al. (2016) in a 2D sandbox contaminated with p-xylene at residual saturation indicated that 65% of the contaminant mass was removed by volatilization.
It should be noted that the degree of volatilization depends on the physico-chemical properties of both the contaminant and the mobilizing fluid (in this case, foam).
Regarding the contaminant, its vapor pressure and Henry’s constant are of main importance. Both values tend to decrease the longer the hydrocarbon chain, and the higher the degree of H-bonds or stacking interactions. For mixtures of contaminants, the molar composition also plays an important role, as dictated by Raoult’s law. Temperature also influences these values and is important to consider, especially when considering coupling foam injection with in situ thermal desorption. The octanol–water partition constant (Kow), which quantifies a contaminant’s affinity for the aqueous phase, is equally important.
Regarding the foam, both the foam quality as well as the extent of micellar solubilization are important. Higher foam quality is associated with increased transport in the gas phase, whereas high concentrations of micelles will stabilize the contaminants in the liquid phase. Some authors have reported that the degree of volatilization of a contaminant tends to decrease the higher the surfactant concentration (Bouzid et al. 2019; Roy et al. 1995), which is likely due to the isolation of the compound within the micelles, making it stabilized in the liquid phase.
17.5 Laboratory Experiments
17.5.1 Column Experiments Examining Foam–Oil Interactions
The various experimental approaches employed in the literature have examined different aspects of foam–oil interactions and oil mobilization mechanisms in porous media. 1D experiments carried out in well-controlled laboratory conditions can provide excellent insights into pressure drop versus injection velocity and imbibition-drainage versus capillary pressure relationships. These types of experiments also allow for easy exploration of different parameters, such as chemical formulation of foams. In the enhanced oil recovery (EOR) literature, the behavior of foam in porous media in the presence of hydrocarbons has been studied in detail. Most of these studies were performed in Bentheim sandstone core, a material whose permeability (on the order of 2–10−12 m2) and mineralogy are considered representative of petroleum reservoirs (Peksa et al. 2015). In order to replicate the typical conditions of an EOR process, the common practice is to perform tests at elevated temperatures and pressures on columns initially saturated with brine.
Farajzadeh et al. (2010) performed foam tests by alternating surfactant (AOS) and gas (N2 or CO2) injection in sandstone cores (SAG method). The front half of the cores were saturated with brine, while the back half of the core were at residual oil saturation (Isopar H, C9–C11). This configuration was used in order to reproduce the water–oil transition encountered in oil reservoirs. They observed that at low pressures (1 bar), the presence of oil does not allow foaming in the core. At higher pressures (90 bar), the CO2 foam reduced gas mobility in the region of low oil saturation; however, upon contact with the oil-containing zone, the gas and surfactant separated, and there was no oil recovery at the outlet. At even higher pressures (137 bar), the foam formed in the front part of the core persisted in contact with the oil, and the resulting increasing pressure increased the recovered mass.
Simjoo et al. (2013a) sought to improve the understanding of how the presence of an organic phase in a porous medium affects the rate of removal of that organic phase by foam. Previous studies often involved cores saturated with oil over only a portion of their length, in order to create a clean “antechamber” in which foam can form in situ prior to contact with oil. In this case, foam injection tests were carried out in sandstone cores containing a residual hexadecane phase along its entire length; this configuration is relevant in an ER context, where injection wells are placed within a source zone in order to displace NAPL and direct it to surrounding recovery wells. Thus, foam was formed by co-injecting gas (N2) and surfactant solution (1 wt%) at a constant total flow rate (Q = 1.1 mL/min, fq = 91%). The surfactants employed included an AOS, as well as a mixture (1:1 by weight) of AOS and a polymeric fluorosurfactant (FC), and all solutions were prepared using brine (0.5 M NaCl). From their tests, Simjoo et al. (2013a) identified two regimes of oil displacement. The first involves an increase in the NCa and the formation of an oil bank. During this regime, oil saturation decreases until a value is reached at which oil cannot be displaced by the viscous force alone. This is when the second regime occurs, where the NCa remains nearly constant, and the oil is moved droplet by droplet as a dispersed phase.
Janssen et al. (2019) investigated a combined foam and alkaline solution injection process in sandstone cores for hexadecane recovery. They sought to understand the effects that different salt contents in a surfactant solution might have on the formation of an oil bank and its subsequent mobilization by foam. The sandstone cores used, initially at residual oil saturation, were previously treated by injection of a solution bank composed of brine (0–2.5 wt% NaCl), an alkali (Na2CO3), a surfactant (internal olefin sulfonate, or IOS), and an alcohol (sec-butanol). This first step led to an increase in capillary number, which allowed the mobilization of oil droplets that coalesced into an oil bank. Downstream of the oil bank, some oil was solubilized and formed a microemulsion. The next step was to simultaneously inject gas (N2) and surfactant solution (IOS and Na2CO3 mixed with brine), which resulted in the formation of a strong foam and efficient oil displacement. The authors hypothesized that upon contact with the oil bank, a decrease in effective porosity caused a local increase in pressure drop, large enough to initiate foam generation. Thus, the results demonstrated that foam formation is possible in the presence of high oil saturation under these experimental conditions.
Tang et al. (2019) conducted sandstone core tests to compare how foams injected in different modes (i.e., pre-generation and co-injection) are affected by an organic phase present at residual saturation. The surfactant solution used was 0.5 wt% AOS with 3 wt% NaCl, and the gas used was N2. A series of tests were performed for each of the two oils studied: (1) hexadecane, and (2) a mixture of hexadecane and oleic acid (80:20 by mass). Tests with hexadecane (known to be weakly destabilizing) revealed similar results for both pre-generation and co-injection. In both cases, formation of a foam bank was observed, with gradual refinement of the foam texture along the core. Tests with the oleic acid/hexadecane mixture (known to be highly destabilizing) revealed significant differences between the propagation of the foams formed by the two injection modes. Co-injection had barely formed foam, even in the presence of low oil saturation (Sor ≈ 0.1). Weak foam began to form near the column outlet, and the foam began a very slow propagation in the opposite direction of injection. On the other hand, the pre-generated foam showed two phases of propagation, the first characterized by weak foam that gradually lowers the oil saturation, to allow for the eventual formation of a strong foam front in the second phase.
17.5.2 Adding Particles to Foam in Presence of Oil
The molar mass and molecular structure of surfactants may influence the stability of foam lamellae in the presence of organic phases (Vikingstad et al. 2006), although general trends remain unclear. Some studies (Osei-Bonsu et al. 2015; Yu et al. 2019) indicate that the rate of liquid drainage from lamellae was slowed for foams created from viscous surfactant solutions. This suggests that additives included in the surfactant solution to increase viscosity could help maintain foam stability in the presence of NAPL. In contrast, in contaminated sand column tests, Forey et al. (2019) observed that the higher the polymer concentration (and thus the viscosity of the surfactant solution), the lower the foam RF, meaning that the foam collapses easily upon contact with the organic phase.
The incorporation of colloidal particles into surfactant solutions to improve the efficiency of the foam injection process has been studied extensively in petroleum engineering (Nguyen et al. 2014; Singh and Mohanty 2017; Sun et al. 2014; Yang et al. 2017) and in environmental settings (Forey et al. 2019; Karthick and Chattopadhyay 2017). It is necessary to optimize the surfactant solution formulation, including particle type (composition, hydrophobic/hydrophilic behavior) and concentration, to avoid the formation of complexes (precipitates and aggregates) that can interfere with foam formation and clog the porous medium. Moreover, solid particles used to reinforce a foam in an environmental context must be non-toxic. Finally, the particles must be small enough not to reduce the permeability of the medium by deposition.
Sun et al. (2014) conducted micropattern and sand column foam injection tests to investigate the effect of adding hydrophobic silica nanoparticles (diameter = 14 nm) on the recoverability of a crude oil. The surfactant solutions used for the sand column tests consisted of sodium dodecyl sulfate (2.2 CMC), NaCl (0.5 wt%), and a variable SiO2 concentration (between 0 and 2 wt%). Foam was generated by co-injection of the surfactant solution and gas (N2) at a constant total flow rate (Qfoam = 0.005 mL/min, fq = 50%). An increasing recovery efficiency was observed for SiO2 concentrations of 0–1 wt%, but for concentrations ≥ 1.5 wt% the improvement was negligible. As confirmed visually in micropatterning, the adsorption of solid particles onto the walls of foam bubbles allows the bubbles to maintain a spherical shape in the medium, indicating that they are more difficult to deform. This increased viscoelasticity allows the bubbles to exert a greater “micro-force” on the trapped droplets and dislodge them in greater numbers, even from dead-end pores.
Karthick and Chattopadhyay (2017) compared the effect of adding hydrophobic and hydrophilic silica nanoparticles for soil column diesel recovery by pre-generated foam injection. The surfactant solution used was Tween-20 (0.1 vol%; non-ionic) and 0.5 wt% SiO2 nanoparticles (diameter = 50–80 nm). The hydrophobic particles had formed aggregates in water and had to be pretreated with tetrahydrofuran. The results revealed recovery rates of 78 and 57.5% with the solutions containing hydrophobic and hydrophilic particles, respectively. The authors attribute this difference to a better adsorption capacity at air–water interfaces in hydrophobic particles. For comparison, a soil washing test with the Tween-20 solution without nanoparticles in the same column recovered only 42% of the initial diesel; no nanoparticle-free foam test was performed.
17.5.3 Layered 2D Sandboxes
Studies in 2D cells are useful for improved understanding, controlling, and modeling of the mechanisms that drive the complex behavior of foam and the redistribution of contaminants. 2D studies are ideal for studying fluid behavior in permeability/wettability contrasted media, which reflect the subsurface anisotropy of complex contaminated sites where usual treatments can be ineffective. The interest of foam in that context is its sweeping efficiency coupled to the processes presented above in the interaction oil/foam paragraphs.
17.5.3.1 Longpré-Girard et al. (2016)
In this study, the custom 2D sandbox used consisted of a stainless steel tank (1.5 cm thick walls) with an internal volume of 2.5 L (26 cm long, 14 cm high and 8 cm thick) and a tempered glass window (1.5 cm thick) to enable visualization of foam flooding experiments (Longpré-Girard et al. 2016). The ability of surfactant foam to enhance mobility control and light NAPL (LNAPL) recovery in a layered heterogeneous porous medium was investigated. The 2D sandbox was filled with two layers, respectively, of coarse and medium silica sand. The surfactant solution used was Ammonyx Lo at a concentration of 0.1% (w/w). The experimental protocol included tracer tests in uncontaminated and contaminated (p-xylene residual saturation of 0.17) conditions, foam injection under uncontaminated conditions, as well as surfactant solution and foam injection under contaminated conditions. The foam was pre-generated and injected into the tank using increasing pressures (from 14.7 to 51.5 kPa). Tracer tests indicated that the permeability contrast between sand layers was increased by LNAPL contamination. Foam injection under uncontaminated conditions presented an S-shaped front that indicated a better mobility control than the piston-shaped front obtained during tracer tests. During foam injection, complete sweep of the sand tank was achieved with 1.8 pore volume (PV) compared to 2.8 PV during tracer injection, thus indicating better mobility control with foam. Pre-flush of the contaminated sand tank with surfactant solution initiated p-xylene mobilization, but no free phase was recovered at the effluent. LNAPL recovery mechanisms involved during foam injection in the contaminated sand tank were 19% by mobilization, 16% by dissolution, and 65% by volatilization. The p-xylene concentration measured in the sand after the foam injection experiment was < 16.3 mg/kg (Fig. 17.10).
17.5.3.2 Effect of Nanoparticles Addition
Singh and Mohanty (2017) performed 2D sandbox foam injection tests containing two layers of sand (6:1 permeability contrast), to study the effect of adding hydrophilic silica nanoparticles (diameter = 10 nm) on the recovery of a crude oil. The foam was generated in a pre-column, using gas (N2) and surfactant solution (0.5 wt% AOS with 0.3 wt% SiO2 nanoparticles), and then injected into the tank at a constant velocity (vfoam = 1.4–10−5 m/s, fq = 80%). The particle-stabilized foam (generated ΔP = 34.5 kPa after 22 VP injected) recovered 9% more oil than the particle-free foam (generated ΔP = 27.6 kPa after 23 VP injected) injected under the same conditions.
The goal of the Forey et al. (2021) study was to investigate how the presence of oil impacts foam formation during a liquid–gas co-injection process. As organic phases, such as LNAPL, usually act as defoaming agents that thwart the use of foam in remediation treatments, the synergy between a biological surfactant (saponin 10 CMC 0.5% wt) and silica colloidal particles was explored to obtain a strong and stable foam. In the investigations, gas–liquid co-injection experiments were carried out in a large 2D porous medium in the absence and in the presence of oil (MACRON 1821 F-4 oil). When no oil was present, although foam visually swept the porous medium in a piston-like way, buoyancy segregation still acts, and a gas/liquid saturation gradient takes place across the flow direction. As the surfactant concentration increases, foam strengthens due to a higher lamellae stability that prevents bubbles to coalesce. If SiO2 colloidal particles are incorporated in the formulation, the resistance factor is slightly enhanced and the water saturation (Sw) is substantially decreased. SiO2 addition probably arises in thicker lamellae without significantly affecting the foam structure, and bubbles are in a shield consisting of solid SiO2 colloidal particles. By repeating the experiment in the presence of a layer initially at residual oil saturation, formed foams are destroyed when they come in contact with oil, thus increasing the local gas mobility while it seems unaffected in the rest of the container. Results showed that foam resistance to oil-induced destruction was less damaging when the surfactant concentration was increased and was damped when SiO2 particles were added to the formulation. With this formulation, the pressure gradient within the tank was similar in the regions with and without oil, indicating that the particles provided efficient mobility control.
17.5.4 Fractured Media
Fractured media are among the most difficult systems to manage, because of their strong heterogeneity and anisotropy, lower connectivity, the size, and the traveling of fractures that make flow very heterogeneous both in magnitude and direction (NASEM 2015). Despite all this, the improved sweep efficiency of foams and the ability to generate them in situ by co-injection has been demonstrated (Brattekås and Fernø 2016). Fatin-Rouge and Visitacion Carillo (2018) compared the recovery of different hydrocarbons with densities ranging from 0.9 to 1.8 g/cm3 in a water-saturated cell made of calcareous rectangular blocks with a network of fractures with size of ~3.5 mm. Pre-generated foams (fq = 95%) were injected an compared to aqueous solutions. Conclusions revealed that foam should be preferred for light and volatile contaminants given the low density of both foam and LNAPLs and the importance of the mobilized fraction in the gas phase of foam.
17.6 Field Applications
Foam has been applied to very few sites, and this was on chlorinated solvents contamination. The cases are presented below as the foam behavior and field processes could be very useful to understand the behavior of foam in presence of floating LNAPL.
17.6.1 Hirasaki et al. (1997a; b)
The first application of foams for in situ remediation of soils at the field-scale was performed on a military base located in Ogden, Utah (Hirasaki et al. 1997b). The contamination consisted of trichloethylene (TCE), a chlorinated solvent used for degreasing metal parts, released to the environment as a result of its improper storage in trenches without impermeable bases. The contaminant was present at a concentration of 668 mg/kg as a residual saturation (no NAPL was detected in the test cell). The water table was located at approximately 7.6 m above ground level. The aquifer consisted of a sand horizon (0–15.2 m, K ˃ 9.8–10−4 m/s), with an underlying clay layer (˃15.2 m, K < 3.9–10−4 m/s) that acted as a capillary barrier and prevented vertical migration of contaminant. An environmental characterization of the site revealed the absence of TCE in areas of high permeability. Thus, the approach of the pilot test was to use the foam to regulate the hydraulic conductivity profile of the aquifer by selectively blocking the high permeability layer to improve the recovery by the surfactant solution in the low-permeability layer.
The test cell had an area of approximately 85 m2, on which 10 wells were constructed, including 3 injection wells, 3 recovery wells, 2 observation wells, and 2 hydraulic control wells. The injection and recovery wells were arranged in rows. Two observation wells were installed in the center of the cell and were used to identify the arrival time of the various fluids. A groundwater pumping and re-injection system was set up to prevent the loss of fluids outside the treatment area. The surfactant solution used was 4 wt% dihexyl sulfosuccinate (Aerosol MA-80I) (˃4 CMC) and 10,250 ppm NaCl, a formulation that was optimized to maximize solubilization. The test lasted 3.2 days and consisted of simultaneous injection of surfactant solution at a constant flow rate (Q = 113 L/min) into two injection wells at a time, and air supplied by a constant pressure compressor (manually adjusted to be between 55 and 76 kPa) into the 3rd injection well. Air injection was alternated between the three wells over a two-hour period. The amount of TCE measured in the soils after treatment was 77 mg/kg, representing a removal of ~88% of the initial mass.
17.6.2 Portois et al. (2018a; b)
A foam injection test was conducted by Portois et al. (2018b) at an active plant site in Spain contaminated by a storage tank leak during the 1980s. The aquifer beneath the site had a very heterogeneous stratigraphy, consisting of an impermeable clay layer from 0 to 5.5 m, a Quaternary aquifer composed of fine to medium sand (K ≈ 10–6 − 4.10–5 m/s) from 5.5 to 7.5 m, and an impermeable clay horizon from 7.5 to 11 m. The contamination was present as a dissolved plume of TCE (max. concentration 300,000 µg/L). Given the presence of low-permeability clay layers, there were concerns about exceeding the maximum safe injection pressure during the pilot test. The approach was therefore to inject the foam into the permeable sand layers, to provide temporary containment around the source area and to limit plume flow downstream. This study was a first step in the development of a method that allows for the delivery of reagents into a source zone using foams.
Left: aerial view of foam injection system showing the position of the wells (black dots), the estimated position of the TCE NAPL source zone (red area), the hydraulic conductivity (K, m.s−1) for the natural terrain and the estimated position and K of the foam areas (in squared black on white) from modeling. Right: value of the fluxes measured downstream the injection area, before and after the foam injection
The test cell had an area of ~1800 m2 in which 6 injection wells (screened interval 5.7–7.7 m) were installed around the periphery of the source zone. The surfactant solution used was a mixture of sodium laureth sulfate, cocamidopropyl betaine, and lauryl glucoside, and air was supplied by a compressor. No surfactant injection was performed prior to foam injection to avoid mobilizing the contaminant. The test was conducted over a 96-h period, during which surfactant solution and air were co-injected into all 6 wells simultaneously. Compliance with the injection pressure limit of 300 kPa (to avoid fracturing) was ensured during the test by manually adjusting the total injection rate (Qfoam = 12.1 − 35.4 L/min), foam quality (95–99%), and surfactant solution concentration (0.3–0.9 wt%, i.e. 4–14 CMC). Blocking capacity was defined in terms of the ease of water flow through the medium: a decrease of more than 100 times the initial Kw was measured around the wells after foam injection. According to a numerical model, the radius of influence of the injected foam was 3.2 m (Fig. 17.11left). After foam injection, down gradient from the source zone, a three to fourfold reduction in the TCE mass discharged was measured (Fig. 17.11right).
17.6.3 Maire et al. (2017, 2018a; b)
Maire et al. (2018a, b) performed the first field-scale application of foam injection for NAPL displacement and recovery by pumping. The study site was an active chlor-alkali plant located in Tavaux, France. The simplified stratigraphy of the aquifer was as follows: a fine alluvial horizon (0–2 m, K ≈ 10–7–10–9 m/s) at the surface, followed by coarse alluvium (2–9.5 m soil, K ≈ 10–2–10–4 m/s), and an underlying impermeable marly bedrock (K ≈ 10–9 m/s). Over the course of a year, the water table varied between 2 and 4 m below ground surface. The contaminant at the site consisted of a complex mixture—which included hexachlorobutadiene (58%), hexachloroethane (16%), pentachlorobenzene (3.5%), and tetrachloroethylene (8%)—present as an 8.3 m3 NAPL lens accumulated at the bottom of the aquifer (pool). In preparation for the pilot test, watertight bentonite cement walls were constructed along the perimeter of the cell (100 m2 square) to prevent the loss of contaminants outside the treatment area. Nine wells were installed in the cell, including a central recovery well, an observation well in each of the four corners of the cell, and four foam injection wells. The foam injection wells were constructed around the central well, at a distance of 3 m from the central well, with injection sections of 9–9.5 m between packers. During the first phase of the test, which lasted ~1 month, groundwater was pumped from the central well and re-injected into the four corners of the cell, with the goal of recovering as much free phase as possible via a submersible pump installed in the central well: this configuration resulted in the recovery of 7.6 m3 of NAPL. The second phase of the test consisted of foam injection in SAG mode, i.e., by alternating the injection of 1/6 VP of surfactant solution (sodium dihexylsulfosuccinate, 4 wt% or 2.7 CMC) followed by 1/3 VP of air. The injection was performed at constant pressure (value not specified).
Since the foam flows radially around an injection well, the NAPL was directed not only to the central well, but also to the four corners of the cell. In addition, due to technical limitations, the injection could only be performed in two wells at a time, instead of all four simultaneously. The configuration of the wells was found to not be optimal for the convergence of the NAPL to the central recovery well. No quantification of NAPL recovery was reported, but verification boreholes completed three weeks after injection revealed the presence of foam and the absence of NAPL droplets in the soils.
17.7 Modeling Foam Behavior
As the different mechanisms described above show, foam presents a complex behavior in porous media. Two main types of models have been proposed so far to simulate foam behavior. Note these approaches do not include multiphase simulation of NAPL transport. Firstly, the so-called bubble generation models describe in detail the generation and coalescence of bubbles according to parameters related to the dynamics of the different fluids present and the porous medium. The so-called local equilibrium (LE) models are an extension of the multiphase models in porous media which modify the properties of the gas phase when it is present in the form of foam. Globally, bubble models lead to a finer description of the processes and can be partly validated by micro-model experiments, even if this requires some averaging. On the contrary, LE models are rather intended for core, 2D pilot or reservoir scale simulations and do not include some detailed mechanisms.
Different articles highlight the convergence of the two types of approaches on generic cases, see in particular the excellent synthesis of Ma et al. (2015) and the Lotfollahi et al. (2016) comparative study. We will use here a LE formulation. This formulation is often referred to as the “STARS” approach because this model was one of the first to develop a generic foam model. However, this type of model is currently included in most reservoir models, and we further present an implementation in the OpenFoam free library.
This modeling part only deals with the specificity of foam flow. For the interaction of foam with contaminants or oil phase, other components of multiphase flow models shall be used. In the oil industry multiphase models like ECLIPSE or others include the interface tension between three phases and shall be able to model foam–oil interactions (e.g., Olabode et al. 2021). To our knowledge there are no models of foam injection with TOUGHREACT (Xu et al. 2012) which lacks of the foam properties part. Foam can be modeled with UTCHEM as a specific fluid, and the model may also simulate the foam–oil interactions. However, Janssen et al. (2020) showed that the parametric approach of UTCHEM could not reproduce the foam behavior observed in experiments.
17.7.1 Foam in Porous Media Theoretical Basis
In the framework of the generalized Darcy theory, the foam is represented as a gas with specific properties, mainly a higher viscosity. The gas permeability is modified according to the following equation:
where \({q}_{g}\) is the Darcy flux of gas, k the permeability, \({k}_{rg}\) the relative permeability for the gas phase, \({k}_{rg}^{f}\) the relative permeability modified to account for foam, P is the pressure, g the gravity, \(\rho\) the density, \(fmmob\) the maximum foam resistance, and Fi are foam factors.
In the STARS approach, each of the Fi function lies between 0 and 1 and the lower it is, the higher the \({k}_{rg}^{f}\) will be, i.e., the lower the foam strength. Thus, this model allows, when one of the factors is zero, to return to a model of classical gas transport. In its formulation, the model also implicitly contains the fact that the presence of foam does not directly modify the capillary curves of water in the porous medium, which had been demonstrated by Bernard and Jacobs 1965 or Vassenden and Holt 1998 and more recently by Eftekhari and Farajzadeh (2017). Thus, the presence of foam mainly leads to a change in Sw which in turn modifies the gas relative permeability.
As shown in the previous sections, the major specificity of the foam is to present a maximum of viscosity for a given water content (often low), with a very fast decrease of apparent viscosity for low water contents (high quality) and much slower for the high water content part (low quality). This specific behavior is reproduced by a factor directly related to the water content, called Fdry for the dry-out effect of the foam at high gas contents. In the STARS model, Fdry is represented by:
where fmdry is the critical water saturation under which foam collapses and epdry controls the abruptness of the foam collapse (Vicard et al. 2022). This function creates a very steep front close to fmdry. Figure 17.12 represents on the left a modeled quality scan (QS) curve and on the middle the corresponding \({k}_{rg}^{f}\) curve on a log scale. We can see that the maximum apparent viscosity point corresponds to a very steep drop in \({k}_{rg}^{f}\). In the kr graph, the krg value for pure gas is presented, and one can see that the “gas as foam” relative permeability is several orders of magnitude lower than the pure gas, the difference depending mainly on fmmob value. Although close to Sw* the value of the water relative permeability is quite low, because the amount of water in the medium is low. This is illustrated by the right panel of the figure where the mobilities of the two phases are presented with the total mobility. It can be seen that close to Sw* the total mobility is very small and is driven by the gas phase while when Sw increases, i.e., in the low-quality region (low fg value in QS curve, high Sw) the water mobility becomes more important and finally drives the foam behavior.
Left: Quality scan curve with the maximum identified; middle: corresponding kr (Sw) curves for the QS curve, large red symbols indicating the correspondence between the points, krg for pure gas, \({k}_{rg}^{f}\) for gas as foam, krw for water; right: mobilities of the foam and water presented as function of Sw. Note that the high fg region corresponds to the low Sw range
The other functions have all the same formula in the STARS model:
where X is a variable, and fi and epi are constant. The functions studied here concern the effect of non-Newtonian behavior with the factor Fcap, the effect of the presence of oil with Foil, and the surfactant concentration Fsurf.
17.7.2 Fitting Foam Model Parameters
The STARS model includes in general two parameters for each factor, but the calibration is difficult because the observation is done on QS curves, whereas the real parameters of the model are the Sw and \({k}_{rg}^{f}\) curves which are difficult to measure in this context. Moreover, the maximum of a QS curve is rarely obtained experimentally, and it does not correspond directly to the fmdry parameter. Therefore, there are different approaches that are all iterative. Ma et al. (2014) propose a method for fitting the fmdry parameters using optimization on a steady-state experiment supplemented with a transient experiment. Zeng et al. (2016) start with a quality scan experiment (fixed total velocity) to determine Fdry parameters and the transition quality point fg* followed by experiments at the given fg* value for several total velocities. However, their approach did not cover all the low-quality regime and lead to very low epdry values which are too low compared to the literature. Abbaszadeh et al. (2018) used a similar approach. Boeije and Rossen (2015b) used the high-quality part of the curve to determine graphically the parameters fmdry and fmmob, then using the curvature of the low-quality part chose a point to obtain a value of epcap and then fmcap. This procedure gave good results but has the disadvantage of not being automatic. Hosseini-Nasab et al. (2018) used a multi-parameter joint calibration, but it was performed on a single curve.
Vicard et al. (2022) proposed a more general approach which aims at calibrating a set of curves obtained for different total velocities. This involved a calibration of the non-Newtonian part using an Oswald's law type approach on the set of curves, followed by an iterative calibration of the parameters of the Fdry curve, differentiated according to the total velocities. This calibration approach gave satisfactory results on three different data sets.
Some authors did not use quality scan curves to retrieve foam model parameters. Ding et al. (2020) performed history matching showing a good fit to the model parameters, including the presence of oil.
The study by Valdez et al. (2021) detailed an estimate of the uncertainties on the major parameters of a quality scan curve. It turns out that with a few points and a single curve, the uncertainty is quite high, but it decreases sharply if the number of points on the curve is increased, justifying the need of several curves to calibrate the various model parameters.
17.7.3 Effects of Permeability
Luo et al (2019) gathered about ten studies with permeability variations highlighting, for most of them, a power law increase of apparent viscosity with permeability. However, as the authors pointed out, most of the studies cannot be used directly to determine the model parameters, as most of the experiments were conducted for a single foam quality. The more detailed study by Farajzadeh et al. (2015), confirmed by Gassara et al. (2017), similarly revealed a power relationship, implying small variations of fmdry as a function of k. In other papers a decrease of fmdry with permeability is also shown. If the apparent viscosity increases with the permeability, the global pressure gradient decreases slightly, as the increase in viscosity does not totally compensate the effect of the permeability. This viscous effect leads, however, to a major effect of the foam, which is the profile stabilization. This effect already reported in the theoretical part is explored using modeling by Wei et al. (2018) whom show blocking of the most permeable channels due to foam. Wang et al. (2019) present results showing an increase in the apparent viscosity of the foam with increasing permeability, up to a peak that occurs around 1 Darcy for a 3:5 mixture of LAO and OA-12 (lauramidopropylamine oxide and N,N-dimethyldodecylamine).
17.7.4 Effects of the Surfactant Type and Concentration
Most studies show major factors (fmdry, fcap, epcap) that differ widely with the type of surfactant and even with their concentrations. Farajzadeh et al. (2015) show, using moradi-araghi data that the model parameters change depending on the surfactant used for the same medium and injection conditions. Jones et al. (2016) show that the concentration of AOS significantly changes the shape of the quality scan curve during nitrogen foam injection. The curves also differ depending on the solution used (brine or fresh water). Zeng et al. (2016) compared an anionic surfactant (AOS1416) with to zwitterionic surfactants (LB and LS) using nitrogen and demonstrates a better foam strength with the anionic AOS. Presently, there is, to our knowledge, no way to extrapolate the parameters value from one surfactant to another.
17.7.5 Effects of Total Velocity
We performed a global study on the role of the total superficial velocity (ut) on the variation of the quality scan curves and associated parameters gathering most of the studies providing such data (Vicard et al. 2022). This was done on three different media as no other studies report quality scan curves for different interstitial velocity. Figure 17.13 shows the major results as the variation of the transition point position between low and high quality with the total interstitial velocity. The value of fg* is constant for the studies in quite high permeability media of Vicard et al. (2021) (k = 5·10–11 m2) and Osterloh and Jante (1992) (k = 6·10–12 m2) while significantly increases with ut for lower permeability in the Alvarez et al. (2001) study (k = 5·10–13 m2). As it is the first analysis of this type, we do not know if this effect is really due to the permeability and if the results in other media would be similar. These three studies illustrate that the value of fg* largely varies according to surfactant (as already stated above), and quite low fg* values were obtained with saponin (Vicard et al. 2022).
Evolution of the breaking point of the quality scan curves as a function of velocity for three studies (Vicard et al. 2022, Alvarez et al. 2001, Osterloh and Jante 1992), left: the value of the gas fraction at the transition point (fg*), on the right value of the pressure gradient (Ut) at the same point (values of the Vicard’s experiments were multiplied by 10 to see them better). Ut in m·s−1
Concerning the pressure gradient (Fig. 17.12b) one thing clearly appears is that the pressure gradient is much higher in Alvarez et al. (2021) and Osterloh and Jante (1992) studies which operate in consolidated media and with non-environmental-friendly surfactant. It is known in the literature that the typical surfactants used in oil industry (AOS, SDS or similar) provide very strong foam and thus high pressure gradients.
In Alvarez et al. (2001), due to the small range of velocity tested, the shear thinning effect appears only at the highest velocity: the pressure gradient decreases (Fig. 17.12b). For the studies in higher permeability media, the relation between the pressure gradients shows a slight shear thinning effect for the Osterloh and Jante (1992) points but a simple linear relationship for Vicard et al. (2022)’s points. Therefore, as could be expected, the shear thinning effect varies with the medium permeability and certainly also with the type of surfactant associated with the pressure gradient value (Fig. 17.13).
17.7.6 Model Results to Understand Foam Behavior
With the limitations provided above, several papers used the STARS approach to detail the foam behavior in porous media.
17.7.6.1 Gravity Effect
Batot et al. (2016) illustrated the gravity effect on vertical 2D simulations (without radial effect) with a STARS-type foam model calibrated on 1D experiments. Using AOS and CO2, the simulations showed a gravitational effect on the injection without foam, while the foam allowed eliminating almost totally this effect, with velocities of the order of 200 m/year (or about 0.6 m/day). However, in order to limit this gravity effect, the tendency is to increase the strength of the foam, which induces a low injectivity. Boeije and Rossen (2015a) showed that, by using SAG, a simplified model can determine an injection optimum that limits the gravity effect.
17.7.6.2 Heterogeneous Media
Tham (2015) compared simulation scenarios with and without foam, showing the profile stabilization effect with foam, but under the conditions presented, the amount of oil recovered in the presence of foam increases by less than 10% compared to the water alternating gas case, thus reducing heterogeneity is not always enough to remove all the oil from the porous media.
17.7.6.3 Foam Flow Around Injection Wells
Gong et al. (2018) pointed out through a simplified model that conventional reservoir models have difficulties in representing the process of foam injection in the vicinity of wells. Their study showed that these simulators, due to the large grid size close to the wellbore, tend to simulate too low injectivity values for both gas and water.
17.7.6.4 Unstability and Transient Effects
In the presence of oil or in coarse media, different authors show that several PVs are needed before obtaining a stable and efficient foam (e.g., Hosseini-Nasab et al. 2018). These processes were modeled by Lotfollahi et al. (2016), in a bubble model, the process being quite complex. To our knowledge this transient effect is not directly modeled in LE models or simplified by a foam formation approach. Instabilities within the foam in a SAG-type process are described by Farajzadeh et al. (2016), who states that they are due to spatially varying mobilities and can be limited by reducing the mobility at the injection point to a value close to the front.
17.8 Applying Foam Models for LNAPL-Contaminated Sites
The vast majority of published studies have been carried out under conditions similar to those of underground reservoirs for enhanced oil recovery (EOR). However, for an environmental remediation application, many differences exist:
-
The permeability of the medium is often higher than deep sediments of EOR.
-
The injection pressures are limited by the sediment resistance close to the soil surface (Maire et al. 2018a, b; Fitzhenry et al. 2022).
-
Most experiments carried out for the EOR use CO2, whereas this being more soluble than air or N2 does not necessarily favor the desired behavior of the foam.
-
The temperatures of oil reservoirs are much warmer, which a priori represents more difficult conditions than those for aquifers at the surface.
It appears that the conditions in ER are very different, which means that it will not be possible to use the same parameters as those used in the EOR conditions. However, different studies show that the model formulation can remain the same, and foam data are getting more abundant for these media.
17.9 Conclusion
This chapter presented the behavior of foam in porous media with the objective to use it as a remediation or blocking agent. The physics of foam is complex which justifies the introduction of several parameters to transform classical multiphase models into foam specific models. These parameters are quite empirical and require numerous experiments. Moreover, the foam parameters depend not only on the porous medium but also on the chemical properties of the foam and the injection velocity. Laboratory experiments helped to understand the behavior of foam and demonstrated that foam is able to remove NAPL content of the porous medium to various levels, often better than with conventional surfactants or other techniques. Other advantages of foam are its low cost and shear-thinning behavior favoring injection front stability and sweeping of the porous media.
Very few experiments have been conducted at field scale, although they have been relatively successful. Several explanations can be considered: (i) before injection, multiple experiments must be performed to select surfactant and foam types, (ii) the well injection itself is complex to be set, and (iii) in environmental application the surfactant choice is limited and those surfactants are less effective than in the case of EOR. Despite these difficulties, the recent increase of interest in foam applications and the increasing technical level reported in the literature suggest that foam injection is a very promising technique for in situ remediation at complex sites.
References
Abbaszadeh M, Varavei A, Solutions IP, Rodrı F, Garza D, Enrique A, Miller CA (2018) Methodology for the development of laboratory-based comprehensive foam model for use in the reservoir simulation of enhanced oil recovery. SPE Reser Evaluat Eng 21:26–28
Alvarez JM, Rivas HJ, Rossen WR (2001) Unified model for steady-state foam behavior at high and low foam qualities. SPE J 6(03):325–333
Batôt G, Delaplace P, Bourbiaux B, Nouvelles IFPE, Pedroni LG, Nabzar L (2016) WAG management with foams: influence of injected gas properties an surfactant adsorption
Bernard GG, Jacobs WL (1965) Effect of foam on trapped gas saturation and on permeability of porous media to water. SPE J 5:295–300
Boeije CS, Rossen WR (2015a) Gas-injection rate needed for SAG foam processes to overcome gravity override. SPE J 20:49–59
Boeije CS, Rossen W (2015b) Fitting foam-simulation-model parameters to data: I. Coinjection of gas and liquid. SPE Reser Evaluat Eng 18:264–272
Bouzid I, Maire J, Fatin-Rouge N (2019) Comparative assessment of a foam-based method for ISCO of coal tar contaminated unsaturated soils. J Environ Chem Eng 7(5):103346
Brattekås B, Fernø MA (2016) New insight from visualization of mobility control for enhanced oil recovery using polymer gels and foams. https://doi.org/10.5772/64586
Cantat I, Cohen-Addad S, Elias F, Graner F, Hohler R, Pitois O, Rouyer F, Saint-Jalmes A (2013) Foams: structure and dynamics. Belin. https://doi.org/10.1093/acprof:oso/9780199662890.001.0001
Denkov ND (2004) Mechanisms of foam destruction by oil-based antifoams. Langmuir ACS J Surf Colloids 20(22):9463–9505
Denkov N, Tcholakova S, Politova-Brinkova N (2020) Physicochemical control of foam properties. Curr Opin Colloid Interf Sci 50:81. https://doi.org/10.1016/j.cocis.2020.08.001
Ding L, Cui L, Jouenne S, Gharbi O, Pal M, Bertin H, Gue D (2020) Estimation of local equilibrium model parameters for simulation of the laboratory foam-enhanced oil recovery process using a commercial reservoir simulator. https://doi.org/10.1021/acsomega.0c03401
Dwarakanath V, Jackson RE, Pope GA (2002) Influence of wettability on the recovery of NAPLs from alluvium. Environ Sci Technol 36(2):227–231
Eftekhari AA, Farajzadeh R (2017) Effect of foam on liquid phase mobility in porous media. Nature Publishing Group, New York, pp 1–8. https://doi.org/10.1038/srep43870
Falls AH, Hirasaki GJ, Patzek TW, Gauglitz PA, Miller DD, Ratulowski T (1988) Development of a mechanistic foam simulator: the population balance and generation by snap-off. SPE Res Eng 3:884–892
Farajzadeh R, Andrianov A, Zitha PLJ (2010) Investigation of immiscible and miscible foam for enhancing oil recovery. Ind Eng Chem Res 49(4):1910–1919
Farajzadeh R, Andrianov A, Krastev R, Hirasaki GJ, Rossen WR (2012) Foam-oil interaction in porous media: implications for foam assisted enhanced oil recovery. Adv Colloid Interf Sci 183–184:1–13
Farajzadeh R, Lotfollahi M, Eftekhari AA, Rossen WR, Hirasaki GJH (2015) Effect of permeability on implicit-texture foam model parameters and the limiting capillary pressure. Energy Fuels 29:3011–3018. https://doi.org/10.1021/acs.energyfuels.5b00248
Farajzadeh R, Eftekhari AA, Hajibeygi H, Kahrobaei S, van der Meer JM, Vincent-Bonnieu S, Rossen WR (2016) Simulation of instabilities and fingering in surfactant alternating gas (SAG) foam enhanced oil recovery. J Nat Gas Sci Eng 34:1191–1204
Fatin-Rouge N, Visitacion Carillo S (2018) Récupération de phases liquides organiques en milieu karstique. Institut UTINAM (UMR 6213)
Fatin-Rouge N (2020) Contaminant mobilization from polluted soils: behavior and reuse of leaching solutions. In: van Hullebusch ED et al (eds) Environmental soil remediation and rehabilitation, applied environmental science and engineering for a sustainable future. Springer, New York. https://doi.org/10.1007/978-3-030-40348-5_1
Fitzhenry E, Martel R, Robert T (2022) Foam injection for enhanced recovery of diesel fuel in soils: sand column tests monitored by CT scan imagery. J Hazard Mater 3:128777. https://doi.org/10.1016/j.jhazmat.2022.128777
Fitzhenry E (2021) Application de la scanographie à l’étude de l’injection de mousse pour le traitement de sols contaminés au diesel, Mémoire de maîtrise, Institut national de la recherche scientifique. Centre Eau Terre et Environnement, Québec, p 174
Forey N, Atteia O, Omari A, Bertin H (2019) Saponin foam for soil remediation: on the use of polymer or solid particles to enhance foam resistance against oil. J Contam Hydrol 27:103560. https://doi.org/10.1016/j.jconhyd.2019.103560
Forey N, Atteia O, Omari A, Bertin H (2021) Use of saponin foam reinforced with colloidal particles as an application to soil remediation: experiments in a 2D tank. J Contam Hydrol 238:103761. https://doi.org/10.1016/j.jconhyd.2020.103761
Friedmann F, Chen WH, Gauglitz PA (1991) Experimental and simulation study of high-temperature foam displacement in porous media. Soc Petr Eng Res Eng 6:37–45
Gassara O, Douarche F, Braconnier B, Bourbiaux B (2017) Calibrating and interpreting implicit-texture models of foam flow through porous media of different permeabilities. J Petrol Sci Eng 159:588–602. https://doi.org/10.1016/j.petrol.2017.09.069
Gong J, Global S, International S (2018) SPE-190435-MS modelling of liquid injectivity in surfactant-alternating-gas foam enhanced oil recovery
Hirasaki GJ, Miller CA, Szafranski R, Lawson JB, Akiya N (1997a) Surfactant/foam process for aquifer remediation. In: Society of petroleum engineers, international symposium on oilfield chemistry. Houston, TX, p 10
Hirasaki GJ, Miller CA, Szafranski R, Tanzil D, Lawson JB, Meinardus H, Jin M, Londergan JT, Jackson RE, Pope GA, Wade WH (1997b) Field demonstration of the surfactant/foam process for aquifer remediation. In: Society of petroleum engineers, annual technical conference and exhibition. San Antonio, TX, p 16
Hirasaki GJ, Lawson JB (1985) Mechanisms of foam flow in porous media: apparent viscosity in smooth capillaries. SPE J 25(02):176–190. https://doi.org/10.2118/12129-PA
Hosseini-nasab SM, Douarche F, Simjoo M, Nabzar L, Bourbiaux B, Zitha PLJ, Roggero F (2018) Numerical simulation of foam flooding in porous media in the absence and presence of oleic phase. Fuel 225:655–662. https://doi.org/10.1016/j.fuel.2018.03.027
Janssen MTG, Pilus RM, Zitha PLJ (2019) A comparative study of gas flooding and foam-assisted chemical flooding in bentheimer sandstones. Transp Porous Media 131(1):101–134
Janssen MTG, Torres Mendez FA, Zitha P (2020) Mechanistic modeling of water-alternating-gas injection and foam-assisted chemical flooding for enhanced oil recovery. Ind Eng Chem Res 59:3606–3616. https://doi.org/10.1021/acs.iecr.9b06356Jones
Jones SA, Laskaris G, Vincent-bonnieu S, Farajzadeh R, Rossen WR (2016) Journal of industrial and engineering chemistry effect of surfactant concentration on foam: from coreflood experiments to implicit-texture foam-model parameters. J Ind Eng Chem 37:268–276. https://doi.org/10.1016/j.jiec.2016.03.041
Jones SA, Getrouw N, Vincent-Bonnieu S (2018) Foam flow in a model porous medium: I. The effect of foam coarsening. Soft Matter 14(18):3490–3496. https://doi.org/10.1039/C7SM01903C
Karthick RA, Chattopadhyay P (2017) Remediation of diesel contaminated soil by tween-20 foam stabilized by silica nanoparticles. Int J Chem Eng Appl 8(3):194–198
Lake LW (1989) Enhanced oil recovery. Prentice Hall, Englewood Cliffs, NJ, p 550
Liontas R, Ma K, Hirasaki GJ, Biswal SL (2013) Neighbor-induced bubble pinch-off: novel mechanisms of in situ foam generation in microfluidic channels. Soft Matter 9(46):10971
Longpré-Girard M, Martel R, Robert T, Lefebvre R, Lauzon JM (2016) 2D sandbox experiments of surfactant foams for mobility control and enhanced LNAPL recovery in layered soils. J Contam Hydrol 193:63–73
Longpré-Girard M, Martel R, Robert T, Lefebvre R, Lauzon J-M, Thomson N (2019) Surfactant foam selection for enhanced light non-aqueous phase liquids (LNAPL) recovery in contaminated aquifers. Transp Porous Media 131(1):65–84
Lotfollahi M, Farajzadeh R, Delshad M, Varavei A, Rossen WR (2016) Comparison of implicit-texture and population-balance foam models. J Nat Gas Sci Eng 31:184–197
Luo H, Ma K, Mateen K, Ren G, Neillo V, Blondeau C, Bourdarot G (2019) A mechanistic foam simulator incorporating systematic dependencies of various foam properties on permeability
Ma K, Farajzadeh R, Miller CA, Lisa S, George B (2014) Sensitivity in simulating steady-state and transient foam flow through porous media. Transp Porous Media 102:325–348. https://doi.org/10.1007/s11242-014-0276-9
Ma K, Ren G, Mateen K, Morel D, Cordelier P (2015) Modeling techniques for foam flow in porous media. SPE J 20:453–470. https://doi.org/10.2118/169104-PA
Maire J, Fatin-Rouge N (2017) Surfactant foam flushing for in situ removal of DNAPLs in shallow soils. J Hazard Mater 321:247–255
Maire J, Brunol E, Fatin-Rouge N (2018a) Shear-thinning fluids for gravity and anisotropy mitigation during soil remediation in the vadose zone. Chemosphere 197:661–669
Maire J, Joubert A, Kaifas D, Invernizzi T, Marduel J, Colombano S, Cazaux D, Marion C, Klein PY, Dumestre A, Fatin-Rouge N (2018b) Assessment of flushing methods for the removal of heavy chlorinated compounds DNAPL in an alluvial aquifer. Sci Total Environ 612:1149–1158
Marchalot J, Lambert J, Cantat I, Tabeling P, Jullien MC (2008) 2D foam coarsening in a microfluidic system. EPL 83(6):64006. https://doi.org/10.1209/0295-5075/83/64006
Mulligan CN, Eftekhari F (2003) Remediation with surfactant foam of PCP-contaminated soil. Eng Geol 70(3–4):269–279
NASEM, National Academies of Sciences, Engineering and Medecine. (2015) Characterization, modeling, monitoring, and remediation of fractured rock. The National Academies Press, Washington, DC
Nguyen P, Fadaei H, Sinton D (2014) Pore-scale assessment of nanoparticle-stabilized CO2 foam for enhanced oil recovery. Energy Fuels 28(10):6221–6227
Olabode OA, Ogbebor VO, Onyeka EO (2021) The effect of chemically enhanced oil recovery on thin oil rim reservoirs. J Petrol Explor Prod Technol 11:1461–1474. https://doi.org/10.1007/s13202-021-01090-9
Osei-Bonsu K, Shokri N, Grassia P (2015) Foam stability in the presence and absence of hydrocarbons: from bubble- to bulk-scale. Colloids Surf A Physicochem Eng Aspects 481:514–526
Osei-Bonsu K, Grassia P, Shokri N (2017) Investigation of foam flow in a 3D printed porous medium in the presence of oil. J Colloid Interf Sci 490:850–858. https://doi.org/10.1016/j.jcis.2016.12.015
Osterloh WT, Jante MJ (1992) Effects of gas and liquid velocity on steady-state foam flow at high temperature. In: Society of petroleum engineers, enhanced oil recovery symposium. Tulsa, Oklahoma, p 12
Parlar M, Parris MD, Jasinski RJ, Robert JA (1995) SPE 29678 an experimental study of foam flow through berea sandstone with applications to foam diversion in matrix acidizing
Peksa AE, Wolf K-HAA, Zitha PLJ (2015) Bentheimer sandstone revisited for experimental purposes. Mar Petrol Geol 67:701–719
Pennell KD, Pope GA, Abriola LM (1996) Influence of viscous and buoyancy forces on the mobilization of residual tetrachloroethylene during surfactant flushing. Environ Sci Technol 30(4):1328–1335
Portois C, Boeije CS, Bertin HJ, Atteia O (2018a) Foam for environmental remediation: generation and blocking effect. Transp Porous Media 124(3):787–801
Portois C, Essouayed E, Annable MD, Guiserix N, Joubert A, Atteia O (2018b) Field demonstration of foam injection to confine a chlorinated solvent source zone. J Contam Hydrol 214:16–23
Ransohoff TC, Radke CJ (1998) Mechanisms of foam generation in glass bead pack. In: Society of petroleum engineers and research engineers, SPE Reservoir Engineering, pp 573–585
Robert T, Martel R, Lefebvre R, Lauzon JM, Morin A (2017) Impact of heterogeneous properties of soil and LNAPL on surfactant-enhanced capillary desaturation. J Contam Hydrol 204:57–65
Roof JG (1970) Snap-off of oil droplets in water-wet pores. SPE J 10(01):85–90
Rosen MJ, Et Kunjappu JT (2012) Surfactants and interfacial phenomena. John Wiley & Sons, Hoboken
Roy D, Kongara S, Valsaraj KT (1995) Application of surfactant solutions and colloidal gas aphron suspensions in flushing naphthalene from a contaminated soil matrix. J Hazard Mater 42(3):247–263
Schramm LL (2000) Surfactants, fundamentals and applications in the petroleum industry. Cambridge University Press, Cambridge
Schramm LL, Novosad JJ (1990) Micro-visualization of foam interactions with a crude oil. Colloids Surf 46(1):21–43
Sheng JJ (2013) Enhanced oil recovery. Field case studies. Gulf Professional Publishing, New York
Simjoo M, Dong Y, Andrianov A, Talanana M, Zitha PLJ (2013a) CT scan study of immiscible foam flow in porous media for enhancing oil recovery. Ind Eng Chem Res 52(18):6221–6233
Simjoo M, Rezaei T, Andrianov A, Zitha PLJ (2013b) Foam stability in the presence of oil: effect of surfactant concentration and oil type. Colloids Surf A Physicochem Eng Aspects 438:148–158
Singh R, Mohanty KK (2017) Foam flow in a layered, heterogeneous porous medium: a visualization study. Fuel 197:58–69
Sun Q, Li Z, Li S, Jiang L, Wang J, Wang P (2014) Utilization of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles. Energy Fuels 28(4):2384–2394
Tang J, Vincent-Bonnieu S, Rossen WR (2019) CT coreflood study of foam flow for enhanced oil recovery: the effect of oil type and saturation. Energy 188:1597
Tham SL (2015) A simulation study of enhanced oil recovery using carbon dioxide foam in heterogeneous reservoirs. In: SPE annual technical conference and exhibition. OnePetro
Valdez AR, Rocha BM, Maria J, Vilela A, Souza OD, Pérez-gramatges A, Weber R (2021) Foam assisted water–gas flow parameters: from core flood experiment to uncertainty quantification and sensitivity. Transp Porous Media 157:1–21. https://doi.org/10.1007/s11242-021-01550-0
Vassenden F, Holt T (1998) Experimental foundation for relative permeability modeling of foam. In: SPE Improved Oil Recovery Conference, Tulsa, April 1998.
Vicard A, Atteia O, Bertin H, Lachaud J. (2022) Estimation of local equilibrium foam model parameters as functions of the foam quality and the total superficial velocity. ACS omega 7(20):16866–76
Vikingstad AK, Aarra MG, Skauge A (2006) Effect of surfactant structure on foam–oil interactions: comparing fluorinated surfactant and alpha oelfin sulfonate in static foam tests. Colloids Surf A Physicochem Eng Aspects 279(1–3):105–112
Wang H, Chen J (2012) Enhanced flushing of polychlorinated biphenyls contaminated sands using surfactant foam: effect of partition coefficient and sweep efficiency. J Environ Sci 24:1270–1277. https://doi.org/10.1016/S1001-0742(11)60881-4
Wang Y, Yue X, Liu K, Zhang B, Ling Q (2019) Effect of permeability on foam mobility and flow resistance distribution: an experimental study. Colloids Surf A Physicochem Eng Aspects 582:123769. https://doi.org/10.1016/j.colsurfa.2019.123769
Wei P, Pu W, Sun L, Pu Y, Wang S, Fang Z (2018) AC SC. J Petrol Sci Eng 163:340–348. https://doi.org/10.1016/j.petrol.2018.01.011
Xu T, Sonnenthal E, Spycher N, Zhang G, Zheng L, Pruess K (2012) TOUGHREACT: a simulation program for subsurface reactive chemical transport under non-isothermal multiphase flow conditions, groundwater reactive transport models 1:74. https://doi.org/10.2174/978160805306311201010074
Yan S, Wei-guo C, Ya-bin L, Xin W, Jian W (2019) Enhanced removal efficiency and influencing factors of nitrobenzene in soil by foam flushing. IOP Conf Ser Earth Environ Sci 295:12047
Yang W, Wang T, Fan Z, Miao Q, Deng Z, Zhu Y (2017) Foams stabilized by in situ-modified nanoparticles and anionic surfactants for enhanced oil recovery. Energy Fuels 31(5):4721–4730
Yu Y, Soukup ZA, Saraji S (2019) An experimental study of in-situ foam rheology: effect of stabilizing and destabilizing agents. Colloids Surf A Physicochem Eng Aspects 28:578
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Atteia, . et al. (2024). Application of Foams as a Remediation and Blocking Agent. In: García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R. (eds) Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons. Environmental Contamination Remediation and Management. Springer, Cham. https://doi.org/10.1007/978-3-031-34447-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-34447-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34446-6
Online ISBN: 978-3-031-34447-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)