Abstract
Due to human activity and, to a lesser extent, natural processes, petroleum hydrocarbons continue to pollute the environment. These contaminants of concern can be found globally and their remediation is key to restoring affected sites to safe and functional status. Conventional treatment of sites contaminated with petroleum hydrocarbons relies heavily on remediation approaches that are often financially prohibitive or may be technically impractical and that sometimes produce undesirable by-products. Using microbes that occur in nature (if not always at the site), can be a viable treatment with distinct advantages. Understanding the environment, contaminants, and natural biological processes occurring are key aspects for effective application of remediation techniques that rely on biological processes. Whether by stimulating the native microbial community, or, secondarily, by augmenting the native community with known degrader populations to degrade the target compounds, bioremediation is a practical, effective, and sustainable natural solution to a wide array of contamination around the globe. This chapter explores approaches to bioremediation of both soil and groundwater contaminated by petroleum hydrocarbons, describing how the approaches work and the benefits and challenges associated with them. It focuses on the use of aerobic and anaerobic microbial bioremediation, phytoremediation, and mycoremediation to address petroleum hydrocarbons.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Petroleum hydrocarbon (PHC) pollution is a major and continuing environmental issue caused by human activity and, to a lesser extent, by natural processes. Water and soil contaminated by PHCs can harm local ecosystems and contribute to widespread environmental deterioration. Conventional treatment techniques for these contaminated sites rely on methods that remove, reduce, or mitigate the toxic effects of PHCs. These include pump-and-treat systems, air sparging, multiphase extraction, soil vapour extraction, and excavation. Excavated materials—typically soil—can be disposed of off-site or treated using one or more remedial technologies, including bioremediation, incineration (e.g. open pit burning), thermal desorption, chemical treatment, and containment systems. All of these treatments have the potential to be financially prohibitive, technically impractical, and may produce undesired by-products (Speight and Arjoon 2012); some of them may result only in transferring contaminants from one media to another, rather than degrading the contaminants to non-toxic end products.
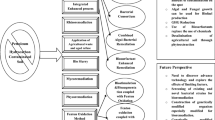
Bioremediation, which uses bacteria or other biological means to degrade contaminants, has emerged at the forefront of treatment technologies for petroleum-contaminated systems over the years (Morgan and Watkinson 1989; Butnariu and Butu 2020; Ławniczak et al. 2020). Because the natural environment contains a diverse consortium of microbial and plant life, harnessing the potential of living organisms to degrade and remediate contamination is a viable and effective treatment option (Fig. 14.1). Bioremediation functions either through biodegradation, which is the mineralization of the organic contaminant into carbon dioxide, water, inorganic compounds, and cell proteins, or it may transform the target organic compound into other simpler organic compounds that are generally less detrimental to the environment, such as methane, fatty acids, alcohols etc. (Atlas 1991; Speight and Arjoon 2012; Balseiro-Romero et al. 2018; Butnariu and Butu 2020).
At contaminated sites, PHCs commonly undergo biphasic biodegradation. In the initial phase of biodegradation, the rate of removal is high and primarily limited by microbial degradation kinetics of the native microbial community. In the second phase, the rate of PHC removal is slower and is primarily limited by bioavailability of the contaminant, with slow desorption of compounds from the soil mineral and organic matter fractions (Huesemann et al. 2004; Megharaj et al. 2011). Remediation timelines vary widely based on site specific conditions and the characteristics of the PHCs (Balseiro-Romero et al. 2018).
Whilst aerobic bioremediation approaches effectively remediate a wide range of PHCs, there are some instances where their application is technically impractical or excessively expensive due to oxygen demand or delivery. In these cases, previously unknown anaerobic approaches can also be considered. For example, whilst anaerobic benzene, toluene, ethyl benzene, and xylene (BTEX) bioremediation was once not expected to occur, it is now known to be possible under a range of anaerobic conditions (Ulrich and Edwards 2003). Whether aerobic or anaerobic, for bioremediation to be effective, microorganisms (naturally present or added through bioaugmentation) must enzymatically break down contaminants and convert them into less hazardous products. This chapter gives an overview of bioremediation processes for PHCs and provides a summary of the current state of its use in the field. PHC contamination occurs in many matrices including soil, water, and vapour. This chapter covers the application of bioremediation processes in both soil and groundwater which are intrinsically linked to each other in the natural environment. The principles of bioremediation are applicable to both and thus are the focus of this chapter.
14.1.1 Background on Petroleum Hydrocarbons
Petroleum is produced through thermal maturation of buried organic material over millions of years. This process involves diagenesis (a short period of biological degradation after deposition), catagenesis (geothermal degradation and cracking), and metagenesis (further decomposition, mainly producing methane) (Varjani 2017; Ławniczak et al. 2020). Petroleum itself can be defined as any hydrocarbon mixture of natural gas, condensate, and crude oil. PHCs are a complex mix of compounds that contain varying proportions of hydrogen atoms on a carbon frame; they may also contain nitrogen, sulphur, and oxygen. After being extracted from the subsurface, naturally occurring raw unrefined oil, known as crude oil, is transported to refineries where it undergoes distillation to produce usable products, including gasoline, diesel, and other refined products (Atlas 1991; Varjani 2017; Truskewycz et al. 2019; Ławniczak et al. 2020). These distillation products are not distinct entities—they are composed of a complex mix of hundreds or thousands PHC compounds of different molecular weights and chemical properties. Hydrocarbon compounds can be sorted into four groups based on their structure: alkanes (or paraffins), cycloalkanes (or naphthenes), alkenes (or olefins), and arenes (or aromatics). Alkanes are saturated aliphatic hydrocarbons, where each carbon atom forms four single bonds with hydrogen and other carbons (a common example is hexane, or octane); cycloalkanes are saturated ring hydrocarbons (e.g. methyl-cyclopentane); alkenes are unsaturated aliphatic hydrocarbons with at least two carbon atoms joined by more than one bond (e.g. ethene); and arenes consist of one or more benzene rings containing hydrocarbons (xylene, e.g. BTEX). Compounds within this final group containing two or more benzene rings are termed as polycyclic aromatic hydrocarbons or PAHs (common examples are naphthalene, phenanthrene, and pyrene) (Colwell et al. 1977; Varjani 2017; Truskewycz et al. 2019; Kuppusamy et al. 2020a, b). Biodegradability trends of PHCs vary based on many factors, including size, weight, bonding, structures (ring and branching), solubility, and the affinity for sorption to organics (Koc) or solids (Kd).
The composition of crude oil can vary based on location, age of the oil field, sources of organic matter, depth, and weathering (Atlas 1991; Ławniczak et al. 2020). Weathering is defined as processes that occur to the PHCs after their release into the soil, surface water, or groundwater. Source variability for environmental contamination can vary even more with potential organic or inorganic additives and with the age of contamination. Because sources and composition of contaminants of concern vary so greatly, environmental investigations often use the generalized term “total petroleum hydrocarbons” (TPH) to encompass all potential groups present at a location. The majority of PHC remedial efforts focuses on widely used fuels such as diesel or gasoline and specifically target BTEX and a limited number of PAHs due to their physical and chemical properties, toxicity, and adverse effect on human health (El-Naas et al. 2014). Other PHC compounds and associated products—including heavier PHCs, petroleum-derived metabolites, and arsenic (Cozzarelli et al. 2016)—may also pose negative impacts on the environment and exposed populations. Some of these impacts are not fully understood yet and their treatment may be particularly challenging.
To decide whether bioremediation is the best choice for remediation (and to decide when, where, and how to apply it), one must first understand which PHCs are present, how they may change over time, and their mobility. An accurate conceptual site model is critical to visualize the source, pathways, and receptors of the contaminated environment; such a model equips practitioners to choose an appropriate remedial plan to achieve clean-up targets. These targets are, in turn, usually developed based on potential endpoint uses, exposure risk to PHCs, and regulatory clean-up criteria for soil, surface water, and groundwater set by regulators and government agencies. Whilst a detailed discussion on the fate and transport of PHCs is not included herein, biodegradation of PHCs in soil and groundwater is key to controlling their fate and transport.
14.2 Microbial Degradation
14.2.1 Factors Controlling Biodegradation
Several factors affect the ability of microorganisms to successfully thrive and degrade target compounds, including the presence of such microbial populations, electron acceptors (e.g. oxygen, nitrate, sulphate, Fe3+), water, and nutrients, as well as the temperature and pH of the media. In addition to considering the organisms necessary for remediation, one must also consider that the specific compounds to be remediated will also affect the ability of microorganisms to biodegrade them; these compound-specific factors include concentration, solubility, and chemical structure. Third, the local environment and particular media will also have an overarching effect on remediation; factors including porosity, permeability, lithology, and groundwater flow impact both the microbial population and compound fate and transport (Fig. 14.1). Consequently, biodegradation rates are typically variable across a plume (Davis, 2023), for instance between “hot spots” and the plume edge.
Microorganisms present must be metabolically capable of breaking down the organic compound of concern, as well as potential by-products of the process. Sufficient moisture in the subsurface is also critical; 30–90% moisture content is commonly required for optimal oil degradation (Vidali 2001). Microbial communities require water not only for cellular processes, but also as a medium for nutrient flux, since nutrients, electron acceptors, and carbon are present in the aqueous phase and since biochemical reactions typically occur in the aqueous phase.
Oxygen presence or absence is another critical aspect for living systems. For aerobic metabolism, oxygen is the terminal electron acceptor and must be present for degradation to occur. If oxygen is consumed faster than it is replenished, anaerobic conditions may occur, and availability of oxygen becomes rate limiting at dissolved oxygen (DO) concentrations below 1–2 mg/L (Shaler and Klecka 1986; Vidali 2001; Speight and Arjoon 2012). For anaerobic metabolism, alternative terminal electron acceptors (sulphate [SO42−], nitrate [NO3–], and ferric iron [Fe3+]) and carbon dioxide required for methanogenic reaction must be present in abundance for degradation to occur (Vidali 2001; Xiong et al. 2015). Because sulphate, nitrate, and some iron species may be more water soluble than oxygen, when oxygen cannot be supplied or sustained, anaerobic processes may have some advantage over aerobic processes. Regardless, bioavailable nutrients (including nitrogen, phosphorus, and sulphur) and trace minerals form the backbone of all necessary enzymes used to break down contaminants and are therefore required for all microbial life to survive (C:N:P = 100:10:10 optimal) (Vidali 2001; Khudur et al. 2015). Salinity is also key for microbial life—too much or too little can inhibit microbial degradation (Ulrich et al. 2009).
Microbial life can function in a wide range of temperatures. However, the optimal temperatures often fall within 0–40 °C (Atlas 1991; Vidali 2001). Temperature affects the biochemical reaction rates possible within an environment, with rates found to increase as temperatures rise from 7 to 35 °C, above which rates may no longer increase (El-Naas et al. 2014). Additionally, temperature impacts both microbial growth and contaminant bioavailability and solubility (Morgan and Watkinson 1989; Atlas 1991; Xiong et al. 2015). Alkalinity/acidity conditions represent another critical parameter, since many microorganisms can only survive in certain, often very narrow, pH ranges. Optimal pH is normally at neutral pH 7, but 6–8 is often acceptable. Additional factors may be affected by pH, like nutrient availability and heavy metal solubility; these factors can impact a community’s capacity to degrade contaminants. The concentration of organic contaminants can also impact a microbial community’s effectiveness in remediation, since high concentrations (approaching solubility in particular) of PHC may impart a toxic effect to key degrading microorganisms and limit their growth, whilst low concentration may be too low to sustain microbial growth (Truskewycz et al. 2019; Ławniczak et al. 2020). Please refer to Chaps. 1, 5, and 9 for a more complete discussion of the complex topic of fate and transport of the contaminant.
Because PHC bioavailability is essential to a microbial community’s capacity for bioremediation, understanding bioavailability is crucial. Although there is much debate regarding the definition of the term, a good working definition for bioavailability is “the fraction of a chemical that is freely available to cross an organism’s cellular membrane from the medium in which the organism inhabits at a given time” (Kuppusamy et al. 2020a, b). Of course, storage, assimilation, transformation, and degradation are only possible once the chemical has been taken within a cell. Two critical factors should be considered with respect to bioavailability of a compound: the mass transfer of the compound from the environment to the microorganism cell and the subsequent rate of uptake and metabolism of the chemical. On a cellular level, bioaccessibility may be defined as what chemicals are available from the outside environment to cross the membrane of a cell, assuming their transport between cell and environment can occur. Whilst a microorganism may be capable of promoting degradation of a contaminant, it can only do so if the contaminant is present and accessible.
Biodegradation of hydrocarbons from the aqueous phase is relatively efficient, in contrast to biodegradation of hydrocarbons that have sorbed to soil organic matter or that have been sequestered in low-permeability layers (Megharaj et al. 2011; Balseiro-Romero et al. 2018). Compound-specific characteristics that affect solubility not only reduce the concentration of PHC compounds in the aqueous phase but also cause preferential sorption to solid matrices and organic matter in the subsurface and subsequent slow release into the environment (Megharaj et al. 2011; Balseiro-Romero et al. 2018; Kuppusamy et al. 2020a, b). The extent to which hydrocarbons have partitioned to the solid phase is a factor that impacts how well biodegradation may occur at a site.
14.2.2 Aerobic and Anaerobic Degradation
Organic compounds—including PHCs—are used by microorganisms as the electron donors required for metabolic pathways under either aerobic or anaerobic conditions; in fact, PHCs are often the sole source of carbon for these organisms (Beauchamp et al. 1989; Liu et al. 2020). Transformation of PHCs is energetically more favourable under aerobic conditions as compared to anaerobic conditions and is often mediated by a wide range of naturally occurring bacteria and fungi. Oxidation of PHCs (and BTEX in particular) in groundwater and soil is well documented (Collins et al. 2002; El-Naas et al. 2014; Yu et al. 2022). PHCs are a relatively reduced chemical species and can serve as the energy source and the electron donor for aerobic microbial metabolism. Oxygen serves as the reactant to oxidize the substrate and as the electron acceptor for microbial metabolism. Aerobic bacteria use different types of oxygenases, including monooxygenase, cytochrome-dependent oxygenase, and dioxygenase, to insert one or two atoms of oxygen into their targets. Because anaerobic degradation of PHCs is less well known, it will be explored in depth below.
Anaerobic biodegradation uses other electron accepting processes that generally fall within two large categories. Microorganisms can either generate energy by coupling substrate oxidation to respiration via reduction of an alternate terminal electron acceptor (i.e. sulphate, nitrate, iron, manganese, or carbon dioxide) or they can generate energy through fermentation (Foght 2008). Alternatively, less common electron acceptors such as carbon dioxide, vanadium, cobalt, and uranium have been found to function for select species of microorganisms. For more information on microorganisms that use these less common electron acceptors and that are found in extreme sites with specific conditions, please refer to Morrill et al. (2014) or Greene et al. (2016).
Though PHCs are a source of carbon that can be used for anaerobic microbial growth, even under optimal conditions, their chemical inertness poses an energetic and mechanistic challenge for successful anaerobic microbial metabolism (Rabus et al. 2016). Microorganisms must overcome the high energy barriers of PHC in their initial activation and cleavage of non-polar carbon-hydrogen bonds. The lower energy activation of PHC under aerobic conditions favours O2-dependent oxygenase-catalysed reactions for this initial C–H bond cleavage (Rabus et al. 2016). Whether aerobic or anaerobic, degradation of PHCs involves a multitude of possible reactions as described below.
The anaerobic transformation of toluene and xylenes is relatively well understood. Because the microbes that metabolize these compounds are widely distributed amongst nitrate- and sulphate-reducers and fermenting organisms, these compounds attenuate naturally in the environment given sufficient time (Toth et al. 2021). Conversely, the very narrow set of microbes that anaerobically degrade benzene have only recently been identified through genome sequencing (Vogt et al. 2011; Luo et al. 2014, 2016; Toth et al. 2021). Deltaproteobacterium ORM2 is one such organism. Toluene and xylene degradation typically occurs before benzene degradation because the responsible organisms tend to be more abundant in nature, however their presence may delay benzene degradation (Toth et al. 2021).
Anaerobic hydrocarbon-degrading microbial populations rely on a synergistic web of activities of diverse groups of microorganisms to achieve degradation of PHCs to less hazardous products, as illustrated by the pathways shown in Fig. 14.2. In the case of BTEX, these organisms include the following:
Anaerobic biodegradation pathways for benzene, toluene, and xylene. While some of the degradation pathways are universal among anaerobic systems, the microbes shown are anaerobic and methanogenic bioaugmentation cultures unique to fermentative and methanogenic systems. Note that the mechanism for initial activation of the benzene ring is still unknown at this time. Figure courtesy of Dr. Courtney Toth, University of Toronto
-
Bacteria that ferment BTEX are the key components of the microbial community utilizing BTEX as a carbon and energy source.
-
Archaea (primarily methanogenic archaea) are critical members of the community by virtue of their role converting acetate (a fermentation product of BTEX) into methane and carbon dioxide.
-
Sulphate-reducing and/or fermentative bacteria that metabolize downstream fermentation products from BTEX (e.g. fatty acids and alcohols) are responsible for generating hydrogen (H2), formate, and acetate, which are in turn metabolized by methanogenic archaea. We refer to these microorganisms as “syntrophs”.
-
Sulphate-reducing and/or fermentative bacteria that metabolize components of dead biomass (e.g. proteins, carbohydrates, and lipids) produce H2, acetate, formate, and other methylated (C1) compounds, which are in turn metabolized by methanogenic archaea. We refer to these organisms collectively as “recyclers” (Lillington et al. 2020). Whilst not directly involved in anaerobic BTEX biodegradation, they generate micronutrients and co-factors required for BTEX-degrading enzymes and microorganisms.
-
Low proportions of other organisms naturally present in many anaerobic ecosystems may contribute to the process in ways that are not yet known.
Anaerobic BTEX-degrading communities are often comprised of microorganisms unique to fermentative and methanogenic systems (e.g. Deltaproteobacteria ORM2, Methanosaeta, and Methanoregula) and may grow only under very specific conditions, including an oxidation reduction potential (ORP) below -100 millivolts (representing iron reducing conditions or lower). In fact, a significant amount of effort is typically required by a remediation practitioner to establish suitable conditions at a contaminated site in which these microorganisms will grow. For instance, the absence or depletion of benzene will cause concentrations of Deltaproteobacteria ORM2 to decrease to extinction, along with all other syntrophic microorganisms dependent on ORM2’s growth and benzene fermentation products. This is because benzene is the only known substrate fermented by ORM2 (Luo et al. 2016; Toth et al. 2021) and benzene concentrations greater than 0.1 mg/L are required to stimulate ORM2 benzene degradation. Since naturally occurring or added electron donors and carbon sources (i.e. volatile fatty acids and other fermentable carbon substrates) will stimulate the proliferation of intrinsic and/or bioaugmented microorganisms, their presence may reduce or completely inhibit BTEX degradation by key organisms. Consequently, electron donors should typically not be added to BTEX-contaminated sites.
In addition, BTEX fermentation does not always require an exogenous or added electron acceptor. Known benzene-degrading microbes may metabolize benzene coupled syntrophically to sulphate reduction (if available) or via methanogenesis (Luo et al. 2016; Toth et al. 2021). Sulphate may also promote the growth of other (facultative) sulphate-reducing bacteria, including Desulfovibrio and Geobacter as it was seen in a recent study (Toth et al. 2021).
14.3 Bioremediation Technologies
The principles of bioremediation technologies are based upon key tenets of microbial life and degradation. This sort of treatment can be accomplished ex situ or in situ. In ex situ remediation, where contaminated soil or groundwater is excavated and removed for treatment; the soil or groundwater can be put to beneficial reuse or disposed of as a non-hazardous waste. In situ remediation, which is increasingly now a preferred approach, causes less disturbance of the area and normally comes with lower costs. Table 14.1 summarizes these technologies.
Creation of a conceptual site model is a first crucial step in understanding what microbial processes are or are not occurring at contaminated site and what can be done. In general, samples relating to aqueous geochemistry and microbial communities are gathered, geologic cores are taken, soil vapour and other parameters are measured, and historical data relating to the site and surrounding areas is reviewed. This step establishes lines of evidence required for completing a feasibility assessment for a bioremediation approach. Additional assessments, such as treatability studies and advanced genomic studies, can also provide useful information.
14.3.1 Biostimulation and Bioaugmentation
For effective and efficient bioremediation, sufficient biomass to degrade the compound(s) of concern is required. This can be achieved through two approaches: biostimulation and bioaugmentation. Biostimulation amendments added to the subsurface stimulate the naturally occurring microbial community to degrade target contaminants. These amendments may include nutrients, such as phosphorus and nitrogen, or other trace minerals and electron acceptors that are absent or scarce, such as oxygen, nitrate, and sulphate (Anderson and Lovley 2000; Wolicka et al. 2009; Megharaj et al. 2011; Speight and Arjoon 2012; Brown et al. 2017; Müller et al. 2021; Primitz et al. 2021). Figure 14.3 shows a nutrient amendment infrastructure commonly required for injections at a treatment site. These amendments may help the microbial community to thrive and therefore increase the rate of biodegradation. For PHCs, this biodegradation typically occurs because these compounds serve as the electron donor necessary for microbial activity. This treatment option assumes the native community in the area was already metabolically capable of degrading the target compound, but was limited by lack of nutrients (Khudur et al. 2015).
Monitored natural attenuation (MNA) can be assessed where the natural populations of the microbial community and nutrients are sufficient for degradation of the target compound. This approach requires regular monitoring to track the remediation but requires no other amendments of the site (Chen et al. 2005; Kao et al. 2006). More information on monitoring natural attenuation processes and more specifically natural source zone depletion (NSZD) are provided in Chaps. 5 and 13.
If the naturally occurring microbial community does not already include microorganisms capable of promoting degradation of PHCs or if the population density is not great enough to support acceptable degradation, bioaugmentation may be an option (Megharaj et al. 2011; Varjani and Upasani 2021; Zang et al. 2021; Zuzolo et al. 2021a, b). Bioaugmentation is the process of amending an existing microbial community with microbial cultures or isolates known to degrade target compounds (Morgan and Watkinson 1989; Singer et al. 2005; Wolicka et al. 2009; Varjani and Upasani 2021). Figure 14.4 demonstrates a common in situ bioaugmentation treatment for groundwater. Once injections are complete, very little permanent equipment remains at the injection site other than sampling points. To treat the whole area and avoid localized effects, the introduced microorganisms or nutrients must be distributed throughout the contaminated matrix. In addition, the bioaugmented microorganisms must also be able to thrive alongside existing native microorganisms, or the treatment effect will be short lived, and the degradation of the compounds may not be sustained (Singer et al. 2005). This is because effective treatment also relies on bioaugmented microorganisms spreading as their population densities increase and moving with ambient groundwater, all of which occurs after injections are complete.
14.3.2 In Situ Biological Technologies
In situ biological technologies influence remediation by changing the environmental conditions of the subsurface to enhance or alter the microbial community present. Common techniques, their benefits, and their limitations are summarized in Table 14.1. In situ treatment technologies include bioventing, biosparging, and low-energy thermal (further explored in Chap. 18) technologies.
A common treatment, bioventing uses wells to supply the contaminated subsurface with air and nutrients to stimulate the natural microbial community to degrade contaminants under aerobic conditions. This process provides enough air movement to stimulate microbial degradation whilst limiting the volatilization and release of hydrocarbons to the atmosphere (Frutos et al. 2010; Johnston et al. 2010; Tzovolou et al. 2015). In biosparging, air is pumped through wells under the groundwater table to increase the dissolved oxygen concentrations within the aqueous phase and promote microbial metabolism. Figure 14.5 demonstrates a common schematic of an in situ biosparging remediation system for PHC-contaminated groundwater. In addition to increasing oxygen in groundwater, this process can enhance mixing within the saturated zone and thereby increase the dissolution of sorbed hydrocarbon into the groundwater (Strzempka et al. 1997; Vidali 2001; Kao et al. 2008; Kabelitz et al. 2009). Low-energy heating is another way to support the microbial community; this technology aims to provide optimal temperature to maximize microbial kinetics whilst also enhancing LNAPL mobility and partitioning of hydrocarbons into other phases (Imhoff et al. 1997; Vermeulen and McGee 2000; Macbeth et al. 2012).
14.3.3 Ex Situ Biological Technologies
Ex situ treatment technologies bring contaminated soil and groundwater to the surface to enhance or alter microbial community and speed degradation. Such technologies include landfarming, composting, biopiles, and bioreactors. Landfarming is a very simple technique that has been widely applied to soil where contamination is generally shallow and accessible from the ground surface. Contaminated soil is excavated, spread over a large area, and periodically tilled to enhance oxygen penetration and keep the system aerobic (Vidali 2001; Bergsveinson et al. 2019). This method takes up a large, open land surface, which may not always be economical or feasible when space is at a premium. Composting is similar to landfarming but involves amending the excavated contaminated soil with organic materials like manure or agricultural waste to increase microbial community diversity and density (Hwang et al. 2001; Megharaj et al. 2011; Syawlia and Titah 2021). Biopiles are a hybrid of landfarming and composting, in which an engineered aerated composting cell is created from the excavated soil. Biopiles may require a smaller surface area footprint than conventional landfarming does (see Fig. 14.6). These large piles are designed to limit the leaching and volatilization loss of hydrocarbons experienced by landfarming and composting whilst promoting microbial diversity, increasing temperature, and optimizing air penetration for both aerobic and anaerobic communities (Singh et al. 2017; Wang et al. 2021; Zhang et al. 2021). Bioreactors are another form of ex situ technology but are often considered a separate category because they are a highly controllable closed treatment system, separate from the natural environment. Both slurry (mixed phase) and aqueous-phase reactors can be used to treat soil excavated from the contaminated site or water pumped from it. Since bioreactors are an external closed environment, parameters can be tightly controlled to foster biodegradation and the process can be maximized for rapid microbial degradation kinetics (Chiavola et al. 2010; Das and Kumar 2018). Figure 14.7 shows potential infrastructure required for treatment of PHC-contaminated groundwater.
14.3.4 Mycoremediation of Complex Organics
Interest in remediation using fungi as a sole degradation driver has grown in recent years. Numerous studies indicate that microbial diversity, including fungal species, is critical for remediation of PHCs (Obuekwe et al. 2005; Mohsenzadeh et al. 2009; Zafra et al. 2014; Lee et al. 2015; Andreolli et al. 2016; Marchand et al. 2017). Fungal species are a critical component to a healthy diverse rhizosphere, as discussed below and illustrated in Fig. 14.1. Though fungal degradation of PHCs can occur in isolation, diverse communities create a mutually beneficial system where fungi and other organisms work together to degrade organic contaminants more efficiently than they could in isolation. Investigations of microbial diversity within highly PHC-contaminated soil at a former petrochemical plant characterized degradation potential of 95 bacterial and 160 fungal identified isolates. Fusarium oxysporum and Trichoderma tomentosum significantly degraded all PAH compounds tested (anthracene, phenanthrene, fluorene, and pyrene). Sordariomycetes has often demonstrated high affiliation with hydrocarbon degradation, and fungal species studied belonging to Sordariomycetes class, Trichoderma, and Fusarium were found to be more efficient degraders than those of other classes studied (Hong et al. 2010; Wu et al. 2010; Argumedo-Delira et al. 2012). Bioaugmentation with six known potential hydrocarbon-degrading fungi was shown to significantly increase degradation over biostimulation of the native community alone in TPH-contaminated soil mesocosms (Medaura et al. 2021). Both bioaugmentation with fungal species and biostimulation with nutrients had TPH degradation rates above unamended contaminated soil (39.90 ± 1.99%, and 24.17 ± 1.31%, respectively). In addition to increased TPH degradation with bioaugmented fungal species, the bacteria within the native soil shifted to a more diverse community, enriched in known hydrocarbon-degrading bacteria orders Cytophagales, Bacteroidales, and Rhodocyclales. This fact indicates that a complex and synergistic relationship between the native community and bioaugmented species can occur, increasing overall capacity and rates of degradation of complex compounds (Medaura et al. 2021).
A study conducted by Argumedo-Delira et al. (2012) tested the tolerance of 11 strains of Trichoderma to naphthalene, phenanthrene, and benzo(α)pyrene. Several fungal strains of Trichoderma tested were capable of tolerating concentrations of phenanthrene and naphthalene above 250 mg/L, as well as benzo(α)pyrene concentrations of 100 mg/L. Although established potential for PAH remediation by fungi is present, enzymatic activity and pathways are not well understood. Andreolli et al. (2016) isolated Trichoderma longibrachiatum from uncontaminated forest soil through selective enrichment for hydrocarbon degraders. In a diesel-contaminated soil microcosm, soil inoculated with Trichoderma longibrachiatum demonstrated the fastest removal of C12-40 hydrocarbon fraction, at 54.2 ± 1.6% in 30 days, compared to 7.3 ± 6.1% removal in controls (Andreolli et al. 2016). Additionally, Andreolli et al. (2016) characterized the potential for PAH removal, with 69–71% removal of phenanthrene, anthracene, pyrene, and fluoranthene, potentially indicating that Trichoderma longibrachiatum is a strong hydrocarbon degrader. This result is promising, not only because of the efficacy of the fungal strain, but also because it indicates fungal species not previously exposed to PHCs can be effective in their biodegradation.
Fungal species are found in all environments; a comprehensive study by Richardson et al. (2019) revealed a genetically diverse microbial community even in oil sands tailings water, which contains a multitude of bitumen-associated organics, including a toxic naphthenic acid fraction (Qin et al. 2019). Next-generation community sequencing revealed that, although limited in classification below the phylum level, two of the most abundant operational taxonomic units of the entire data set were fungi (Richardson et al. 2019). This presence of major fungal activity within the water fraction of the tailings pond indicates that fungi are able to resist such harsh and toxic environments and, potentially, are also able to metabolize organic compounds found in association with bitumen. Repas et al. (2017) isolated Trichoderma harzianum from plant roots growing in coarse tailings and found it had the capacity to remediate complex petrochemical residues present within the tailings. The fungal isolate T. harzianum was isolated in OSPW by Miles et al. (2019) and demonstrated the ability to withstand high salinity conditions (≥ 60 g/L), a pH range of 2–9, and a naphthenic acid fraction compound-inhibitory concentration of 2400 mg/L. Further testing revealed this isolate, sourced from the environment, was able to grow on an agar plate using a single pure drop of naphthenic acids as its sole source of carbon; this indicates a strong potential for fungal remediation of toxic organic compounds like naphthenic acids (Miles et al. 2019, 2020). With vast fungal diversity and ubiquitous presence in the soil and groundwater environment, in situ mycoremediation is a compelling treatment method for toxic, otherwise recalcitrant organic contaminants in soil and groundwater.
14.3.5 Phytoremediation of Petroleum Hydrocarbons
Phytoremediation is a remediation technology in use since at least the 1980s to degrade, extract, contain, or immobilize contaminants from soil, groundwater, and other media using plants (Landmeyer 2012). Whilst a wide array of contaminants can be effectively treated using phytoremediation, PHCs are particularly amenable to this strategy, and the use of phytoremediation for PHCs has increased in recent decades. Phytoremediation sites commonly look like natural landscapes with rows of various species of trees and/or monitoring well stick-ups. This aspect is an additional benefit of this technique: it maintains the aesthetic of the natural environment and may even limit the amount of reclamation necessary after remediation.
Effective phytoremediation of PHCs typically occurs through several mechanisms, which may happen simultaneously, including rhizodegradation, phytodegradation, and to a lesser extent, phytovolatilization. Rhizodegradation refers to the microbial biodegradation of contaminants in the soil surrounding plant roots (the so-called rhizosphere effect), whilst phytodegradation describes contaminant degradation within plant tissue. Phytovolatilization, defined as root uptake and transfer of contaminants or their metabolites to atmosphere through plant transpiration, and other plant-related mechanisms may also contribute to PHC treatment in some circumstances as illustrated in Fig. 14.1 (McCutcheon and Schnoor 2003; Landmeyer 2012). Additionally, for sites with impacted groundwater, phytohydraulics—the interception of impacted groundwater and control of contaminant plume migration through transpiration—can be an important contributor to site remediation by reducing or preventing plume migration (Landmeyer 2012). Other specific mechanisms may also be involved, depending on contaminant types, the impacted medium (e.g. soil or groundwater), and the design of the phytoremediation system.
Of particular significance to PHC phytoremediation is rhizodegradation because it is often the single greatest contributor to phytoremediation effectiveness at PHC-impacted sites (Landmeyer 2012). Rhizodegradation may be thought of as plant-assisted bioremediation. A plant’s roots release a variety of compounds known as root exudates, including simple sugars, polysaccharides, amino acids, and microbial growth factors. Exudates encourage the colonization and proliferation of bacteria and fungi nearby (Barac et al. 2004; Al-Zaban et al. 2021). As the plant thrives, the rhizosphere becomes a zone of enhanced microbial density and activity as compared to surrounding bulk soil (Zuzolo et al. 2021a, b; Zuzolo et al. 2021a, b). Notably, some root exudates may serve to induce the expression of microbial enzymes useful for cometabolic biodegradation of PHCs (Dagher et al. 2019; Davin et al. 2019).
The interaction between plant roots and their associated microbial communities and the mechanisms by which they interact is highly complex. Though its full significance to PHC phytoremediation is beyond the scope of this chapter, interested readers would be well served to further explore this topic using the references cited above.
Several factors must be considered in order to successfully design and apply phytoremediation at PHC-impacted sites (Susarla et al. 2002). These include the concentration, distribution, depth, and types of contaminants, as well as hydrogeological and soil conditions and remedial goals. These aspects and their interplay affect the choice of appropriate plants, the configuration of plantings, and locations chosen for implementation. Properties of the soil and groundwater also influence phytoremediation success; these include nutrient availability, particle size and soil classification, bulk density, salinity, redox potential, pH, cation exchange capacity, organic matter content, and the presence of microorganisms for degradation (Gerhardt et al. 2009). As with PHC remediation achieved through bacterial degradation, phytoremediation is largely dependent on environmental factors. However, plants often have a higher tolerance to rapid changes in the environment, including temperature, moisture, and salinity.
As a general rule, for phytoremediation be effective, plant roots must grow close to impacted media. As a result, impacted soil at depths below typical rooting depths may not be effectively treated with this approach. Though it depends on plant species and site conditions, roots generally cannot be expected to naturally penetrate deeper than 5–10 ft below ground surface (bgs) (McCutcheon and Schnoor 2003). PHC-impacted groundwater also must be accessible to plant roots for a remediation to occur through phytohydraulics. In some cases, an engineered phytoremediation approach has been demonstrated to effectively target impacted groundwater at depths greater than 5–10 ft bgs (Geosyntec Consultants 2022).
As with all technologies in this chapter, phytoremediation requires a proper understanding of this unique and evolving field, and less-experienced practitioners may want to consult with phytoremediation specialists to ensure appropriate system design, implementation, operation, and maintenance to aid in long-term effectiveness. Successful application of phytoremediation depends upon a complete conceptual site model that takes into account all available phytoremedial options. Phytoremediation alone, or in conjunction with other treatment technologies, has proven an effective remediation option available.
14.4 Conclusion
Given the continuing global demand for petroleum products, understanding the environmental implications of PHC contamination is critical. Remediation of PHCs released into the environment will continue for decades, if not centuries. Although PHCs are a complex mix of organic compounds, natural microbial communities have adapted to these carbon sources and can attenuate or remediate them, though they often need human intervention to achieve remedial goals. Harnessing the natural potential of microorganisms, fungi, and plants, under many different conditions, is key to the future of remediation.
With knowledge gained from the study of natural systems, practitioners can and should incorporate nature-based solutions as they develop technologies and treatment plans for PHC-impacted sites. As regulators increasingly mandate sustainable and green treatment technologies for such sites, practitioners must be well versed in these biologically mediated solutions and able to create a well-characterized conceptual site model based on a comprehensive understanding of the current environment and how it can be amended.
Treatment options for PHC-contaminated sites have grown drastically in the last 40 years. PHC compounds previously thought to be recalcitrant to anaerobic biological treatment have been proven amenable to it, given the correct conditions and organisms are present. Identification and analysis of PHCs has improved, and, in the near future, cheaper, faster, and more accurate analyses will likely enhance conceptual site models and improve remediation treatment plans. In addition, improvements and innovations regarding effective distribution of amendments to the subsurface will also increase effectiveness. In fact, as the leading edge of science advances, biological treatment of contaminated sites could become the principal treatment option for PHC contamination. For all this to occur, a bias towards traditional remedial technologies on the part of developers and landowners must be overcome, and a broader understanding and acceptance of biological treatments by regulators and by the public must be achieved.
By 2030, major growth within this field of study is expected to occur and technologies described in this chapter as innovative or exploratory may likely be commonplace. Once biological mechanisms for PHC remediation are better understood, these technologies can be applied with better confidence and efficacy. The broader application of molecular biological tools (discussed in Chap. 10), including microbial community analysis and advanced chemical analysis, (including compound-specific isotope analyses, as presented in Chap. 11) will contribute greatly to better harnessing biological processes for both in situ and ex situ remediation. Genetically modified organisms may well be designed and used to remediate PHCs in difficult environmental conditions (such as high salinity, low temperature, high clay content, or low oxygen) once they are accepted by the public and regulators and proven with successful field demonstrations.
Given the pace of growth in the field, in a few short years, biologically mediated remediation of PHC-contaminated sites will likely be possible on shorter timelines and at lower costs. Future innovation and research will lead to the coupling of biologically mediated remediation options with compatible and synergistic technologies to achieve even better remedial outcomes. Biotic and abiotic solutions can be applied in tandem to reduce costs, since abiotic remediation alone often occurs in a non-linear fashion, with the last phase of contaminant removal typically taking significantly more time, resources, and money than the first stages; adding a biotic component could vastly reduce cost and increase sustainability.
The work in the laboratory and the field to expand our understanding of bioremedial technologies, their effectiveness, and their applications is ever developing. Research into complex sites, difficult conditions, and complex contaminant mixtures will lead to better, cleaner, and greener solutions for practitioners to apply to contaminated environments.
References
Al-Zaban MI, AlHarbi MA, Mahmoud MA (2021) Hydrocarbon biodegradation and transcriptome responses of cellulase, peroxidase, and laccase encoding genes inhabiting rhizospheric fungal isolates. Saudi J Biolog Sci 28(4):2083–2090
Anderson RT, Lovley DR (2000) Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environ Sci Technol 34(11):2261–2266
Andreolli M, Lampis S, Brignoli P et al (2016) Trichoderma longibrachiatum Evx1 is a fungal biocatalyst suitable for the remediation of soils contaminated with diesel fuel and polycyclic aromatic hydrocarbons. Environ Sci Pollut Res 23(9):9134–9143
Argumedo-Delira R, Alarcón A, Ferrera-Cerrato R et al (2012) Tolerance and growth of 11 Trichoderma strains to crude oil, naphthalene, phenanthrene and benzo[a]pyrene. J Environ Manag 95:S291–S299
Atlas RM (1991) Microbial hydrocarbon degradation—bioremediation of oil spills. J Chem Technol Biotechnol 52(2):149–156
Balseiro-Romero M, Monterroso C, Casares JJ (2018) Environmental fate of petroleum hydrocarbons in soil: review of multiphase transport, mass transfer, and natural attenuation processes. Pedosphere 28(6):833–847
Barac T, Taghavi S, Borremans B et al (2004) Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol 22(5):583–588
Beauchamp EG, Trevors JT, Paul JW (1989) Carbon sources for bacterial denitrification. Adv Soil Sci 10:113–142
Bergsveinson J, Perry BJ, Simpson GL et al (2019) Spatial analysis of a hydrocarbon waste-remediating landfarm demonstrates influence of management practices on bacterial and fungal community structure. Microb Biotechnol 12(6):1199–1209
Brown DM, Okoro S, van Gils J et al (2017) Comparison of landfarming amendments to improve bioremediation of petroleum hydrocarbons in Niger Delta soils. Sci Total Environ 596–597:284–292
Butnariu M, Butu M (2020) Bioremediation: a viable approach for degradation of petroleum hydrocarbon. Springer International Publishing, pp 195–223
Chen KF, Kao CM, Wang JY et al (2005) Natural attenuation of MTBE at two petroleum-hydrocarbon spill sites. J Hazard Mater 125(1–3):10–16
Chiavola A, Baciocchi R, Gavasci R (2010) Biological treatment of PAH-contaminated sediments in a sequencing batch reactor. J Hazard Mater 184(1–3):97–104
Collins C, Laturnus F, Nepovim A (2002) Remediation of BTEX and trichloroethene—current knowledge with special emphasis on phytoremediation. Environ Sci Pollut Res 9(1):86–94
Colwell RR, Walker JD, Cooney JJ (1977) Ecological aspects of microbial degradation of petroleum in the marine environment. CRC Crit Rev Microbiol 5(4):423–445
Cozzarelli IM, Schreiber ME, Erickson ML, Ziegler BA (2016) Arsenic cycling in hydrocarbon plumes: secondary effects of natural attenuation. Groundwater 54(1):35–45
Dagher DJ, de la Providencia IE, Pitre FE et al (2019) Plant identity shaped rhizospheric microbial communities more strongly than bacterial bioaugmentation in petroleum hydrocarbon-polluted sediments. Front Microbiol 10
Das AJ, Kumar R (2018) Bioslurry phase remediation of petroleum-contaminated soil using potato peels powder through biosurfactant producing Bacillus licheniformis J1. Int J Environ Sci Technol 15(3):525–532
Davin M, Starren A, Marit E et al (2019) Investigating the effect of Medicago sativa L. and Trifolium pratense L. root exudates on PAHs bioremediation in an aged-contaminated soil. Water Air Soil Pollut 230(12)
Davis GB (2023) Reviewing the Bioremediation of Contaminants in Groundwater: Investigations over 40 Years Provide Insights into What's Achievable. Frontiers in Bioscience-Elite 15(3):16
El-Naas MH, Acio JA, El Telib AE (2014) Aerobic biodegradation of BTEX: progresses and prospects. J Environ Chem Eng 2(2):1104–1122
Foght J (2008) Anaerobic biodegradation of aromatic hydrocarbons: pathways and prospects. J Mol Microbiol Biotechnol 15(2–3):93–120
Frutos FJG, Escolano O, Garcia S et al (2010) Bioventing remediation and ecotoxicity evaluation of phenanthrene-contaminated soil. J Hazard Mater 183(1–3):806–813
Geosyntec Consultants (2022) Phytoremediation using treeWell® technology, 27 Apr 2022
Gerhardt KE, Huang X-D, Glick BR et al (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176(1):20–30
Greene A, Wright M, Aldosary H (2016) Bacterial diversity and metal reducing bacteria in Australian thermal environments. In: The spotlight: recent progress in the understanding of beneficial and harmful microorganisms. Microb Infect 32
Hong JW, Park JY, Gadd GM (2010) Pyrene degradation and copper and zinc uptake by Fusarium solani and Hypocrea lixii isolated from petrol station soil. J Appl Microbiol 108(6):2030–2040
Huesemann MH, Hausmann TS, Fortman TJ (2004) Does bioavailability limit biodegradation? A comparison of hydrocarbon biodegradation and desorption rates in aged soils. Biodegradation 15(4):261–274
Hwang EY, Namkoong W, Park JS (2001) Recycling of remediated soil for effective composting of diesel-contaminated soil. Compost Sci Utiliz 9(2):143–148
Imhoff PT, Frizzell A, Miller CT (1997) Evaluation of thermal effects on the dissolution of a nonaqueous phase liquid in porous media. Environ Sci Technol 31(6):1615–1622
Johnston CD, Woodbury R, Bastow TP et al (2010) Biosparging successfully limited fugitive VOCs while remediating residual weathered gasoline in a shallow sand aquifer. In: 7th international groundwater quality conference, vol 342, pp 225–228
Kabelitz N, Machackova J, Imfeld G et al (2009) Enhancement of the microbial community biomass and diversity during air sparging bioremediation of a soil highly contaminated with kerosene and BTEX. Appl Microbiol Biotechnol 82(3):565–577
Kao CM, Huang WY, Chang LJ et al (2006) Application of monitored natural attenuation to remediate a petroleum-hydrocarbon spill site. Water Sci Technol 53(2):321–328
Kao CM, Chen CY, Chen SC et al (2008) Application of in situ biosparging to remediate a petroleum-hydrocarbon spill site: field and microbial evaluation. Chemosphere 70(8):1492–1499
Khudur LS, Shahsavari E, Miranda AF et al (2015) Evaluating the efficacy of bioremediating a diesel-contaminated soil using ecotoxicological and bacterial community indices. Environ Sci Pollut Res 22(19):14809–14819
Kuppusamy S, Maddela NR, Megharaj M et al (2020a) Ecological impacts of total petroleum hydrocarbons. In: Total petroleum hydrocarbons. Springer, Cham
Kuppusamy S, Maddela NR, Megharaj M et al (2020b) Total petroleum hydrocarbons: environmental fate, toxicity, and remediation. Springer, Cham
Landmeyer JE (2012) Introduction to phytoremediation of contaminated groundwater. [Electronic resource]: historical foundation, hydrologic control, and contaminant remediation. Springer
Ławniczak Ł, Woźniak-Karczewska M, Loibner AP et al (2020) Microbial degradation of hydrocarbons—basic principles for bioremediation: a review. Molecules 25(4):856
Lee H, Yun SY, Jang S et al (2015) Bioremediation of polycyclic aromatic hydrocarbons in creosote-contaminated soil by Peniophora incarnata KUC8836. Bioremediat J 19(1):1–8
Lillington SP, Leggieri PA, Heom KA et al (2020) Nature’s recyclers: anaerobic microbial communities drive crude biomass deconstruction. Curr Opin Biotechnol 62:38–47
Liu X, Li Z, Zhang C et al (2020) Enhancement of anaerobic degradation of petroleum hydrocarbons by electron intermediate: performance and mechanism. Bioresour Technol 295
Luo F, Gitiafroz R, Devine CE et al (2014) Metatranscriptome of an anaerobic benzene-degrading, nitrate-reducing enrichment culture reveals involvement of carboxylation in benzene ring activation. Appl Environ Microbiol 80(14):4095–4107
Luo F, Devine CE, Edwards EA (2016) Cultivating microbial dark matter in benzene-degrading methanogenic consortia. Environ Microbiol 18(9):2923–2936
Macbeth TW, Truex MJ, Powell T et al (2012) Combining low-energy electrical resistance heating with biotic and abiotic reactions for treatment of chlorinated solvent DNAPL source area
Marchand C, St-Arnaud M, Hogland W et al (2017) Petroleum biodegradation capacity of bacteria and fungi isolated from petroleum-contaminated soil. Int Biodeterior Biodegradation 116:48–57
McCutcheon SC, Schnoor JL (2003) Phytoremediation: transformation and control of contaminants. Wiley-Interscience
Medaura MC, Guivernau M, Moreno-Ventas X et al (2021) Bioaugmentation of Native Fungi, an efficient strategy for the bioremediation of an aged industrially polluted soil with heavy hydrocarbons. Front Microbiol 12
Megharaj M, Ramakrishnan B, Venkateswarlu K et al (2011) Bioremediation approaches for organic pollutants: a critical perspective. Environ Int 37(8):1362–1375
Miles SM, Hofstetter S, Edwards T et al (2019) Tolerance and cytotoxicity of naphthenic acids on microorganisms isolated from oil sands process-affected water. Sci Total Environ 695:133749
Miles SM, Asiedu E, Balaberda A-L et al (2020) Oil sands process affected water sourced Trichoderma harzianum demonstrates capacity for mycoremediation of naphthenic acid fraction compounds. Chemosphere 258:127281
Mohsenzadeh F, Naseri S, Mesdaghinia A et al (2009) Identification of petroleum resistant plants and rhizospheral fungi for phytoremediation of petroleum contaminated soils. J Jpn Petrol Inst 52(4):198–204
Morgan P, Watkinson RJ (1989) Hydrocarbon degradation in soils and methods for soil biotreatment. Crit Rev Biotechnol 8(4):305–333
Morrill PL, Brazelton WJ, Kohl L et al (2014) Investigations of potential microbial methanogenic and carbon monoxide utilization pathways in ultra-basic reducing springs associated with present-day continental serpentinization: the Tablelands, NL, CAN. Front Microbiol 5
Müller C, Knöller K, Lucas R et al (2021) Benzene degradation in contaminated aquifers: enhancing natural attenuation by injecting nitrate. J Contam Hydrol 238:103759
Obuekwe CO, Badrudeen AM, Al-Saleh E et al (2005) Growth and hydrocarbon degradation by three desert fungi under conditions of simultaneous temperature and salt stress. Int Biodeterior Biodegradation 56(4):197–205
Primitz JV, Vazquez S, Ruberto L et al (2021) Bioremediation of hydrocarbon-contaminated soil from Carlini Station, Antarctica: effectiveness of different nutrient sources as biostimulation agents. Polar Biol 44(2):289–303
Qin R, Lillico D, How ZT et al (2019) Separation of oil sands process water organics and inorganics and examination of their acute toxicity using standard in-vitro bioassays. Sci Total Environ 695
Rabus R, Boll M, Heider J et al (2016) Anaerobic microbial degradation of hydrocarbons: from enzymatic reactions to the environment. J Mol Microbiol Biotechnol 26(1–3):5–28
Repas TS, Gillis DM, Boubakir Z et al (2017) Growing plants on oily, nutrient-poor soil using a native symbiotic fungus. PLoS ONE 12(10)
Richardson E, Bass D, Smirnova A et al (2019) Phylogenetic estimation of community composition and novel eukaryotic lineages in Base Mine Lake: an oil sands tailings reclamation site in Northern Alberta. J Eukaryot Microbiol
Shaler TA, Klecka GM (1986) Effects of dissolved oxygen concentration on biodegradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol 51(5):950–955
Singer AC, van der Gast CJ, Thompson IP (2005) Perspectives and vision for strain selection in bioaugmentation. Trends Biotechnol 23(2):74–77
Singh P, Jain R, Srivastava N et al (2017) Current and emerging trends in bioremediation of petrochemical waste: a review. Crit Rev Environ Sci Technol 47(3):155–201
Speight JG,Arjoon KK (2012) Bioremediation of petroleum and petroleum products. Wiley, Incorporated, Somerset, USA
Strzempka CP, Woodhull PM, Vassar TM et al (1997) In-situ biosparging and soil vapor extraction for JP-4 contaminated soils and groundwater: a case study. In: 4th International in situ and on-site bioremediation symposium, vol 4(1), pp 245–250
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organic chemical contamination. Ecol Eng 18(5):647–658
Syawlia RM, Titah HS (2021) Removal of hydrocarbons from contaminated soils using bioremediation by aerobic co-composting methods at ship dismantle locations. J Ecol Eng 22(6):181–190
Toth CRA, Luo F, Bawa N et al (2021) Anaerobic benzene biodegradation linked to the growth of highly specific bacterial clades. Environ Sci Technol 55(12):7970–7980
Truskewycz A, Gundry TD, Khudur LS et al (2019) Petroleum hydrocarbon contamination in terrestrial ecosystems-fate and microbial responses. Molecules 24(18)
Tzovolou DN, Theodoropoulou MA, Blanchet D et al (2015) In situ bioventing of the vadose zone of multi-scale heterogeneous soils. Environ Earth Sci 74(6):4907–4925
Ulrich AC, Edwards EA (2003) Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ Microbiol 5(2):92–102
Ulrich AC, Guigard SE, Foght JM et al (2009) Effect of salt on aerobic biodegradation of petroleum hydrocarbons in contaminated groundwater. Biodegradation 20(1):27–38
Varjani SJ (2017) Microbial degradation of petroleum hydrocarbons. Biores Technol 223:277–286
Varjani S,Upasani VN (2021) Bioaugmentation of Pseudomonas aeruginosa NCIM 5514-A novel oily waste degrader for treatment of petroleum hydrocarbons. Bioresour Technol 319
Vermeulen F, McGee B (2000) In situ electromagnetic heating for hydrocarbon recovery and environmental remediation. J Can Pet Technol 39(8):24–28
Vidali M (2001) Bioremediation. An overview. Pure Appl Chem 73(7):1163–1172
Vogt C, Kleinsteuber S, Richnow HH (2011) Anaerobic benzene degradation by bacteria. Microb Biotechnol 4(6):710–724
Wang M, Garrido-Sanz D, Sansegundo-Lobato P et al (2021) Soil microbiome structure and function in ecopiles used to remediate petroleum-contaminated soil. Front Environ Sci 9
Wolicka D, Suszek A, Borkowski A et al (2009) Application of aerobic microorganisms in bioremediation in situ of soil contaminated by petroleum products. Biores Technol 100(13):3221–3227
Wu Y-R, Luo Z-H, Vrijmoed LLP (2010) Biodegradation of anthracene and benz[a]anthracene by two Fusarium solani strains isolated from mangrove sediments. Biores Technol 101(24):9666–9672
Xiong S, Li X, Chen J et al (2015) Crude oil degradation by bacterial consortia under four different redox and temperature conditions. Appl Microbiol Biotechnol 99(3):1451–1461
Yu B, Yuan Z, Yu Z et al (2022) BTEX in the environment: an update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem Eng J 435:134825
Zafra G, Absalón ÁE, Cuevas MDC et al (2014) Isolation and selection of a highly tolerant microbial consortium with potential for PAH biodegradation from heavy crude oil-contaminated soils. Water Air Soil Pollut 225(2)
Zang T, Wu H, Zhang Y et al (2021) The response of polycyclic aromatic hydrocarbon degradation in coking wastewater treatment after bioaugmentation with biosurfactant-producing bacteria Pseudomonas aeruginosa S5. Water Sci Technol 83(5):1017–1027
Zhang K, Wang S, Guo P et al (2021) Characteristics of organic carbon metabolism and bioremediation of petroleum-contaminated soil by a mesophilic aerobic biopile system. Chemosphere 264
Zuzolo D, Guarino C, Tartaglia M et al (2021a) Plant-soil-microbiota combination for the removal of total petroleum hydrocarbons (TPH): an in-field experiment. Front Microbiol 11
Zuzolo D, Sciarrillo R, Postiglione A et al (2021b) The remediation potential for PAHs of Verbascum sinuatum L. combined with an enhanced rhizosphere landscape: a full-scale mesocosm experiment. Biotechnol Rep (Amst) 31:e00657
Acknowledgements
The authors would like to acknowledge and thank Dr. Courtney Toth of University of Toronto and Vertex Environmental Inc. for their contributions to the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Miles, S.M., Gestler, R., Dworatzek, S.M. (2024). Bioremediation of Petroleum Hydrocarbons in the Subsurface. In: García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R. (eds) Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons. Environmental Contamination Remediation and Management. Springer, Cham. https://doi.org/10.1007/978-3-031-34447-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-34447-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34446-6
Online ISBN: 978-3-031-34447-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)