Abstract
White-tailed deer are geographically widespread and occupy a variety of ecosystems from semi-desert shrubland and grasslands to forests. They have a relatively high reproductive potential but recruitment may be limited in semiarid rangelands where annual variation in precipitation is high. They eat browse and forbs but mast may seasonally comprise most of the diet. White-tailed deer select areas with a mixture of woody vegetation and areas dominated by herbaceous vegetation. They use woody vegetation for cover and often forage in adjacent herbaceous-dominated areas. They are highly adaptable and can adjust to changes in vegetation resulting from rangeland management practices; however, excessive grazing reduces habitat quality. Brush management minimally affects white-tailed deer and their habitat when adequate resources such as thermal cover, hiding cover, and browse-and-mast-producing vegetation remain on the landscape. Empirical evidence that creating mosaics of herbaceous-dominated foraging patches and woody cover improves demographics or productivity is equivocal; however, managing for increased spatial heterogeneity in vegetation may increase fawn survival. Chronic wasting disease is a major threat to white-tailed deer populations. White-tailed deer use behavioral adaptations to reduce excessive heat loads resulting from climate change in the southern part of their range. Paradoxically, populations are expanding in the northern part of their range in part because of milder winters. Hunting is the primary tool to manage white-tailed deer populations. Combining recreational hunting with livestock production increases revenue for ranchers. Ironically, white-tailed deer are often a nuisance in eastern forests, but they can be an economically important asset on rangelands.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
A remarkable ability to adapt to different environments and to human presence is a hallmark trait of white-tailed deer (Odocoileus virginianus; Fig. 18.1). White-tailed deer populations persist and reproduce in environments ranging from the temperate forests of the eastern United States to the western deserts, grasslands, shrublands, and woodlands and range from Alaska to Mexico in North America. They occupy landscapes ranging from relatively undisturbed National Parks to the suburbs of major cities (Potratz et al. 2019). Although white-tailed deer is among the most studied and managed wildlife species in North America, less research has been conducted in western rangelands than in other parts of their distribution. The majority of research on rangeland-associated populations has occurred in the Southern Great Plains and Tamaulipan Vegetation Region of Texas. Throughout this chapter, I have included information from other regions whenever possible and examples from non-rangeland areas when appropriate.
2 General Life History and Population Dynamics
White-tailed deer have a relatively high reproductive potential. Number of offspring produced by females depends on many factors including age, nutritional status, environment, deer density, and behavioral interactions (Verme 1969; DeYoung 2011; DeYoung et al 2019). Under favorable conditions, adult female white-tailed deer may produce an average of two or more offspring/year. Under extremely favorable conditions, female fawns may breed, and some adult females may have triplets (Ozoga 1987; DeYoung 2011). Age at which fecundity declines is unclear; some reports suggest it does not decline until well into maturity (DelGiudice et al. 2007).
Males reach sexual maturity at 1.5 years of age (Sauer 1984; DeYoung and Miller 2011). White-tailed deer populations commonly lack older males because of heavy harvest of yearlings and younger males by hunters. In populations with an older age structure, males 3.5 years and older sire about 70% of the fawns in a given year with 1.5- and 2.5-year-old males siring the remainder (DeYoung et al. 2009; DeYoung and Miller 2011).
Fawns use bed sites in grassland and areas dominated by woody vegetation (Grovenburg et al. 2010; Michel et al. 2020; Fulbright et al. 2023). Bed sites tend to have more grass cover and taller grasses than the surrounding habitat (Uresk et al. 1999). Woody plant cover is important along with grass cover at daytime fawn bed sites in the Tamaulipan Vegetation Region (Hyde et al. 1987; Fulbright et al. 2023; Fig. 18.2). Bed site cover may help fawns to avoid predation; however, the importance of bed site cover in evading predators such as coyotes (Canis latrans) is unclear. For example, increasing bed site cover was only weakly related to fawn survival in a recent study not conducted on rangeland (Chitwood et al. 2015). In contrast, white-tailed deer in a separate study avoided recently burned areas during fawn-rearing possibly because hiding cover was lacking (Cherry et al. 2017). Bed site cover may be important for thermoregulation during midday in warm environments (Fulbright et al. 2023). Woody cover that supplies shade and cooler temperatures during fawning and fawn-rearing could be particularly important in hot environments. In cooler environments, thermal cover may be important to help fawns avoid hypothermia. In the northern Great Plains bed sites in Conservation Reserve Program grasslands provided more cover and were warmer during summer than bed sites in wheat fields where mortality of fawns may have resulted from hypothermia (Grovenburg et al. 2012b).
Growth and development of white-tailed deer depend on sex and environmental factors including latitude and habitat quality (Ditchkoff 2011). In general, deer in environments with good nutrition reach maximum body mass at older ages than deer in resource-limited environments (Strickland and Demarais 2000; Monteith et al. 2009). Further illustrating the effect of environment, adult white-tailed deer from the Black Hills of South Dakota are smaller than those from more productive rangelands in eastern South Dakota (Monteith et al. 2009). Body mass of adult white-tailed deer along the Gulf Coastal Prairie is smaller and males have smaller antlers than adult white-tailed deer in the western Tamaulipan Vegetation Region (Rankins et al. 2021). One of the possible reasons deer in the western Tamaulipan Vegetation Region are larger is that digestible energy in browse and mast is greater than along the coast.
Photoperiod regulates seasonal timing of antler growth (Demarais and Strickland 2011). Yearling bucks may be spikes (unbranched antlers) or they may have branched antlers. The percentage of spikes in the yearling cohort strongly depends on nutrition (DeYoung et al. 2019). Antler size increases with age and reaches an asymptote at around five years old (Monteith et al. 2009; Hewitt et al. 2014).
Mortality of white-tailed deer follows a U-shaped curve with highest mortality in fawns and old (> 5 years) adults with higher survival rates in between (DeYoung 2011). Predation is the primary cause of fawn mortality on rangelands with coyotes being the primary predator (Bartush and Lewis 1981; Kie and White 1985; Whittaker and Lindzey 1999; Grovenburg et al. 2012b). Mule deer (O. hemionus)—white-tailed deer hybrids may be more susceptible to predation than nonhybrids because their gait is slower and mechanically less efficient (Lingle 1993).
Annual natural mortality of adult females tends to be low in the northern Great Plains (Dusek et al. 1992; Grovenburg et al. 2011). Annual survival of adult female white-tailed deer was 88% in in the northern Great Plains during the winters of 2014 and 2015, with mortality increasing as winter progressed (Moratz et al. 2018). Predation and hunting are the major causes of mortality of adult female white-tailed deer in the northern Great Plains (Dusek et al. 1992; Moratz et al. 2018). Enhancing nutrition by providing pelleted feed high in protein and energy increased survival of adult males and females in the Tamaulipan Vegetation Region, demonstrating that nutrition is a limiting factor for white-tailed deer populations in that region (DeYoung et al. 2019).
White-tailed deer population dynamics are linked to vegetation dynamics (DeYoung et al. 2019). The conventional paradigm in rangeland vegetation dynamics, the equilibrium model, assumes heavy grazing results in a shift in plant community composition to less palatable plants or those more tolerant of herbivory. The non-equilibrium model of vegetation dynamics is an alternative paradigm where abiotic factors such as variable precipitation drive plant community and ecosystem characteristics with plant–herbivore interactions weakly linked (Briske et al. 2003). Consequently, herbivore populations are density-independent, particularly in regions where the coefficient of variation in annual rainfall exceeds 30–33% (Briske et al. 2003; Derry and Boone 2010). Rangeland ecosystems can exhibit both equilibrium and non-equilibrium vegetation dynamics (Briske et al. 2003; Derry and Boone 2010). The conventional rangeland model of vegetation dynamics parallels density dependence theory in that loss of palatable plants results in a decline in forage quality and availability (DeYoung et al. 2019).
Biologists often use deer management models that assume populations act in a density dependent fashion. However, density-dependent population behavior is often difficult to detect (McCullough 1999). Research on non-equilibrium models of vegetation dynamics has focused on livestock and the effects of environmental stochasticity on white-tailed deer population dynamics has received little attention (DeYoung et al. 2019). DeYoung et al. (2008) hypothesized that high annual variation in precipitation, low soil fertility, and severe winters that limit populations may obscure density dependence in white-tailed deer and predicted that simple density-dependent models may not be useful in more than half of the range of white-tailed deer in the United States. In the Tamaulipan Vegetation Region, DeYoung et al. (2019) examined the effects of three different white-tailed deer densities on population growth rates, fawn and adult survival, and deer morphometrics where the coefficient of variation in annual precipitation exceeded 30%. They concluded that white-tailed deer in the region were only weakly density dependent and that in the absence of several consecutive wet years harvest of females would be additive, not compensatory mortality (DeYoung et al. 2019). One reason for weak density dependence in the region was that cycles of drought followed by periods of high precipitation had a much stronger effect on vegetation than increasing white-tailed deer density from 10 deer 81 ha−2 to 40 deer 81 ha−2 (DeYoung et al. 2019). Environmental stochasticity is a characteristic of many rangeland ecosystems; however, research linking models of vegetation dynamics with white-tailed deer population dynamics in systems other than the Tamaulipan Vegetation Region is lacking. Additional research on white-tailed deer in different rangeland ecosystems is needed to determine the utility of simple density dependent models in management.
Home range sizes of white-tailed deer vary from < 100 ha to > 1000 ha depending on a variety of factors including, sex, age, season, and population density (DeYoung and Miller 2011). Home range size is generally larger in drier, unproductive areas than in more productive, mesic environments (Stewart et al. 2011). Males typically have larger home ranges than females (DeYoung and Miller 2011; Stewart et al. 2011) and home range sizes of males tend to be larger during the breeding season than at other times of the year in low-density populations. Home range size declines when white-tailed deer population density increases. In the Tamaulipan Vegetation Region, for example, home ranges during late gestation, summer lactation, and early rut were 2.4 times larger when deer density was 10 deer 81 ha−2 than when density was 40 deer 81 ha−2 (Fulbright et al. 2023).
White-tailed deer form relatively small groups and rarely come together in large herds (DeYoung and Miller 2011). Females form groups that typically consist of an older matriarch and several generations of her offspring. Males 1.5-years and older form bachelor groups during the non-breeding season but are solitary during the breeding season. Males and females often separate spatially and use areas with different habitat characteristics during the non-breeding season (Stewart et al. 2011). In contrast to mule deer, groups of white-tailed deer do not consistently change group size or formation when confronted with predators (Lingle 2001).
White-tailed deer are crepuscular and are usually most active during early morning and late evening (Wiemers et al. 2014). Most of their active time, except during the breeding season, is spent foraging and searching for food. White-tailed deer consume forage amounting to 2–4% of their live body weight on a dry-matter basis (Halls 1978). Deer have a small rumen to body mass ratio relative to other ruminants. Consequently, they are concentrate feeders that select the most nutritious plants and plant parts (Hewitt 2011). They depend on a relatively short retention time of plant parts in the rumen so they can process the readily digestible nutrients and then quickly pass the undigested material making space for additional forage. White-tailed deer forages are typically classified as browse, forbs, grass, and mast (Hewitt 2011). However, they also consume flowers, dead leaves, and fungi (Darr et al. 2019). They select forbs over browse and grasses when forbs are available (Fulbright and Ortega-S 2013). On rangelands, forbs are often ephemeral. Browse often composes a major part of white-tailed deer diets when forbs are unavailable. Mast may be the dominant dietary component during certain seasons, particularly when acorns or honey mesquite (Prosopis glandulosa) mast are available. For example, mast including honey mesquite pods and prickly pear fruits formed up to 90% of deer diets during summer in a study in the Tamaulipan Vegetation Region (Fulbright et al. 2023). Flowers may also be an important dietary component when they are available composing up to 48% of deer diets based on a study in the Tamaulipan Vegetation Region (Darr et al. 2019).
White-tailed deer eat a variety of plant species and plant parts (Fulbright and Ortega-S 2013). Plant species vary in mineral content and in protein and energy. Consuming a diverse diet may aid in optimizing the nutrient content of their diet (Provenza et al. 2003). Many rangeland shrub genera and species are high in secondary compounds that have anti-nutrition effects on herbivores. Examples of genera with species high in secondary compounds include oaks (Quercus spp.), sagebrush (Artemisia spp.), sumac (Rhus spp.), and acacias (Acacia spp.). Consuming a diverse diet may help to neutralize anti-nutrition effects of secondary compounds and improve the nutrient content of white-tailed deer diets (Provenza et al. 2003, 2009).
3 Species and Population Status
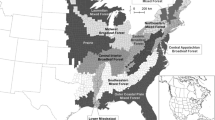
White-tailed deer occupy a large geographic area from Alaska and Canada to South America (Heffelfinger 2011; Fig. 18.3). About 38 subspecies of white-tailed deer occur within this geographic range. In contrast to range contractions for elk (Cervus canadensis; Chap. 20), the geographic range of white-tailed deer has expanded, particularly along the northern fringe of its range (Heffelfinger 2011). Climate change may be involved in range expansion along with human-imposed changes in the landscape such as forest cutting and cultivated agriculture expansion.
Several techniques are used to monitor populations of white-tailed deer. Older methods include pellet-group counts, track counts, night spotlighting, and mark-resight (DeYoung 2011). Helicopter surveys are commonly used in rangelands. Infrared or motion-triggered cameras also are used to estimate population density and can be used in combination with mark-resight techniques (Moore et al. 2014). Camera surveys and N-mixture modeling have been shown to be highly effective methods of estimating white-tailed deer populations (Keever et al. 2017). N-mixture modeling does not require capturing and marking individual deer. The procedure estimates detection probability and abundance with covariates that vary in time and space. Infrared thermal imaging is a technology with potential use for monitoring white-tailed deer populations (DeYoung 2011). Distance sampling can be used to estimate deer densities corrected from imperfect detection using a variety of survey methods including night spotlighting, helicopter surveys, and surveys using infrared thermal imaging (Montague et al. 2017; Peterson et al. 2020). However, conventional distance sampling assumes a monotonically decreasing detection probability with distance from the survey route, which may be violated for deer surveys occurring in areas with variable topography or vegetation cover. Hierarchical distance sampling models that allow and adjust for site-specific covariates on detectability may produce similar parameter estimates to N-mixture modeling (Christensen et al. 2021a).
White-tailed deer are typically undercounted in part because of visual obstruction from vegetation or rough topography. Sightability models are commonly used for aerial surveys from fixed-wing aircraft and helicopters to account for visibility bias. Sightability models use logistic regressions to model detections and non-detections of deer and develop correction factors to account for behavioral and environmental factors that influence rate of detection of animals (Anderson et al. 1998). Precision of sightability models for white-tailed deer declines with increased distance of deer from transects and vegetation obstruction (Dyal et al. 2021). Surveys using cameras and infrared thermal imaging (FLIR, Forward Looking Infrared) produced similar point estimates and detection probabilities (Haus et al. 2019). Use of infrared thermal imaging from unmanned aerial systems (drones) is a promising technology for monitoring white-tailed deer (Chrétien et al. 2016). Current limitations of the technology are limited flight distance of unmanned aerial systems and regulations.
4 Habitat Associations
White-tailed deer occupy a variety of different plant communities and ecosystems ranging from grasslands to forests, and from semi-desert shrubland to suburbs (Figs. 18.1 and 18.3). The fossil record of Odocoileus goes back four million years and fossils occur throughout most of their contemporary range (Heffelfinger 2011). Their success in a variety of settings over a long period of time is testimony to the adaptability of the species. In rangelands, white-tailed deer are most abundant in areas where woody vegetation dominates part of the landscape (Fulbright 2011). In the Great Plains, for example, white-tailed deer are strongly associated with wooded riparian corridors or bottomland areas (Compton et al. 1988). Similarly, Columbian white-tailed deer (O. v. leucurus) in Oregon were generally associated with riparian systems (Smith 1987). In western Texas, white-tailed deer densities increased with increasing woody plant cover (Wiggers and Beasom 1986). Highest densities of Columbian white-tailed deer occurred in areas with ≥ 50% woody vegetation (Smith 1987). In the Tamaulipan Vegetation Region, areas most heavily used by white-tailed deer had ≥ 85% woody canopy cover (Pollock et al. 1994). White-tailed deer typically bed in patches dominated by woody cover and forage where herbaceous vegetation dominates (Volk et al. 2007).
Because they forage in areas with herbaceous vegetation, white-tailed deer typically select vegetation communities that have a mixture of woody-plant-dominated and herbaceous-dominated patches (van der Hoek et al. 2002; Volk et al. 2007). Use of areas dominated by woody vegetation by white-tailed deer varies seasonally and with time of day. In the Tamaulipan Vegetation Region, for example, white-tailed deer used areas with 60–97% woody canopy cover during summer (Steuter and Wright 1980). In January, however, woody plant canopy cover did not influence use by white-tailed deer. In another study, vegetation height was strongly and positively related to relative probability of use during the day (Wiemers et al. 2014). Conversely, vegetation height was negatively related to relative probability of use at night. The negative relationship at night occurred because white-tailed deer were feeding in areas of herbaceous vegetation. A similar temporal and spatial pattern of habitat use was reported in the Great Plains in Kansas (Volk et al. 2007). In the Kansas study, white-tailed deer used areas dominated by woody vegetation at a course (6.25 ha) scale and avoided open grasslands. However, at a fine spatial scale deer that were foraging used open grassland. In the Tamaulipan Vegetation Region, vegetation height was less positively related to relative probability of use in the morning than at midday and was unrelated to relative probability of use in the evening (Wiemers et al. 2014). Selection of woody vegetation during summer and during midday is driven in part by the need for thermal cover to reduce heat loads. In the Great Plains of Colorado, Whittaker and Lindzey (2004) suggested that security cover was the primary driver of white-tailed habitat use. Woody vegetation provides both thermal and security cover and disentangling the two uses is difficult.
5 Rangeland Management
Grazing by domestic livestock is the dominant land use on rangelands. Consequently, responses of white-tailed deer habitat and populations to livestock and associated management practices including brush management, fencing, and water development are important considerations when managing white-tailed deer on rangelands.
5.1 Livestock Grazing
White-tailed deer management on rangeland is prone to error if the influence of domestic livestock is ignored. Cattle grazing and foraging by white-tailed deer are sometimes viewed as complimentary land uses because cattle primarily consume grass whereas white-tailed deer consume primarily forbs and browse (Fulbright and Ortega-S 2013). In fact, cattle grazing has been suggested as a tool to reduce grasses and increase forbs for wildlife (Lyons and Wright 2003). Nevertheless, the effect of cattle grazing on white-tailed deer and their habitat depends on factors such as season, management decisions regarding grazing intensity and stocking rate, and environment.
Livestock grazing may affect white-tailed deer through (1) competition resulting from diet overlap, (2) modifying species composition of plant communities, (3) social interactions, and (4) negative impacts on fawn production and survival. Diet overlap between cattle and white-tailed deer is greater during stress periods (e. g., winter and drought) and on overgrazed rangelands (Fulbright and Ortega-S 2013). Based on a quantitative review of literature on cattle grazing and white-tailed deer and mule deer, cattle and deer diet overlap ranged from 0.6 to 65% (Hines et al. 2021). Diet overlap was greatest during winter and spring. Diet overlap between cattle and deer increased 0.5% with every 0.1 AUY (Animal Unit Year) ha−1 increase in cattle stocking rate.
Domestic sheep (Ovis aries) and, to a lesser degree, goats (Capra aegagrus) compete with white-tailed deer for forbs (Bryant et al. 1979) and dietary overlap varies seasonally. For example, potential competition for forbs among goats, sheep, and white-tailed deer in the southern Great Plains (Edward’s Plateau of Texas) is greatest during winter and early spring (Bryant et al. 1979). The effects of competition on population dynamics of white-tailed deer are unknown but likely vary depending on timing, stocking rates, and local conditions. In the Tamaulipan Vegetation Region, white-tailed deer were able to shift diet composition to less palatable shrubs when Angora goats depleted shrubs that were more palatable to deer (Ekblad et al. 1993). Consequently, both Angora goats and white-tailed deer were able to stabilize the nutrient content of their diet regardless of diet overlap. Indices of diet overlap between white-tailed deer and Angora goats in the Tamaulipan Vegetation Region study ranged from 0.75 to 0.88 when goats were stocked at 0, 2, 4, and 6 goats ha−1.
Long-term, heavy livestock grazing can shift composition of plant communities from palatable plant species toward greater abundance of less palatable plant species. In general, a shift from grassland to dominance of woody vegetation may favor occupancy by white-tailed deer. Rangeland in the southern Great Plains (Edward’s Plateau of Texas) with a history of heavy grazing had larger standing crop of browse than less heavily grazed rangeland (Bryant et al. 1981). White-tailed deer spent more time foraging, however, on heavily grazed than on lightly grazed rangeland suggesting that palatable forage was scarcer on heavily grazed rangeland. In addition, diet samples from lightly grazed rangeland were higher in crude protein and phosphorus, and, except for winter, in digestible energy. Forb diversity is greater on lightly than on heavily grazed rangeland in the southern Great Plains (Edward’s Plateau; Warren and Krysl 1983).
Season, soil properties, and geographic location influence how cattle grazing affects standing crop or percent canopy cover of forbs (Hines et al. 2021). Forbs were more likely to decrease than increase in response to cattle grazing going from south to north across North America, possibly because the likelihood of grazing reducing forbs increased with cooler temperatures and shorter growing seasons. Forbs were more likely to increase in response to grazing going east and south across North America. In the drier ecosystems of western North America, variation in precipitation and amount of annual precipitation likely influenced forb response more than cattle grazing.
In theory, managing rangelands for increased plant species richness should benefit white-tailed deer because of the importance of plant diversity in optimizing the nutrient content of ruminant diets. Based on the intermediate disturbance hypothesis, plant species diversity may peak under moderate grazing intensities (Gao and Carmel 2020). On semiarid rangelands, however, plant species diversity may decline with increasing grazing intensity with the shape of the relationship depending on evolutionary history of grazing (Milchunas et al. 1988). Based on published literature, using livestock to increase species richness appears to be less applicable in semiarid rangeland ecosystems than in subhumid and humid parts of the distribution of white-tailed deer. Based on a meta-analysis of published papers, Gao and Carmel (2020) found that moderate grazing caused a slight increase in plant species richness in subhumid and humid areas, but plant species richness declined in arid and semiarid areas. Response of plant species richness to grazing intensity depended on the type of livestock. For example, in arid and semiarid areas species richness declined with grazing intensity with a mix of sheep and goats but grazing by sheep alone did not influence plant species richness.
The influence of grazing systems on white-tailed deer is unclear due to a lack of replicated research. In the southern Great Plains (Texas Edward’s Plateau), white-tailed deer densities were greater under a seven-pasture, short duration system than under a Merrill three-herd, four-pasture system (Reardon et al. 1978). Results of several studies have suggested that continuous year-long grazing benefitted deer more than rotational grazing systems (Cohen et al. 1989; Martinez et al. 1997; Ortega et al. 1997a, b). In the Tamaulipan Vegetation Region, white-tailed deer avoided intense concentrations of cattle in short-duration grazing cells (Cohen et al. 1989). Precipitation and topo-edaphic conditions mediate the effects of livestock grazing management on wildlife responses (e.g., Lipsey and Naugle 2017) and additional research is needed to evaluate the effects of livestock management (e.g., grazing system, stocking rates and timing) on white-tailed deer in other rangeland ecosystems.
White-tailed deer avoid areas grazed by livestock if areas that are not grazed are available to them. In a review of 70 published papers on cattle-deer interactions which included mule deer, Hines et al. (2021) found that in two-thirds of the papers deer either increased home-range size or used an alternative vegetation community if cattle were present. In the Tamaulipan Vegetation Region, spatial distribution of deer and cattle overlapped in productive areas, but the two species used the areas at different times (Cooper et al. 2008). White-tailed deer tended to move if a cow approached to within 46 m. In Oregon, white-tailed deer avoided pastures grazed by cattle but used the grazed pastures two or three months after cattle removal (Gavin et al. 1984). Possibly, white-tailed deer used the previously grazed pastures because of greater plant species richness. Cattle and white-tailed deer heavily use riparian areas; however, white-tailed deer avoid these areas when cattle are present (Compton et al. 1988; Cooper et al. 2008). There is little spatial overlap between white-tailed deer and cattle in rocky areas, dense shrub communities with little herbaceous vegetation, and areas distant from water because these areas are avoided by cattle (Owens et al. 1991; Cooper et al. 2008).
White-tailed deer in Oregon selected areas with little or no use by cattle or sheep for fawning (Smith and Coblentz 2010). Females in areas with livestock made large shifts in their activity center for fawning; three of seven females established home ranges geographically separate from their annual home range. Females in areas with little or no livestock made small activity center shifts for fawning and used sites within their annual home range. Possibly, females shifted home ranges in areas with livestock because of reductions in height and cover of vegetation resulting from livestock grazing.
Cattle stocking rate had little influence on white-tailed deer densities under average precipitation and temperature conditions based on computer-simulation models of trends in white-tailed deer densities (Glasscock 2001). Combined effects of low winter temperatures, low precipitation, and heavy stocking rates caused rapid declines in deer densities. In contrast, number of fawns surviving to a year old declined with increasing stocking rates consisting of a combination of cattle, sheep, and goats in the southern Great Plains (Edward’s Plateau of Texas; McMahan and Ramsey 1965). In Oklahoma and Arkansas fetuses female−1 declined with increasing cattle stocking rate (Jenks and Leslie 2003). White-tailed deer had 2 fetuses female−1 with no grazing, compared to 1.4 and 1.2 fetuses female−1 with moderate and heavy grazing, respectively.
5.2 Brush Management and Vegetation Manipulation
Brush management includes removal, reduction, or manipulation of woody vegetation (Hamilton et al. 2004). Brush management methods can be grouped broadly as fire, mechanical, and chemical approaches. Brush management has traditionally been applied to meet livestock needs such as increasing herbaceous forage (Fulbright et al. 2018). More recently, approaches to brush management have taken wildlife responses into account or have included improving white-tailed deer habitat as a goal. Efficacy of brush management in reducing woodland expansion in the Great Plains was questioned by Scholtz et al. (2021); brush management treatments were generally short-lived and woody cover showed little reduction at regional scales meaningful to population management.
Documenting whether or not brush management improved habitat quality for white-tailed deer is difficult. Vegetation metrics such as an increase in food plants, particularly forbs, or forage quality are often used to infer improved habitat quality (Fulbright et al. 2018). Basing inferences on forbs is inadequate because mast of woody plants such as mesquite is the primary item in the diet during summer and during drought when forbs are sparse. Ironically, killing mesquite is often a primary goal of brush management. Further, food plants are but one part of white-tailed deer habitat. Other habitat characteristics such as thermal and hiding cover are critically important. Consequently, animal metrics such as demographic characteristics or productivity metrics such as fecundity or body mass are more reliable indicators of changes in habitat quality than vegetation metrics (Van Horne 1983; Fulbright et al. 2018). Unfortunately, vegetation metrics are used more often to infer changes in habitat quality because animal data are expensive and time-consuming to collect.
5.3 Fire
Controlled and prescribed fire may alter food resources, cover, and patterns of habitat use by white-tailed deer. In the Southern Great Plains and Tamaulipan Vegetation Region, prescribed fire was the brush management approach that most consistently resulted in an increase in white-tailed deer food plants based on a review of literature published between 1966 and 2011 (Fulbright and Ortega-S 2013; Fig. 18.4). White-tailed deer are likely attracted to burned areas because forage quality (e.g., crude protein) of vegetation recovering post-fire is typically higher than mature, unburned vegetation (Fulbright and Ortega-S 2013). White-tailed deer may temporarily concentrate in burned patches. For example, use of resprouting shrubs in burned patches relative to use of shrubs in unburned areas peaked 12 to 20 weeks post-fire and remained higher up to 30 weeks post-fire (Fulbright et al. 2011). Although white-tailed deer are attracted to burned areas, they may maintain portions of their home range in unburned areas based on research in non-rangeland environments (Cherry et al. 2018).
Burning may alter predator–prey relationships. For example, although burning increased high-quality forage in a forested area of Georgia, white-tailed deer avoided recently burned areas (Cherry et al. 2017). Similar results have been reported on rangeland. For example, white-tailed deer in the northern Great Plains also avoided burned areas during the first winter after fire (Dubreuil 2003). In both studies, researchers attributed avoidance of recently burned areas to a lack of cover increasing susceptibility to predation. Predators may be attracted to burned areas where prey concentrate. Although not documented on rangeland, panthers (Felis concolor) in Florida are attracted to prescribed burns < 1 year old possibly because of higher numbers of white-tailed deer and other prey (Dees et al. 2001).
Males may use burned areas differently than females. Differential use between sexes may influence how beneficial burning is in managing white-tailed deer habitat; however, research on this topic is minimal on rangelands. Female white-tailed deer in Georgia and North Carolina avoided recently burned patches possibly because of the lack of cover (Lashley et al. 2015; Cherry et al. 2017). Males may be more prone to take advantage of the improved forage quality after fire (Lashley et al. 2015). In the eastern Great Plains of Oklahoma, male and female white-tailed deer exhibited differential selection for fire and herbicide treatments (Leslie et al. 1996). For example, based on pooling two years of data, male deer avoided a treatment with fire and no herbicides during spring and autumn. In comparison, females used the fire with no herbicide treatment in proportion to availability during all seasons. Males selected a treatment with a combination of triclopyr application and fire during summer and autumn. Females avoided the treatment during autumn and winter and selected the triclopyr and fire treatment during spring.
Evidence that fire temporarily improves habitat quality for white-tailed deer is largely based on vegetation metrics and from a restricted geography outside of rangelands. For example, a one-year fire return interval in pine-hardwood forests in Alabama increased estimated nutritional carrying capacity (Glow et al. 2019). There is limited evidence that fire may benefit measures of white-tailed deer productivity such as fawn survival or antler size. In one of the few studies on fire and deer productivity, fawn biomass, and antler size of two-year-old males were greater during the initial year after fire (Springer 1977).
5.4 Mechanical
Mechanical treatments on rangeland include brush management and activities such as haying and mowing. Brush management ranges from removal of individual woody plants by hand-grubbing to use of heavy equipment to uproot plants (Hamilton et al. 2004). Selective removal of individual plants to reduce woody plant density has been referred to as “brush sculpting” (Ansley et al. 2003). Forms of brush management such as roller chopping remove top growth of woody plants leaving the crowns and roots of the plants intact. Re-sprouting woody plant species such as honey mesquite produce sprouts from buds in the crowns and quickly produce new sprouts following top removal.
White-tailed deer can shift diet composition and maintain diet quality when brush management has altered vegetation composition. For example, woody vegetation re-establishes after root-plowing in a decade or two, but the re-established woody plant community may lack woody plant species important for browse (Fulbright and Beasom 1987; Ruthven et al. 1993, 1994). Seventeen years after root plowing in the eastern Rio Grande Plains of Texas, root-plowed sites were dominated by huisache (Vachellia farnesiana) compared to mesquite-mixed brush in untreated areas. White-tailed deer tended to eat more browse and less huisache mast and forbs in untreated than in root-plowed sites (Ruthven et al. 1994). However, reproductive measures and population status of white-tailed deer were similar in untreated and root-plowed areas. The temporal scales of mechanical treatment effects likely vary across rangeland types in relation to a variety of local conditions (e.g., soil properties, precipitation, production potential), but information in most rangeland systems are lacking.
Effects of mechanical brush management on white-tailed deer vary depending on scale and pattern of application. Clearing large tracts of brushland to create extensive grassland with no woody cover reduces white-tailed deer densities (McMahan and Inglis 1974; Darr and Klebenow 1975). Conversely, reducing woody vegetation canopy cover to 50–70% may not reduce white-tailed deer densities (Rollins et al. 1988), although effects of treatment scale and proximity of untreated woody vegetation were not taken into account.
Brush management is often done in strips or other patterns to create mosaics of woody plant-dominated patches and interspersed herbaceous-dominated patches (Archer et al. 2011). Root-plowing to create a mosaic consisting of an alternating sequence of 85-m-wide woody-plant-dominated strips separated by 95-m-wide root plowed strips in the Tamaulipan Vegetation Region had little effect on white-tailed deer home range size or placement (Dykes 2022).
As with fire, males may use brush management treatments differently than females (Stewart et al. 2003). In the Tamaulipan Vegetation Region, for example, adult females used roller chopped strips more than untreated strips regardless of season. In contrast, adult males used roller chopped strips more than untreated strips during autumn but not during spring.
There is no clear scale or pattern of clearing woody plants and creating woody-plant strips or clusters that is optimal for white-tailed deer. A wide range of scales and patterns may exist across which demographic or measures of white-tailed deer productivity are similar. Selection of scales and patterns by range and wildlife managers is based on economics and aesthetics more than knowledge of optimum treatment designs (Fulbright and Ortega-S 2013; Fulbright et al. 2018). From an economic perspective, clearing linear strips is more cost-effective than creating shrub clusters. In regard to aesthetics, humans perceive savanna-like landscapes as more pleasing than woody-plant dominated landscapes (Ulrich et al. 1991). The human-value orientation influencing woody plant management decisions is well illustrated by use of terms such as “brush sculpting.” It is difficult to disentangle what is beneficial to white-tailed deer from what is perceived as aesthetically pleasing by humans because of our incomplete knowledge of the effects of brush management on white-tailed deer at the population level.
As with fire, documentation that mechanical brush management improves habitat quality for white-tailed deer is largely based on vegetation metrics. A knowledge gap exists regarding the question of whether or not mechanical brush management can be used to improve habitat quality based on demographic or productivity metrics of white-tailed deer.
Mowing of grassland to reduce vegetation height has been used to increase use by white-tailed deer (Washburn and Seamans 2007). However, hayed grassland may be avoided by fawns until vegetation regrows enough to provide cover (Grovenburg et al. 2012a).
5.5 Chemical
Chemical treatments are usually applied on rangeland to manage woody vegetation, but they have also been used to manipulate grassland structure and composition for wildlife (Washburn and Seamans 2007). Herbicides can be broadly grouped as soil applied or foliar applied. Methods of application range from applying herbicide to the trunks or canopy of individual woody plants to large-scale broadcast applications from aircraft. Herbicides can be applied in a brush sculpting fashion to kill individual woody plants. Herbicides can also be applied in patterns of alternating treated and untreated strips (Fulbright and Garza 1991). A slightly more complex mosaic pattern created with herbicides is the variable rate pattern (Scifres et al. 1988). Variable rate patterns of herbicide application were developed to increase grass for livestock while leaving adequate woody vegetation for wildlife. In the variable rate pattern, a checkerboard of patches of woody vegetation receives different rates of herbicides. The result is that only a portion of woody plants are killed in some patches and woody plants are totally killed in others.
White-tailed deer may temporarily leave areas aerially treated with broadcast herbicides. One of the possible reasons for this is that some herbicides kill forbs; in addition, herbicides may reduce browse and mast. White-tailed deer densities may return to pre-treatment densities once forbs reestablish (Beasom and Scifres 1977). In South Texas, treating 100% of an area resulted in a 40% reduction in white-tailed deer densities between 15 and 27 months after treatment. However, densities returned to pre-treatment levels 27 months post-treatment.
Herbicide treatments applied in a mosaic fashion may have a neutral to potentially beneficial effect on habitat quality. For example, applying tebuthiuron in alternating treated and untreated strips had little overall effect on white-tailed deer nutritional status in live-oak (Quercus fusiformis) dominated rangeland in South Texas (Fulbright and Garza 1991). In Oklahoma, treating Cross Timbers and Prairies vegetation in a mosaic of herbicide treatments and annual spring burning resulted in greater white-tailed deer body mass and dietary nitrogen concentrations (Soper et al. 1993).
5.6 Managing for Heterogeneity
Standing crop and species composition of herbaceous vegetation in rangeland systems varies in space and time. A traditional paradigm in rangeland management has been focused on stabilizing or increasing livestock productivity by increasing rangeland homogeneity (Fuhlendorf and Engle 2001; Wilcox et al. 2021). A paradigm shift in contemporary rangeland management is to increase spatial heterogeneity (Fuhlendorf et al. 2017). One approach is use of pyric-herbivory to increase spatial heterogeneity by incorporating a combination of fire and grazing to create a mosaic of patches differing in grazing intensity and time since fire (McGranahan et al. 2012). In the southern Great Plains, livestock productivity decreased with declining precipitation in more homogeneous environments (Allred et al. 2014). In heterogeneous environments, livestock productivity was unrelated to precipitation.
White-tailed deer may also benefit from increased landscape heterogeneity resulting from pryric-herbivory. For example, fire and grazing have been used to reduce grass canopy cover and increase forbs (Ramirez-Yanez et al. 2007). Theoretically, creating a mosaic of different forb guilds and successional states increases the diversity of foods available to deer, perhaps conferring nutritional benefits. Fawn survival may be higher in heterogeneous landscapes than in more homogeneous landscapes (Rohm et al. 2007; Grovenburg et al. 2012c; Gulsby et al. 2017; Kilburn 2018). Reasons for higher fawn survival in heterogeneous landscapes are unclear. Several explanations have been proposed, such as higher quality of food in heterogeneous areas allowing females to have smaller home ranges and additional time for defense and nursing of fawns, high availability of food for predators in heterogeneous areas buffering predation, and reduced susceptibility of fawns to predation because females travel more rapidly and further in homogeneous environments (Rohm et al. 2007; Gulsby et al. 2017; Kilburn 2018).
Much of the research on pyric-herbivory has been done in the tallgrass prairies of the Great Plains. Rangelands that are more arid or semiarid often have more inherent heterogeneous spatial structure consisting of patches of bare ground or sparse herbaceous vegetation and patches of perennial grasses or woody plants (Aguiar and Sala 1999, van de Koppel et al. 2002; Segoli et al. 2012). Spatial redistribution of surface water or nutrients is an important ecosystem process to maintain productivity in these systems. The focus of management in more arid and semiarid systems may be maintaining heterogeneity and ecosystem function rather than trying to create it.
Habitat heterogeneity in rangelands is temporal as well as spatial. High variation in precipitation drives variation in the composition and structure of vegetation and, as a result, the abundance of deer foods. In the Tamaulipan Vegetation Region, for example, annual precipitation across six study sites during 2012–2019 varied from 28.9 to 84.5 cm (Fulbright et al. 2021). Standing crop of forbs selected by white-tailed deer during that period varied more than four-fold, from 82 to 442 kg ha−1. On rangelands with highly variable precipitation, season, soil texture, and precipitation may have a greater impact on standing crop of forbs than grazing by ungulates (Fulbright et al. 2021).
Most studies of the influence of white-tailed deer on vegetation have been conducted in the mid-western and eastern United States. Herbivory by white-tailed deer in the eastern portion of their range strongly influences composition of understory vegetation (Frerker et al. 2014, Habeck and Schultz 2015). On rangelands, effects of foraging by white-tailed deer on plant community composition appears to be less dramatic (DeYoung et al. 2019; Bloodworth et al. 2020). Research on rangelands is limited, however, and additional research is needed to clarify how deer impact vegetation in different rangeland plant communities and ecosystems where vegetation dynamics may follow equilibrium or non-equlibrium dynamics, or a combination.
5.7 Habitat Restoration
In the Northern Great Plains, restoration of herbaceous vegetation through the Conservation Reserve Program (CRP) has increased white-tailed deer occurrence and abundance (Nagy-Reis et al. 2019). Fawns in the northern Great Plains selected CRP over other vegetation types (Grovenburg et al. 2012a). Interestingly, revenues from hunting as a result of Conservation Reserve Program plantings override the net economic effect of losses in crop production revenues (Bangsund et al. 2004).
5.8 Water Development
White-tailed deer drink from earthen ponds and concrete water troughs associated with water wells constructed for livestock (Prasad and Guthery 1986; Fulbright et al. 2023). However, the importance of these water sources to white-tailed deer is unclear. We do not know for sure if white-tailed deer require free-standing water or if they can meet their needs with preformed water (dietary moisture) in forage. However, we do know that white-tailed deer use free-standing water when it is available.
White-tailed deer drank free-standing water from concrete troughs in South Texas in exclosures with no livestock (Fulbright et al. 2023). Male white-tailed deer drank an average of 1.57 gallons of water month−1. Females consumed an average of 1.33 gallons month−1 with a minimum and maximum monthly average of 0.08 and 4.8 gallons, respectively. In the Tamaulipan Vegetation Region, white-tailed deer avoided concrete water troughs at the center of short-duration grazing cells possibly because of heavy use by livestock and increased human presence (Prasad and Guthery 1986).
5.9 Fencing
Livestock fencing is a semipermeable barrier to white-tailed deer (Burkholder et al. 2018). White-tailed deer in the northern Great Plains preferred to crawl under fences rather than jumping over them. In the northern Great Plains, odds of a white-tailed deer successfully crossing a fence increased with increasing height of the bottom wire (Jones et al. 2020). Increasing height reduces the number of deer jumping over the fence; 14% fewer deer jump fences 1.8 m tall compared to 1.5 m tall (VerCauteren et al. 2010). White-tailed deer can become entangled in wire fences but the relative importance of fences as a cause of mortality on rangeland is unclear. Entanglement in fences was a minor cause of mortality in the Tamaulipan Vegetation region (Webb et al. 2007). Webb et al. (2007) tracked 48 mature male white-tailed deer for two years; out of 21 mortalities they recorded one that resulted from fence entanglement.
6 Impacts of Disease
Diseases are an important management concern for deer because outbreaks of some diseases can reduce populations; whereas, others are transmissible to humans and livestock. Epizootic hermorrhagic disease is the most important cause of viral-related mortality in white-tailed deer (Christiansen et al. 2021b); however, direct population-level effects are poorly documented (Gaydos et al. 2004). Epizootic hemorrhagic disease is transmitted by biting midges (Culicoides spp.; Stevens et al. 2015). Losses of white-tailed deer in the northern Great Plains from epizootic hermorrhagic disease are normally minor but can be large (South Dakota Game, Fish, and Parks 2022). The disease was implicated in a population decline in the northern Great Plains during the late 1970s (Dusek et al. 1989). Epizootic hermorrhagic disease also affects cattle; however, they rarely exhibit clinical signs of the disease (Campbell and VerCauteren 2011; Stevens et al. 2015). Baiting and feeding of deer increase the probability of direct transmission of the disease from infected animals (Rivera et al. 2021). In addition, white-tailed deer kept in breeding pens have increased prevalence of epizootic hemorrhagic disease (Rivera 2021). In one study, presence of captive white-tailed deer resulted in higher infection rates of epizootic hemorrhagic disease among cattle (Becker et al. 2020).
Bluetongue is a viral disease closely related to epizootic hemorrhagic disease and is also transmitted by biting midges in the genus Culicoides (Campbell and VerCauteren 2011). All ruminants are susceptible to being infected by bluetongue but the disease is most common in sheep (Sperlova and Zendulkova 2011). Although bluetongue is less common in white-tailed deer than epizootic hemorrhagic disease, serious outbreaks sometimes occur. For example, up to 10,000 white-tails perished from the disease in an outbreak in Idaho in 2011 (Phillips 2015).
Bacterial diseases in white-tailed deer include anthrax, dermatophilosis, brain abscesses, bovine tuberculosis, paratuberculosis, leptospirosis, salmonella, and lyme disease (Campbell and VerCauteren 2011). Of these, anthrax is the deadliest. Anthrax is relatively uncommon with the most frequent outbreaks in white-tailed deer occurring in southwestern Texas (Blackburn and Goodin 2013; Mullins et al. 2015). Population-level effects of dermatophilosis and bacterial dermatologic diseases in free-ranging white-tailed deer are probably minimal (Nemeth et al. 2014).
Bovine tuberculosis primarily affects cattle; however, white-tailed deer can contract the disease and are the primary maintenance host of the disease in North America (Carstensen et al. 2008; Campbell and VerCauteren 2011). The disease is endemic to a five-county area in Michigan and an area around Riding Mountain National Park in Manitoba, Canada (Atwood et al. 2007; Brook et al. 2013). Potential for transmission of the disease from white-tailed deer to cattle can be reduced by protecting cattle feeders from white-tailed deer, reducing deer densities, and other strategies than minimize contact between deer and cattle (Campbell and VerCauteren 2011; Brook et al. 2013). White-tailed deer are not an important reservoir for paratuberculosis, which is uncommon in wild ruminants (Campbell and VerCauteren 2011).
In Mexico, 5.6% of white-tailed deer tested had antibodies against leptospirosis (Cantu-C et al. 2008). Probability of deer testing positive for leptospirosis was 3.6 times greater where cattle were continuously grazed than if they were rotationally grazed. In a survey in the United States, about 40% of white-tailed deer tested had titers to the serovars of Leptospira; however, only 3% of the animals tested demonstrated recent infection (Pedersen et al. 2018). Pedersen et al. (2018) concluded that white-tailed deer could be important contributors to the cycle of infection of leoptospirosis and may be involved in transmission of the disease to livestock.
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy. It is not caused by a bacterium or a virus, but rather a prion which is a misfolded form of a protein. First discovered in captive deer in the 1960s, the first case of CWD in free-ranging wildlife was discovered in mule deer in Colorado in 1980. The disease has spread widely since 1980 and affects white-tailed deer from southern Canada to Texas. Chronic wasting disease is of particular concern on rangelands because white-tailed deer in semiarid environments depend on high adult survivorship to counter low fawn recruitment. Using simulation models, Foley et al. (2016) found that CWD increases additive mortality. Annually, white-tailed deer with CWD are 4.5 times more likely to die than those testing negative for the disease (Edmunds et al. 2016). Chronic wasting disease has the potential to limit white-tailed deer populations if the disease becomes endemic (Edmunds et al. 2016).
White-tailed deer serve as hosts for cattle fever ticks (Fulbright and Ortega-S 2013). This creates a challenge for tick eradication because white-tailed deer are free ranging and highly mobile (Currie et al. 2020). Cattle fever ticks were considered eradicated from the United States in 1943 (Thomas et al. 2020). However, since 2008 fever tick infestations in Texas near the border with Mexico have increased. Researchers in the region have been developing ways to reduce fever tick infestations in white-tailed deer. For example, consumption of ivermectin-medicated corn reduces the probability of infestation with cattle fever ticks in white-tailed deer (Currie et al., 2020).
White-tailed deer are highly susceptible to acute respiratory syndrome coronavirus 2 (SARS-Co-V-2; Palmer et al. 2021). Consequently, they are a potential reservoir of the disease that could be transmitted to humans (Palermo et al. 2022).
7 Ecosystem Threats
Continued spread of chronic wasting disease is a major threat to white-tailed deer. The disease is spreading rapidly in North America (Escobar et al. 2020). In the United States, the disease has been reported in wild cervids from Idaho, Montana, and South Dakota south to Texas (Centers for Disease Control 2022). Where the disease is well established, infection rates in free-ranging deer and elk may exceed 10% and cases with infection rates > 25% have been reported. Infection rates are highest in captive herds, reaching 80–90% in certain cases (Haley and Hoover 2015). Chronic wasting disease has been detected in > 175 captive cervid facilities (Carlson et al. 2018). Spread of chronic wasting disease occurs through natural movements of infected animals, movement of infected captive cervids by humans, and escape of infected animals from captive facilities (Carlson et al. 2018; Rivera et al. 2019).
Energy development also alters white-tailed deer habitat on rangelands. Published results of research on the effects of energy development on white-tailed deer is limited. In North and South Dakota, oil and gas development did not appear to alter survival and health of white-tailed deer (Moratz 2016). However, oil and gas development did alter distribution of white-tailed deer (Gullikson 2019); white-tailed deer avoided well pads and avoided areas with oil field development at the population level during summer. For similar reasons, renewable energy development has the potential to reduce white-tailed deer habitat on rangelands. However, the effects on white-tailed deer of wind farms and solar parks and their associated road networks and infrastructure are unknown.
A variety of exotic ungulates have been introduced in areas occupied by white-tailed deer. Potential negative interactions between exotics and white-tailed deer on western US rangelands is primarily restricted to Texas. There were more than two million exotic animals of about 135 species in Texas in the early twenty-first century (Gill 2020). Axis deer (Axis axis), fallow deer (Dama dama), and sika deer (Cervus nippon) are among the most abundant exotics that potentially compete directly with white-tailed deer. These exotic deer species can consume a diet high in grass (Henke et al. 1988). White-tailed deer, in contrast, cannot digest grass as efficiently so exotic deer species have a competitive advantage when forbs and browse are limited in availability. Competition between sika deer and white-tailed deer in Maryland resulted in white-tailed deer consuming lower quality forage (Kalb et al. 2018). As a result of their competitive ability, exotic deer species have the potential to reduce productivity of white-tailed deer and displace them from higher quality habitat (Faas and Weckerly 2010).
Climate change presents a paradox for white-tailed deer with differing effects in the southern and northern parts of their range. In the southern part of their geographic distribution, warming may cause changes in white-tailed deer behavior to cope with higher temperatures. White-tailed deer have few physiological adaptations to reduce heat loads. They can reduce heat loads by panting, but panting results in water loss, which may be maladaptive in dry rangeland environments. White-tailed deer therefore rely primarily on behavioral adaptations such as reducing activity and seeking shade to deal with excessive heat. In Minnesota, white-tailed deer were active at temperatures between 6 and 16 °C but became less active as temperatures warmed above 16 °C (Beier and McCullough 1990). Although white-tailed deer are crepuscular, warming temperatures may cause them to be more active at night when temperatures are cooler. White-tailed deer increased the amount of time they fed at night to avoid hot daytime temperatures in Mississippi (Wolff et al. 2020). In the hot, dry rangelands of the Tamaulipan Vegetation Region, white-tailed deer selected taller vegetation with the lowest operative temperature during morning and midday (Wiemers et al. 2014). Higher temperatures resulting from climate change may make availability of thermal cover even more important to white-tailed deer. White-tailed deer may also alter their behavior to deal with effects of extreme climatic events such as hurricanes that are predicted by climatologists to increase in strength and frequency with climate change (Abernathy et al. 2019).
Climate change may be at least partly responsible for expansion of the range of white-tailed deer in the northern part of their geographic distribution. Dawe and Boutin (2016) modeled the effect of climate change on white-tailed deer distribution in the boreal forest of North America. Their model predicted that during the first half of the twenty-first century the range of white-tailed deer will expand 100 km further north in northeastern Alberta. In Ontario, Kennedy-Slaney et al. (2018) used simulation models to predict that northward expansion of white-tailed deer will not be limited by severe winters by 2100. Weiskopf et al. (2019) predicted that white-tailed deer will become more abundant in the midwestern United States as a result of climate change. Factors facilitating greater abundance included increased survival because warmer temperatures reduced snowpacks.
8 Conservation and Management Actions
Chronic wasting disease is an insidious threat to white-tailed deer throughout the United States. The disease has been reported in 27 states (Centers for Disease Control and Prevention 2022). The primary mechanism of spread for CWD is movement of live animals by humans (Miller and Fischer 2016). Approaches to containing the spread of the disease include local population reduction, regulating the translocation of white-tailed deer and other cervids by humans, and bans on baiting and feeding (Campbell and VerCauteren 2011). Culling of host animals and restrictions on export of meat are also recommended (Mysterud et al. 2021).
Hunting is the primary tool for managing white-tailed deer populations throughout their range (Woolf and Roseberry 1998; Brown et al. 2000; McShea 2012). About 10 million hunters pursued white-tailed deer annually during 2010–2013 (Hewitt 2015). Hunting white-tailed deer provides significant economic benefit to ranchers and other landowners in rangelands of the western United States. In the United States, about 33% of the private land is leased or owned for wildlife-related recreation (Macaulay 2016). Ranching enterprises with a combination of livestock production and hunting have a higher internal rate of return than enterprises with only livestock or only hunting (Genho et al. 2003) and fee hunting will likely compose a larger component of the diverse economies that maintain private ranching operations in the future (Chap. 27). In addition, potential for wildlife-related recreation adds more to real estate values than potential for agricultural production (Baen 1997; Haggerty et al. 2018).
Management of white-tailed deer in the western United States is typically of low intensity on public and private land (Jacobson et al. 2011). However, popularity of more intensive white-tailed deer management is growing. Intensive management typically occurs on private lands and is directed at increasing antler size and managing for older males (Jacobson et al. 2011). Tools of intensive management including high fences that restrict deer ingress and egress and supplemental feeding (Knox 2011). Intensive management has evolved into a deer breeding industry in which captive deer are bred for large antlers. Objections to intensive deer management including privatization of deer, which are considered a publically-owned resource in the United States; lack of fair-chase hunting; reducing the wildness of deer; and exacerbating the spread of chronic wasting disease and other diseases.
9 Research and Management Needs
Comparisons of the effect of different white-tailed deer population densities on population dynamics is needed in rangeland ecosystems with highly variable precipitation and low soil fertility to determine the usefulness of simple density dependent population models for management. Demographic responses of white-tailed deer to different livestock grazing intensities and grazing strategies represent a gap in our knowledge of white-tailed deer-livestock interactions. In particular, livestock grazing may reduce hiding cover for fawns, making them more susceptible to predation. Greater understanding of these interactions is important because dual white-tailed deer hunting and livestock production offer greater returns to ranchers than livestock alone in areas with huntable white-tailed deer populations.
Differential use by males and females may dictate benefits of prescribed burning in managing white-tailed deer habitat. Research on differential use of burned areas and the effects of fire on survival, productivity, and population growth of white-tailed deer is lacking in rangeland environments. Demographic and productivity responses of white-tailed deer to mechanical and chemical brush management on rangelands are also a gap in our knowledge.
Non-native grasses are often planted following brush management. Non-native grasses also have invaded large areas of white-tailed deer habitat on rangelands (Fulbright et al. 2013). White-tailed deer have been implicated in the spread of non-native plants in the eastern United States (Averill et al. 2018). Research is needed to determine if white-tailed deer have a role in dissemination of non-native plant seeds on rangelands.
There is a knowledge gap regarding spatial heterogeneity of vegetation and white-tailed deer nutrition and population ecology on rangelands, especially on fawn survival. In addition, we need to develop a better understanding of the influence of white-tailed deer foraging on vegetation dynamics in different rangeland ecosystems.
There is reason for concern about the impacts of renewable energy development because there is evidence of negative effects of wind farms on other deer species. For example, roe deer (Capreolus capreolus) in Poland have elevated stress levels in response to large wind farms (Klich et al. 2020). They avoid the interior part of wind farms and avoid proximity to wind turbines (Łopucki et al. 2017). Extensive road networks associated with wind farms increase potential for invasion of non-native plants (Keehn and Feldman 2018) that may degrade white-tailed deer habitat.
References
Abernathy HN, Crawford DA, Garrison EP, Chandler RB, Conner ML, Miller KV, Cherry MJ (2019) Deer movement and resource selection during hurricane Irma: implications for extreme climatic events and wildlife. Proc Royal Soc B 286. https://doi.org/10.1098/rspb.2019.2230
Aguiar MR, Sala OE (1999) Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol Evol 14:273–277
Anderson C Jr, Moody D, Smith B, Lindzey F, Lanka R (1998) Development of sightability models for summer elk surveys. J Wildl Manag 62:1055–1066. https://doi.org/10.2307/380255
Allred BW, Scasta JD, Hovick T, Fuhlendorf SD, Hamilton RG (2014) Spatial heterogeneity stabilizes livestock productivity in a changing climate. Agric Ecosyst Environ 193:37–41. https://doi.org/10.1016/j.agee.2014.04.020
Ansley RJ, Kramp BA, Jones DL (2003) Converting mesquite thickets to savanna through foliage modification with clopyralid. J Range Manag 56:72–80
Archer SR, Davies KW, Fulbright TE, McDaniel KC, Wilcox BP, Predick KI (2011) Brush management as a rangeland conservation strategy: a critical evaluation. In: Briske DD (ed) Conservation benefits of rangeland practices: assessment, recommendations, and knowledge gaps. USDA Natural Resources Conservation Service, Washington, DC, pp 105–170
Atwood TC, VerCauteren KC, DeLiberto TJ, Smith HJ, Stevenson JS (2007) Coyotes as sentinels for monitoring bovine tuberculosis prevalence in white-tailed deer. J Wildl Manag 71:1545–1554. https://doi.org/10.2193/2006-441
Averill KM, Mortensen DA, Smithwick EAH, Kalisz S, McShea WJ, Bourg NA, Parker JD, Royo AA, Abrams MD, Apsley DK, Blossey B, Boucher DH, Caraher KL, DiTommaso A, Johnson SE, Masson R, Nuzzo VA (2018) A regional assessment of white-tailed deer effects on plant invasion. AoB PLANTS 10. https://doi.org/10.1093/aobpla/plx047
Baen J (1997) The growing importance and value implications of recreational hunting leases to agricultural land investors. J Real Estate Res 14:399–414. https://doi.org/10.1080/1083557.1997.12090909
Bangsund DA, Hodur NM, Leistritz FL (2004) Agricultural and recreational impacts of the conservation reserve program in Noarth Dakota, USA. J Environ Manage 71:293–303. https://doi.org/10.1016/j.jenvman.2003.12.017
Bartush WS, Lewis JC (1981) Mortality of white-tailed deer fawns in the Wichita Mountains. Proc Oklahoma Acad Sci 61:23–27
Beasom SL, Scifres CJ (1977) Population reactions of selected game species to aerial herbicide applications in South Texas. J Range Manag 30:138–143. https://doi.org/10.2307/3897757
Becker ME, Roberts J, Schroeder ME, Gentry G, Foil LD (2020) Prospective study of epizootic hemorrhagic disease virus and bluetongue virus transmission in captive ruminants. J Med Entomol 57:1277–1285. https://doi.org/10.1093/jme/tjaa027
Beier P, McCullough DR (1990) Factors influencing white-tailed deer activity patterns and habitat use. Wildl Monogr 109:3–51
Blackburn JK, Goodin DG (2013) Differentiation of springtime vegetation indices associated with summer anthrax epizootics in West Texas, USA, deer. J Wildl Dis 49:699–703. https://doi.org/10.7589/2012-10-253
Bloodworth KJ, Ritchie ME, Komatsu KJ (2020) Effects of white-tailed deer exclusion on the plant community composition of an upland tallgrass prairie ecosystem. J Veg Sci 31:899–907. https://doi.org/10.1111/jvs.12910
Briske DD, Fuhlendorf SD, Smeins FE (2003) Vegetation dynamics on rangelands: a critique of the current paradigms. J Appl Ecol 40:601–614
Brook RK, Vander Wal E, van Beest FM, McLachlan S (2013) Evaluating use of cattle winter feeding areas by elk and white-tailed deer: implications for managing bovine tuberculosis transmission risk from the ground up. Preventative Vet Med 108:137–147. https://doi.org/10.1016/j.prevetmed.2012.07.017
Brown TL, Decker DJ, Riley SJ, Enck JW, Lauber TB, Curtis PD, Mattfeld GF (2000) The future of hunting as a mechanism to control white-tailed deer populations. Wildl Soc Bull 28:797–807
Bryant FC, Kothmann MM, Merrill LB (1979) Diets of sheep, angora goats, Spanish goats, and white-tailed deer under excellent range conditions. J Range Manag 32:412–417
Bryant FC, Taylor CA, Merrill LB (1981) White-tailed deer diets from pastures in excellent and poor range condition. J Range Manag 34:193–200
Burkholder EN, Jakes AF, Jones PF, Hebblewhite M, Bishop CJ (2018) To jump or not to jump: mule deer and white-tailed deer fence crossing decisions. Wildl Soc Bull 42:420–429. https://doi.org/10.1002/wsb.898
Campbell TA, VerCauteren KC (2011) Diseases and parasites. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 219–249
Cantu-C A, Ortega-S JA, Mosqueda J, Garcia-Vasquez Z, Henke SE, George JE (2008) Prevalence of infectious agents in free-ranging white-tailed deer in northeastern Mexico. J Wildl Dis 44:1002–1007. https://doi.org/10.7589/0090-3558-44.4.1002
Carlson CM, Hopkins MC, Nguyen NT, Richards BJ, Walsh DP, Walter WD (2018) Chronic wasting disease: status, science, and management support by the U. S. Geological Survey. US Department of the Interior. https://pubs.usgs.gov
Carstensen M, Butler E, DonCarlos M, Cornicelli L (2008) Managing bovine tuberculosis in white-tailed deer in northwestern Minnesota: a 2008 progress report. Mich Bovine Tuberc Bibliography Database 18. https://digitalcommons.unl.edu/michbovinetb/18
Centers for Disease Control and Prevention (2022) Chronic wasting disease. https://www.cdc.gov/prions/cwd/index.html
Cherry MC, Warren RJ, Conner LM (2017) Fire-mediated foraging tradeoffs in white-tailed deer. Ecosphere 8:e01784. https://doi.org/10.1002/ecs2.1784
Cherry MC, Chandler RB, Garrison EP, Crawford DA, Kelly BD, Shindle DB, Godsea KG, Miller KV, Conner LM (2018) Wildfire affects space use and movement of white-tailed deer in a tropical pyric landscape. For Ecol Manage 409:161–169. https://doi.org/10.1016/j.foreco.2017.11.007
Chitwood MC, Lashley MA, Kilgo JC, Pollock KH, Moorman CE, DePerno CS (2015) Do biological and bedsite characteristics influence survival of neonatal white-tailed deer? PLoS ONE 10:e0119070. https://doi.org/10.1371/journal.pone.0119070
Chrétien L, Théau J, Ménard P (2016) Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl Soc Bull 40:181–191. https://doi.org/10.1002/wsb.629
Christensen S, Farr M, Williams D (2021a) Assessment and novel application of N-mixture models for aerial surveys of wildlife. Ecosphere 12:e03725. https://doi.org/10.1002/ecs2.3725
Christensen SJ, Williams DM, Rudolph BA, Porter WF (2021b) Spatial variation of white-tailed deer (Odocoileus virginianus) population impacts and recovery from epizootic hemorrhagic disease. J Wildl Dis 57:82–93. https://doi.org/10.7589/JWD-D-20-00030
Cohen WE, Drawe DL, Bryant FC, Bradley LC (1989) Observations on white-tailed deer and habitat response to livestock grazing in South Texas. J Range Manag 42:361–365
Compton BB, Mackie RJ, Dusek GL (1988) Factors influencing distribution of white-tailed deer in riparian habitats. J Wildl Manag 52:544–548. https://doi.org/10.2307/3801607
Cooper SM, Perotto-Baldivieso HL, Owens MK, Meek MG, Figueroa-Pagán (2008) Distribution and interaction of white-tailed deer and cattle in a semi-arid grazing system. Agric Ecosyst Environ 127:85–92. https://doi.org/10.1016/j.agee.2008.03.004
Currie CR, Hewitt DG, Ortega-S JA, Shuster GL, Campbell TA, Lohmeyer KH, Wester DG, Pérez de León A (2020) Efficacy of white-tailed deer (Odocoileus virginianus) treatment for cattle fever ticks in southern Texas. J Wildl Dis 56:588–596. https://doi.org/10.7589/2015-11-304
Darr GW, Klebenow DA (1975) Deer, brush control, and livestock on the Texas Rollings Plains. J Range Manag 25:115–119
Darr RL, Williamson KM, Garver LW, Hewitt DG, DeYoung CA, Fulbright TE, Gann KR, Wester DB, Draeger DA (2019) Effects of enhanced nutrition on white-tailed deer foraging behavior. Wildl Monogr 202:27–34. https://doi.org/10.1002/wmon.1040
Dawe KL, Boutin S (2016) Climate change is the primary driver of white-tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecol Evol 6:6435–6451. https://doi.org/10.1002/ece3.2316
Dees CS, Clark JD, Van Manen FT (2001) Florida panther habitat use in response to prescribed fire. J Wildl Manag 65:141–147. https://doi.org/10.2307/3803287
DelGiudice GD, Lenarz MS, Powell MC (2007) Age-specific fertility and fecundity in northern free-ranging white-tailed deer: evidence for reproductive senescence? J Mammal 88:427–435. https://doi.org/10.1644/06-MAMM-A-164R.1
Demarais S, Strickland BK (2011) Antlers. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 107–145
Derry JF, Boone RB (2010) Grazing systems are a result of equilibrium and non-equlibrium dynamics. J Arid Environ 74:307–309. https://doi.org/10.1016/j.jaridenv.2009.07.010
DeYoung CD (2011) Population dynamics. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 147–180
DeYoung RW, Miller KV (2011) White-tailed deer behavior. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 311–351
DeYoung CD, Draw DL, Fulbright TE, Hewitt DG, Stedman SW, Synatzske DR, Teer JG (2008) Density dependence in deer populations: relevance for management in variable environments. In: Fulbright TE, Hewitt DG (eds) Wildlife science: linking ecological theory and management applications. CRC Press, Boca Raton, FL, pp 203–222
DeYoung RW, Demarais S, Gee KL, Honeycutt RL, Hellickson MW, Gonzales RA (2009) Molecular evaluation of the white-tailed deer (Odocoileus virginianus) mating system. J Mammal 90:946–953. https://doi.org/10.1644/08-MAMM-A-227.1
DeYoung CD, Fulbright TE, Hewitt DG, Wester DB, Draeger DA (2019) Linking white-tailed deer density, nutrition, and vegetation in a stochastic environment. Wildl Monogr 202:1–63. https://doi.org/10.1002/wmon.1040
Ditchkoff SS (2011) Anatomy and physiology. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 43–79
Dubreuil RP (2003) Habitat selection of white-tailed deer and mule deer in the southern Black Hills, South Dakota. MS Thesis, South Dakota State University, Brookings, USA
Dusek GL, MacKie RJ, Herriges JD Jr, Compton BB (1989) Population ecology of white-tailed deer along the lower Yellowstone River. Wildl Monogr 104:3–68
Dusek GL, Wood AK, Stewart ST (1992) Spatial and temporal patterns of mortality among female white-tailed deer. J Wildl Manag 56:645–650. https://doi.org/10.2307/3809455
Dyal JR, Miller KV, Cherry MJ, D’Angelo GJ (2021) Estimating sightability for helicopter surveys using surrogates of white-tailed deer. J Wildl Manag 85:887–896. https://doi.org/10.1002/jwmg.22040
Dykes JL (2022) Thermal ecology of white-tailed deer on southwestern rangelands. PhD dissertation, Texas A&M University-Kingsville
Edmunds DR, Kauffman MJ, Schumaker BA, Lindzey FG, Cook WE, Kreeger TJ, Grogan RG, Cornish TE (2016) Chronic wasting disease drives population decline of white-tailed deer. PLoS ONE 11:e0161127. https://doi.org/10.1371/journal.pone.0161127
Ekblad RL, Stuth JW, Owens MK (1993) Grazing pressure impacts on potential foraging competition between Angora goats and white-tailed deer. Small Rumin Res 11:195–208. https://doi.org/10.1016/0921-4488(93)90045-J
Escobar LE, Pritzkow S, Winter SN, Grear DA, Kirchgessner MS, Dominguez-Villegas E, Machado G, Peterson A, Soto C (2020) The ecology of chronic wasting disease in wildlife. Biol Rev Camb Philos Society 95:393–408. https://doi.org/10.1111/brv.12568
Faas CJ, Weckerly FW (2010) Habitat interference by axis deer on white-tailed deer. J Wildl Manag 74:698–706. https://doi.org/10.2193/2009-135
Foley AM, Hewitt DG, DeYoung CA, DeYoung RW, Schnupp MJ (2016) Modeled impacts of chronic wasting disease on white-tailed deer in a semi-arid environment. PLoS ONE. https://doi.org/10.1371/journal.pone.016359
Frerker K, Sabo A, Waller D (2014) Long-term regional shifts in plant community composition are largely explained by local deer impact experiments. PLoS ONE 9:e115843. https://doi.org/10.1371/journal.pone.0115843
Fulbright TE (2011) Managing white-tailed deer: western North America. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 537–563
Fulbright TE, Beasom SL (1987) Long term effects of mechanical treatments on white tailed deer browse. Wildl Soc Bull 15:560–564
Fulbright TE, Garza A Jr (1991) Forage yield and white-tailed deer diets following live oak control. J Range Manag 44:451–455
Fulbright TE, Ortega-S JA (2013) White-tailed deer habitat: ecology and management on rangelands, 2nd edn. Texas A&M University Press, College Station, TX
Fulbright TE, Dacy EC, Drawe DL (2011) Does browsing reduce shrub survival and vigor following summer fires? Acta Oecologica 37:10–15. https://doi.org/10.1016/j.actao.2010.10.007
Fulbright TE, Hickman KR, Hewitt DG (2013) Exotic grass invasion and wildlife abundance and diversity, south-central United States. Wildl Soc Bull 37:503–509. https://doi.org/10.1002/wsb.312
Fulbright TE, Davies KW, Archer SR (2018) Wildlife responses to brush management: a contemporary evaluation. Rangel Ecol Manage 71:35–44. https://doi.org/10.1016/j.rama.2017.07.001
Fulbright TE, Drabek DJ, Ortega-S JA, Hines SL, Saenz R III, Campbell TA, Hewitt DG, Wester DB (2021) Forb standing crop response to grazing and precipitation. Rangel Ecol Manage 79:175–185. https://doi.org/10.1016/j.rama.2021.08.007
Fulbright TE, DeYoung CA, Hewitt DG, Draeger DA (2023) Advanced white-tailed deer management: the nutrition—density sweet spot. Texas A&M University Press, College Station, TX
Fuhlendorf SD, Engle DM (2001) Restoring heterogeneity on rangelands: ecosystem management based on evolutionary grazing patterns: we propose a paradigm than enhances heterogeneity instead of homogeneity to promote biological diversity and wildlife habitat on rangelands grazed by livestock. Bioscience 51:625–632. https://doi.org/10.1641/0006-3568(2001)051[0625:RHOREM]2.0.CO;2
Fuhlendorf SD, Fynn RWS, McGranahan A, Twidwell D (2017) Heterogeneity as the basis for rangeland management. In: Briske DD (ed) Rangeland systems: processes, management and challenges. Springer Series on Environmental Management, Cham, Switzerland, pp 169–196. https://doi.org/10.1007/978-3-319-46709-2
Gavin TA, Suring H, Vohs PA Jr, Meslow EC (1984) Population characteristics, spatial organization, and natural mortality in Columbian white-tailed deer. Wildl Monogr 91:1–41
Gao J, Carmel Y (2020) Can the intermediate disturbance hypothesis explain grazing-diversity relations at a global scale? Oikos 129:493–502. https://doi.org/10.1111/oik.06338
Gaydos JK, Crum JM, Davidson WR, Cross SS, Owen SF, Stallknecht DE (2004) Epizootiology of an epizootic hemorrhagic disease outbreak in West Virginia. J Wildl Dis 40:383–393. https://doi.org/10.7589/0090-3558-40.3.383
Genho P, Hunt J, Rhyne M (2003) Managing for the long term while surviving the short term. In: Forgason CA, Bryant FC, Genho P (eds) Ranch management: integrating cattle, wildlife, and range. King Ranch, Kingsville, Texas, USA, pp 81–107
Gill C (2020) Escaped exotic animals are changing the Texas landscape. https://pitchstonewaters.com/escaped-exotics-animals-are-changing-the-texas-landscape/. Accessed 14 Jan 2021
Glasscock SN (2001) Analysis of vegetation dynamics, wildlife interactions, and management strategies in a semi-arid rangeland system: the Welder Wildlife Refuge model (Texas). PhD dissertation, Texas A&M University, College Station
Glow MP, Ditchkoff SS, Smith MD (2019) Annual fire return interval influences nutritional carrying capacity of white-tailed deer in pine-hardwood forests. Forest Sci 65:483–491. https://doi.org/10.1093/forsci/fxy063
Grovenburg TW, Jacques CN, Klaver RW, Jenks JA (2010) Bed site selection by neonate deer in grassland habitats on the Northern Great Plains. J Wildl Manag 74:1250–1256. https://doi.org/10.1111/j.1937-2817.2010.tb01245.x
Grovenburg TW, Swanson CC, Jacques CN, Deperno CS, Klaver RW, Jenks JA (2011) Female white-tailed deer survival across ecoregions in Minnesota and South Dakota. Am Midl Nat 165:426–435. https://doi.org/10.1674/0003-0031-165.2.426
Grovenburg TW, Klaver RW, Jenks JA (2012a) Spatial ecology of white-tailed deer fawns in the Northern Great Plains: implications of loss of conservation reserve program grasslands. J Wildl Manag 76:632–644. https://doi.org/10.1002/jwmg.288
Grovenburg TW, Klaver RW, Jenks JA (2012b) Survival of white-tailed deer fawns in the grasslands of the Northern Great Plains. J Wildl Manag 76:944–956. https://doi.org/10.1002/jwmg.339
Grovenburg TW, Monteith KL, Klaver RW, Jenks JA (2012c) Predator evasion by white-tailed deer fawns. Anim Behav 84:59–65. https://doi.org/10.1016/j.anbehav.2012.04.005
Gullikson BS (2019) Effects of energy development on movements, home ranges, and resource selection of white-tailed deer in the western Dakotas. PhD dissertation, South Dakota State University
Gulsby WD, Kilgo JC, Vukovich M, Martin JA (2017) Landscape heterogeneity reduces coyote predation on white-tailed deer fawns. J Wildl Manag 81:601–609. https://doi.org/10.1005/jwmg.21240
Habeck CW, Schultz AK (2015) Community-level impacts of white-tailed deer on understory plants in North American forests: a meta-analysis. AoB Plants 7:plv119. https://doi.org/10.1093/aobpla/plv119
Haggerty JH et al (2018) Land use diversification and intensification on Elk winter range in greater yellowstone: framework and agenda for social-ecological research. Rangeland Ecol Manage 71:171–174. https://doi.org/10.1016/j.rama.2017.11.002
Haley NJ, Hoover EA (2015) Chronic wasting disease of cervids: current knowledge and future perspectives. Ann Rev Animal Biosci 3:305–325. https://doi.org/10.1146/annurev-animal-022114-111001
Hamilton WT, McGinty A, Ueckert DN, Hanselka CW, Lee MR (2004) Brush management: past, present, future. Texas A&M University Press, College Station
Halls LK (1978) White-tailed deer. In: Schmidt JL, Gilber DL (eds) Big game of North America. Stackpole Books, Harrisburg, PA, pp 43–65
Haus J, Eyler T, Bowman J (2019) A spatially and temporally concurrent comparison of popular abundance estimators for white-tailed deer. Northeast Nat 26:305–324. https://doi.org/10.1656/045.026.0207
Heffelfinger JR (2011) Deer of the Southwest: a complete guide to the natural history, biology, and management of southwestern mule deer and white-tailed deer. Texas A&M University Press, College Station
Henke SE, Demarais S, Pfister JA (1988) Digestive capacity of white-tailed deer and exotic ruminants. J Wildl Manag 52:595–598. https://doi.org/10.23007/3800913
Hewitt DG (2011) Nutrition. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 75–105
Hewitt DG (2015) Hunters and the conservation and management of white-tailed deer (Odocoileus virginianus). Int J Environ Stud 72:839–849. https://doi.org/10.1080/00207233.2015.1073473
Hewitt DG, Hellickson MW, Lewis JS, Wester DB, Bryant FS (2014) Age-related patterns of antler development in free-ranging white-tailed deer. J Wildl Manag 78:979–984. https://doi.org/10.1002/jwmg.741
Hines SL, Fulbright TE, Ortega-S AJ, Webb SL, Hewitt DG, Boutton TW (2021) Compatibility of dual enterprises for cattle and deer in North America: a quantitative review. Rangeland Ecol Manag 74:21–31 https://doi.org/10.1016/j.rama.2020.10.005
Hyde KJ, DeYoung CA, Garza A Jr (1987) Bed sites of white-tailed deer fawns in South Texas. In: Proceedings of the annual conference of the southeastern association of fish and wildlife agencies, vol 41, pp 288–293
Jacobson HA, DeYoung CA, DeYoung RW, Fulbright TE, Hewitt DG (2011) Management on private property. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 453–479
Jenks JA, Leslie DM (2003) Effect of domestic cattle on the condition of female white-tailed deer in southern pine-bluestem forests, USA. Acta Theriol 48:131–144
Jones PF, Jakes AF, MacDonald AM, Hanlon JA, Eacker DR, Martin BH, Hebblewhite M (2020) Evaluating responses by sympatric ungulates to fence modifications across the northern Great Plains. Wildl Soc Bull 44:130–131. https://doi.org/10.1002/wsb.1067
Kalb DM, Bowman JL, DeYoung RW (2018) Dietary resource use and competition between white-tailed deer and introduced sika deer. Wildl Res 45:457–472. https://doi.org/10.1071/WR17125
Keehn JE, Feldman CR (2018) Disturbance affects biotic community composition at desert wind farms. Wildl Res 45:383–396. https://doi.org/10.1071/WR17059
Keever A, McGowan C, Ditchkoff S, Acker P, Grand J, Newbolt C (2017) Efficacy of N-mixture models for surveying and monitoring white-tailed deer populations. Mammal Res 62. https://doi.org/10.1007/s13364-017-0319-z
Kennedy-Slaney L, Bowman J, Walpole AA, Pond BA (2018) Northward bound: the distribution of white-tailed deer in Ontario under a changing climate. Wildl Res 45:220–228. https://doi.org/10.1071/WR17106
Kie JG, White M (1985) Population dynamics of white-tailed deer (Odocoileus virginianus) on the Welder Wildlife Refuge, Texas. Southwest Nat 30:105–118. https://doi.org/10.2307/3670664
Kilburn J (2018) Spatial variability in abundance, detectability, and survival of white-tailed deer across a heterogenous landscape of fear. MS Thesis, University of Connecticut
Klich D, Łopucki R, Ścibior A, Gołębiowska D, Wojciechowska M (2020) Roe deer stress response to a wind farms: methodological and practical implications. Ecol Ind 117. https://doi.org/10.1016/j.ecolind.2020.106658
Knox WM (2011) The antler religion. Wildl Soc Bull 35:45–48. https://doi.org/10.1002/wsb.5
Lashley MA, Chitwood MC, Kays R, Harper CA, DePerno CS, Moorman CE (2015) Prescribed fire affects female white-tailed deer habitat use during lactation. For Ecol Manage 348:220–225. https://doi.org/10.1016/j.foreco.2015.03.041
Leslie DM Jr, Soper RB, Lochmiller RL, Engle DM (1996) Habitat use by white-tailed deer on cross timbers rangeland following brush management. J Range Manag 49:401–406
Lingle S (1993) Escape gaits of white-tailed deer, mule deer, and their hybrids: body configuration, biomechanics, and function. Can J Zool 71:708–724. https://doi.org/10.1139/z93-095
Lingle S (2001) Anti-predator strategies and grouping patterns in white-tailed and mule deer. Ethology 107:295–314. https://doi.org/10.1046/j.1439-0310.2001.00664.x
Lipsey MK, Naugle DE (2017) Precipitation and soil productivity explain effects of grazing on grassland songbirds. Rangel Ecol Manage 70:331–340. https://doi.org/10.1016/j.rama.2016.10.010
Łopucki R, Klich D, Gielarek S (2017) Do terrestrial animals avoid areas close to turbines in functioning wind farms in agricultural landscapes? Environ Monit Assess 189:343. https://doi.org/10.1007/s10661-017-6018-z
Lyons RK, Wright BD (2003) Using livestock to manage wildlife habitat. AgriLife Extension Publication B-6136, Texas A&M University, College Station
Macaulay L (2016) The role of wildlife-associated recreation in private land use and conservation: providing the missing baseline. Land Use Policy 58:218–233. https://doi.org/10.1016/j.landusepol.2016.06.024
Martinez MA, Molina V, Gonzalez-S F, Marroquin JS, Navar Ch J (1997) Observations of white-tailed deer and cattle diets in Mexico. J Range Manag 50:253–257. https://doi.org/10.2307/4003725
McCullough DR (1999) Density dependence and life-history strategies of ungulates. J Mammal 80:1130–1146. https://doi.org/10.2307/1383164
McGranahan DA, Engle DM, Fuhlendorf SD, Winter SJ, Miller JR, Debinski DM (2012) Spatial heterogeneity across five rangelands managed with pyric-herbivory. J Appl Ecol 49:903–910. https://doi.org/10.1111/j.1365-2664.2012.02168.x
McMahan CA, Inglis JM (1974) Use of rio grande plain brush types by white-tailed deer. J Range Manag 27:369–374. https://doi.org/10.2307/3896494
McMahan CA, Ramsey CW (1965) Response of deer and livestock to controlled grazing in Central Texas. J Range Manag 18:1–7
McShea WJ (2012) Ecology and management of white-tailed deer in a changing world. Ann N Y Acad Sci 1249:45–56. https://doi.org/10.1111/j.1749-6632.2011.06376.x
Michel ES, Gullikson BS, Brackel KL, Schaffer BA, Jenks JA, Jensen WF (2020) Habitat selection of white-tailed deer fawns and their dams in the Northern Great Plains. Mammal Res 68:825–833. https://doi.org/10.1007/s13364-020-00519-6
Milchunas DG, Sala OE, Lauenroth WK (1988) A generalized model of the effects of grazing by large herbivores on grassland community structure. Am Nat 132:87–106. https://doi.org/10.1086/284839
Miller MW, Fischer JR (2016)The first five (or more) decades of chronic wasting disease: lessons for the five decades to come. In: Transactions of the North American wildlife and natural resources conference, vol 81, pp 1–12
Montague DM, Montague RD, Fies ML, Kelly MJ (2017) Using distance-sampling to estimate density of white-tailed deer in forested, mountainous landscapes in Virginia. Northeast Nat 24:505–519. https://doi.org/10.1656/045.024.0409
Monteith KL, Schmitz LE, Jenks JA, Delger JA, Bowyer RT (2009) Growth of male white-tailed deer: consequences of maternal effects. J Mammal 90:651–660. https://doi.org/10.1644/08-MAMM-A-191R1.1
Moore MT, Foley AM, DeYoung CD, Hewitt DG, Fulbright TE, Draeger DA (2014) Evaluation of population estimates of white-tailed deer from camera survey. J Southeast Assoc Fish Wildl Agencies 1:127–132
Moratz KL (2016) Effect of oil and gas development on survival and health of white-tailed deer in the western Dakotas. PhD Dissertation, South Dakota State University
Moratz KL, Gullikson BS, Michel ES, Jenks JA, Grove DM, Jensen WF (2018) Assessing factors affecting adult female white-tailed deer survival in the northern Great Plains. Wildl Res 45:679–684. https://doi.org/10.1071/WR18032
Mullins JC, Van Ert M, Hadfield T, Nikolich MP, Hugh-Jones ME, Blackburn JK (2015) Spatio-temporal patterns of an anthrax outbreak in white-tailed deer, Odocoileus virginianus, and associated genetic diversity of Bacillus anthracis. BMC Ecol 15:23. https://doi.org/10.1186/s12898-015-0054-8
Mysterud A, Benestad SL, Rolandsen CM, Våge J (2021) Policy implications of an expanded chronic wasting disease universe. J Appl Ecol 58:281–285. https://doi.org/10.1111/1365-2664.13783
Nagy-Reis MB, Lewis MA, Jensen WF, Boyce MS (2019) Conservation reserve program is a key element for managing white-tailed deer populations at multiple spatial scales. J Environ Manage 248:109299. https://doi.org/10.1016/j.jenvman.2019.109299
Nemeth NM, Ruder MG, Gerhold RW, Brown JD, Munk BA, Oesterle PT, Kubiski SV, Keel MK (2014) Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol 51:633–640
Ortega IM, Soltero-Gardea S, Bryant FC, Drawe DL (1997a) Evaluating grazing strategies for cattle: deer and cattle food partitioning. J Range Manag 50:622–630
Ortega IM, Soltero-Gardea S, Bryant FC, Drawe DL (1997b) Evaluating grazing strategies for cattle: nutrition of cattle and deer. J Range Manag 50:631–637
Owens ML, Launchbaugh KL, Holloway JW (1991) Pasture characteristics affecting spatial distribution of utilization by cattle in mixed brush communities. J Range Manag 44:118–123
Ozoga JJ (1987) Maximum fecundity in supplementally-fed northern Michigan white-tailed deer. J Mammal 68:878–879. https://doi.org/10.2307/1381573
Palermo PM, Orbegozo J, Watts DM, Morrill JC (2022) SARS-CoV-2 neutralizing antibodies in white-tailed deer from Texas. Vector-Borne Zoonotic Dis 22:62–64. https://doi.org/10.1089/vbz.2021.0094
Palmer MV, Martins M, Falkenberg S, Buckley A, Caserta LC, Mitchell PK, Cassmann ED, Rollins A, Zylich NC, Renshaw RW, Guarino C (2021) Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol 95:e00083–21.17.6. https://doi.org/10.1128/JVI.00083-21
Pedersen K, Anderson TD, Maison RM, Wiscomb GW, Pipas MJ, Sinnett DR, Baroch JA, Gidlewski T (2018) Leptospira antibodies detected in wildlife in the USA and the US Virgin Islands. J Wildl Dis 54:450–459. https://doi.org/10.7589/2017-10-269
Peterson MK, Foley AM, Tri AN, Hewitt DG, DeYoung RW, DeYoung CA, Campbell TA (2020) Mark-recapture distance sampling for aerial surveys of ungulates on rangelands. Wildl Soc Bull 44:713–723. https://doi.org/10.1002/wsb.1144
Phillips R (2015) Fish and Game confirms outbreak of bluetongue disease in whitetails. Idaho Fish and Game. https://idfg.idaho.gov/
Pollock MT, Whittaker DG, Demarais S, Zaiglan RE (1994) Vegetation characteristics influencing site selection by male white-tailed deer in Texas. J Range Manag 47:235–239
Potratz EJ, Brown JS, Gallo T, Anchor C, Santymire RM (2019) Effects of demography and urbanization on stress and body condition in urban white-tailed deer. Urban Ecosystems. https://doi.org/10.1007/s11252-019-00856-8
Prasad NLNS, Guthery FS (1986) Wildlife use of water under short duration and continuous grazing. Wildl Soc Bull 14:450–454
Provenza FD, Villalba JJ, Dziba LE, Atwood SB, Banner RE (2003) Linking herbivore experience, varied diets, and plant biochemical diversity. Small Rumin Res 49:257–274. https://doi.org/10.1016/S0921-4488(03)00143-3
Provenza FD, Villalba JJ, Wiemeier RW, Lyman T, Owens J, Lisonbee L, Clemensen A, Welch KD, Gardner DR, Lee ST (2009) Value of plant diversity for diet mixing and sequencing in herbivores. Rangelands 31:45–49. https://doi.org/10.2111/1551-501X-31.1.45
Ramirez-Yanez LE, Ortega-S, Brennan LA, Rasmussen GA (2007) Use of prescribed fire and cattle grazing to control guineagrass. In: Proceedings of the 23rd tall timbers fire ecology conference: fire in grassland and shrubland ecosystems. Tall Timbers Research Station Tallahassee, FL, USA, pp 240–245
Rankins ST, DeYoung RW, Foley AM, Ortega-S JA, Fulbright TE, Hewitt DG, Schofield LR, Campbell TA (2021) Energy content of browse: a regional driver of white-tailed deer size. In: Abstracts of the 44th annual meeting of the Southeast Deer Study Group
Reardon PO, Merrill PA, Taylor CA Jr (1978) White-tailed deer preferences and hunter success under various grazing systems. J Range Manag 31:40–42
Rivera NA, Brandt AL, Novakofski JE, Mateus-Pinilla NE (2019) Veterinary medicene: research and reports 10:123–139. https://doi.org/10.2147/VMRR.S197404
Rivera NA, Varga C, Ruder MG, Dorak SJ, Roca AL, Novakofski JE, Mateus-Pinilla NE (2021) Bluetongue and epizootic hemorrhagic disease in the United States of America at the wildlife-livestock interface. Pathogens 10:915. https://doi.org/10.3390/pathogens10080915
Rohm JH, Nielsen CK, Woolf A (2007) Survival of neonatal white-tailed deer in an exurban population. J Wildl Manag 71:940–944. https://doi.org/10.2193/2006-116
Rollins D, Bryant FC, Waid DD, Bradley LC (1988) Deer response to brush management in central Texas. Wildl Soc Bull 16:277–284
Ruthven DC III, Fulbright TE, Beasom SL, Hellgren EC (1993) Long-term effects of root plowing on vegetation in the eastern South Texas Plains. J Range Manag 46:351–354
Ruthven DC III, Hellgren EC, Beasom SL (1994) Effects of root plowing on white-tailed deer condition, population status, and diet. J Wildl Manag 58:59–70. https://doi.org/10.2307/3809549
Sauer PR (1984) Physical characteristics. In: Halls LK (ed) White-tailed deer: ecology and management. Stackpole Books, Harrisburg, PA, pp 73–90
Scifres CJ, Hamilton WT, Koerth BH, Flinn RC, Crane RA (1988) Bionomics of patterned herbicide application for wildlife enhancement. J Range Manag 41:317–321
Scholtz R, Fuhlendorf SD, Ulden DR, Allred BW, Jones MO, Naugle DE, Twidwell D (2021) Challenges of brush management treatment effectiveness in southern Great Plains, United States. Rangel Ecol Manage 77:57–63. https://doi.org/10.1016/j.rama.2021.03.007
Segoli M, Ungar ED, Shachak M (2012) Fine-scale spatial heterogeneity of resource modulation in semi-arid “Islands of Fertility.” Arid Land Res Manag 26:344–354. https://doi.org/10.1080/15324982.2012.694397
Smith WP (1987) Dispersion and habitat use by sympatric Columbian white-tailed deer and Columbian black-tailed deer. J Mammal 68:337–347. https://doi.org/10.2307/1381473
Smith WP, Coblentz BE (2010) Cattle or sheep reduce fawning habitat available to Columbian white-tailed deer in western Oregon. Northwest Sci 84:315–326. https://doi.org/10.3955/046.084.0401
Soper RB, Lochmiller RL, Leslie DM, Jr Engle DM (1993) Condition and diet quality of white-tailed deer in response to vegetation management in central Oklahoma. In: Proceedings of the Oklahoma academy of science, vol 73, pp 53–61
South Dakota Game, Fish, and Parks (2022) Epizootic hermorrhagic disease (EHD). https://gfp.sd.gov
Sperlova A, Zendulkova D (2011) Bluetongue: a review. Vet Med 9:430–452
Springer MD (1977) The influence of prescribed burning on nutrition in white-tailed deer in the Coastal Plain of Texas. PhD dissertation, Texas A&M University, College Station, USA
Steuter AA, Wright HA (1980) White-tailed deer densities and brush cover on the Rio Grande Plain. J Range Manag 33:328–331
Stevens G, McCluskey B, King A, O’Hearn E, Mayr G (2015) Review of the 2012 epizootic hemorrhagic disease outbreak in domestic ruminants in the United States. PLoS ONE 10:e0133359. https://doi.org/10.1371/journal.pone.0133359
Stewart KM, Fulbright TE, Drawe DL, Bowyer RT (2003) Sexual segretation in white-tailed deer: responses to habitat manipulations. Wildl Soc Bull 31:1210–1217
Stewart KM, Bowyer RT, Weisburg PJ (2011) Spatial use of landscapes. In: Hewitt DG (ed) Biology and management of white-tailed deer. CRC Press, Boca Raton, FL, pp 181–217
Strickland BK, Demarais S (2000) Age and regional differences in antlers and mass of white-tailed deer. J Wildl Manag 64:903–911. https://doi.org/10.2307/3803198
Thomas DB, Klafke G, Busch JD, Olafson PU, Miller RA, Mosqueda J, Stone NE, Scoles G, Wagner DM, Perez-De-Leon A (2020) Tracking the increase of acaricide resistance in an invasive population of cattle fever ticks (Acari:Ixodidae) and implementation of real-time PCR assays to rapidly genotype resistance mutations. Ann Entomol Soc Am 113:298–309. https://doi.org/10.1093/aesa/saz053
Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M (1991) Stress recovery during exposure to natural and urban environments. J Environ Psychol 11:201–230. https://doi.org/10.1016/S0272-4944(05)80184-7
Uresk DW, Benzon TA, Severson KE, Benkobi L (1999) Characteristics of white-tailed deer fawn beds, Black Hills, South Dakota. Great Basin Naturalist 59:348–354
Van der Hoek D, Knapp AK, Briggs JM, Bokdam J (2002) White-tailed deer browsing on six shrub species of tallgrass prairie. Great Plains Res 12:141–156
Van de Koppel J, Rietkerk M, van Langevelde F, Kumar L, Klausmeier CA, Fryxell JM, Hearne JW, van Andel J, de Ridder N, Skidmore A, Stroosnijder L, Prins HHT (2002) Spatial heterogeneity and irreversible vegetation change in semiarid grazing systems. Am Nat 159:209–218. https://doi.org/10.1086/324791
Van Horne B (1983) Density as a misleading indicator of habitat quality. J Wildl Manag 47:893–901. https://doi.org/10.2307/3808148
VerCauteren KC, VanDeelen, Lavelle MJ, Hall WH (2010) Assessment of abilities of white-tailed deer to jump fences. J Wildl Manag 74:1378–1381. https://doi.org/10.1111/j.1937-2817.2010.tb01260.x
Verme LJ (1969) Reproductive patterns of white-tailed deer related to nutritional plane. J Wildl Manag 33:881–887. https://doi.org/10.2307/3799320
Volk MD, Kaufman DW, Kaufman GA (2007) Diurnal activity and habitat associations of white-tailed deer in tallgrass prairie of eastern Kansas. Trans Kans Acad Sci 110:145–154. https://doi.org/10.1660/0022-8443(2007)110(145:DAAHAO12.0.CO:2
Warren RJ, Krysl LJ (1983) White-tailed deer food habitats and nutritional status as affected by grazing and deer-harvest management. J Range Manag 36:104–109
Washburn BE, Seamans TW (2007) Wildlife responses to vegetation height management in cool-season grasslands. Rangel Ecol Manage 60:319–323. https://doi.org/10.2111/1551-5028(2007)60[319:WRTVHM]2.0.CO;2
Webb SL, Hewitt DG, Hellickson MW (2007) Survival and cause-specific mortality of mature male white-tailed deer. J Wildl Manag 71:555–558. https://doi.org/10.2193/2006-189
Weiskopf SR, Ledee OE, Thompson LM (2019) Climate change effects on deer and moose in the Midwest. J Wildl Manag 83:769–781. https://doi.org/10.1002/jwmg.21649
Whittaker DG, Lindzey FG (1999) Effect of coyote predation on early fawn survival in sympatric deer species. Wildl Soc Bull 27:256–262
Whittaker DG, Lindzey FG (2004) Habitat use patterns of sympatric deer species on Rocky Mountain Arsenal, Colorado. Wildl Soc Bull 32:1114–1123. https://doi.org/10.2193/0091-7648(2004)032[1114:HUPOSD]2.0CO;2
Wiemers DW, Fulbright TE, Wester DB, Ortega-S JA, Rasmussen GA, Hewitt DG, Hellickson MW (2014) Role of thermal environment in habitat selection by male white-tailed deer during summer in Texas, USA. Wildl Biol 20:47–56. https://doi.org/10.2981/wlb.13029
Wiggers EP, Beasom SL (1986) Characterization of sympatric or adjacent habitats of 2 deer species in west Texas. J Wildl Manag 50:129–134. https://doi.org/10.2307/3801502
Wilcox BP, Fuhlendorf SD, Walker JW, Twidwell D, Wu XB (2021) Saving imperiled grassland biomes by recoupling fire and grazing: a case study from the Great Plains. Front Ecol Environ. https://doi.org/10.1002/fee.2448
Woolf A, Roseberry JL (1998) Deer management: our profession’s symbol of success or failure? Wildl Soc Bull 26:515–521
Wolff CL, Demarais S, Brooks CP, Barton BT (2020) Behavioral plasticity mitigates the effect of warming on white-tailed deer. Ecol Evol 10:2579–2587. https://doi.org/10.1002/ece3.6087
Acknowledgements
I thank David Hewitt and Mike Cherry for reviewing early drafts of the manuscript. This chapter is CKWRI manuscript number 22-104.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Fulbright, T.E. (2023). White-Tailed Deer. In: McNew, L.B., Dahlgren, D.K., Beck, J.L. (eds) Rangeland Wildlife Ecology and Conservation . Springer, Cham. https://doi.org/10.1007/978-3-031-34037-6_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-34037-6_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34036-9
Online ISBN: 978-3-031-34037-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)