Abstract
Knowledge about deer spatial use is essential for damage mitigation, conservation, and harvest management. We assess annual and seasonal home range sizes in relation to habitat composition for red deer (Cervus elaphus) in Sweden, using GPS-data from two regions with different management systems. We compare our findings with reviewed data on red deer home range sizes in Europe. Annual and seasonal home ranges during calving, hunt, and winter-spring, decreased with increasing proportion forest. Female annual home ranges in a mixed agricultural-forest landscape were three times larger than in a forest-dominated landscape. Core areas (50% Kernels) were approximately 1/5 of the full annual and seasonal home ranges (95% Kernels) regardless of habitat composition. Home range size in the forest-dominated landscape showed little inter-seasonal variation. In the agricultural-forest landscape, home ranges were larger during calving, hunt, and winter-spring compared to summer and rut. In the forest-dominated landscape, management areas are large enough to cover female spatial use. In the agricultural-forest landscape, female spatial use covers several license units. Here, the coordinated license system is needed to reach trade-offs between goals of conservation, game management, and damage mitigation. Males had in general larger home ranges than females, and the majority of the males also made a seasonal migration to and from the rutting areas. The license system area in the agricultural-forest landscape is large enough to manage migrating males. In the forest landscape, a coordination of several management areas is needed to encompass male migrations. We conclude that management needs to adapt to deer spatial use in different types of landscapes to reach set goals.
Similar content being viewed by others
Introduction
Knowledge about deer spatial use is profound for an effective deer management (Jarnemo 2008; Meisingset et al. 2018; Fattorini et al. 2020). Intra-specific variations in home range size among cervids (Hewison et al. 1998; Kie et al. 2002; Anderson et al. 2005) suggest that environmental factors play an important role and needs to be considered in management. A home range must contain resources enough to meet the requirements of energy and cover for the animal, often implying multiple habitat patches to satisfy their needs. Forage scarcity, or a patchy distribution of forage, is suggested to induce movements over larger areas (Ford 1983; O’Neill et al. 1988; Saïd and Servanty 2005). Consequently, spatial resource heterogeneity on a landscape level may impact home range size (Kie et al. 2002; Anderson et al. 2005; Reinecke et al. 2014), movements (Frair et al. 2005; Kie et al. 2005; Coulon et al. 2008), and animal distribution (Clutton-Brock and Harvey 1978; Beier and McCullough 1990; Kie et al. 2002). During the course of the year, the availability and distribution of forage may vary. The need for cover may also change due to climate (Borkowski and Ukalska 2008; van Beest et al. 2012; Bobek et al. 2016), predator presence, or because of the onset of the hunting season (Naugle et al. 1997; Sunde et al. 2009; Jarnemo and Wikenros 2014). These seasonal variations may thus modify home range sizes (Richard et al. 2011; Van Beest et al. 2011; Reinecke et al. 2014).
Red deer (Cervus elaphus) is one example of a species where studies reveal a large variation in home range size across Europe (Table 1). Average annual home range sizes can vary from less than 100 ha (Lovari et al. 2007) to more than 7000 ha (Zlatanova et al. 2019), and average seasonal home ranges from 26 ha (Bocci et al. 2010) to well above 5000 ha (Náhlik et al. 2009). Home range size of red deer has been shown to be affected by age (Froy et al. 2018), sex (Szemethy et al. 1998; Reinecke et al. 2014; Zlatanova et al. 2019), resident or shifter/migratory strategies (Luccarini et al. 2006; Bocci et al. 2010; Kropil et al. 2015; Meisingset et al. 2018), climate factors such as snow cover and temperature (Schmidt 1993; Luccarini et al. 2006; Rivrud et al. 2010), forage abundance and supplemental feeding (Schmidt 1993; Richard et al. 2011; Reinecke et al. 2014), and human disturbance such as hunting and other outdoor activities (Jeppesen 1987; Lovari et al. 2007; Gillich et al. 2021). Jerina (2012) found that annual home range sizes of red deer were more affected by anthropogenic factors such as roads and supplemental feeding than by natural factors such as temperature and snow cover. Laguna et al. (2021) observed differences in ranges between areas with and without hunting, as well as an impact of human land use. It has also been suggested that the comparably large home ranges observed in the broad-leaved forests in Eastern Europe, may be an effect of the presence of large carnivores (Kamler et al. 2008; Zlatanova et al. 2019). The effect of climate factors and forage abundance suggest that home range sizes could vary between seasons, and a seasonal impact has indeed been shown in some studies (Kamler et al. 2008; Náhlik et al. 2009; Richard et al. 2011). It seems also likely that climate effects, and the distribution of forage and cover, vary between different types of landscapes, and that landscape structure and habitat composition can also affect home range size (Reinecke et al. 2014; Bevanda et al. 2015; Borkowski et al. 2016). From a background of large variations in home range sizes in Europe and several impacting factors, red deer home range sizes in Sweden are difficult to predict. Located in northernmost Europe, stretching from the nemoral zone in the south to the northern boreal zone in the north, shifting from a landscape dominated by modern agriculture in the south to one dominated by homogenous coniferous forests in the north, and with approximately 1000 km between the southernmost and the northernmost red deer population, there is a potential for large variations in home range sizes and movement patterns among red deer in Sweden.
Red deer is a highly important game species (Milner et al. 2006; Apollonio et al. 2010), but as one of the large natural herbivores it is also a keystone species in the ecosystems, shaping vegetation (De Vires 1995; Svenning 2002; Kuijper et al. 2010) and being an important prey for large carnivores (Jȩdrzejewski et al. 2002; Gazzola et al. 2005; Nowak et al. 2005). Numbers of red deer increase in Europe, resulting in increased interactions with other species, the environment, and human activities such as agriculture and forestry, and thus stressing the need for an evidence-informed management to minimize such impacts and an understanding of how the deer interact with factors in the landscape in order to find effective countermeasures (Apollonio et al. 2017; Linnell et al. 2020; Valente et al. 2020; Carpio et al. 2021). Damage on crops and forest plantations by deer can be affected by factors in the surrounding landscape (Jarnemo et al. 2014; Sorensen et al. 2015; Takarabe and Iijima 2020). Knowledge about deer spatial use and how it varies between different seasons can thus be one key to the understanding of spatiotemporal variation in damage risk and thus where and when damage prevention should be prioritized. The most severe and widespread damage is probably bark stripping on trees—a complex problem where damage levels can be affected by many factors, e.g. population density, forage availability, supplemental feeding, crop intake, landscape structure, climate, and human disturbance (Gill 1992; Gerhardt et al. 2013; Jarnemo et al. 2014, 2022), and show large variations between areas and seasons (Verheyden et al. 2006; Spake et al. 2020). Traditionally, damage mitigations have focused on reducing deer population densities alongside with fencing and supplemental feeding. More recent findings, however, conclude that an increased harvest is not necessarily effective, as other factors (see above) than density affect damage levels, wherefore management needs to adopt a more holistic approach where other factors in addition to population density are integrated (Reimoser 2003; Kuijper 2011; Jarnemo et al. 2014).
One harvest-problem is that local management units are too small to manage a population of its own (Jarnemo 2008; Kropil et al. 2015; Meisingset et al. 2018). Conflicting management aims, competition over deer between hunting units, the same deer being subject to different harvest regimes, that deer may cause damage in areas harbouring deer off-season but not during the hunting season, and that unknown spatial use and migrations may bias monitoring, complicate management with the possible consequence that goals are not reached (Jarnemo 2008; Putman 2012; Torres-Porras et al. 2014; Fattorini et al. 2020). Being subject to intensive trophy hunting, males are often over-harvested (Clutton-Brock and Albon 1989; Buckland et al. 1996; Milner-Gulland et al. 2004). Males generally have larger home ranges than females (Kamler et al. 2008; Reinecke et al. 2014; Zlatanova et al. 2019), and can also perform seasonal migrations between a rut area and a winter-summer area (Jarnemo 2008; Kropil et al. 2015), which could further increase a male over-harvest as they face different hunting regimes. In combination with a reluctance to harvest females, this leads to populations with a sex ratio highly skewed towards females and with a low average age for males (Beddington 1974; Ginsberg and Milner-Gulland 1994; Langvatn and Loison 1999), with possible negative effects on population dynamics (Mysterud et al. 2002) and a potential for high population growth (Caughley 1977). To efficiently counteract damage, to obtain a goal-oriented harvest, and to manage deer on a population scale, it is thus important that sizes of management units are coordinated with deer spatial use in an integrated management system (Clutton-Brock et al. 2002; Jarnemo 2008; Meisingset et al. 2018; Fattorini et al. 2020).
With the main objectives to analyse home range sizes in relation to habitat composition within the home range and to relate home range sizes to hunting regimes, we investigated annual and seasonal home range sizes in two hunted populations of red deer in two different regions in Sweden. The regions included a homogeneous forest landscape with a system of independent red deer management areas ranging in size from 2800 to 10,000 ha, and a mixed landscape dominated by agriculture with a license-regulated harvest coordinated in an area of approximately 260,000 ha. Previous studies have revealed significant differences between these regions regarding red deer damage to forest plantations (Månsson and Jarnemo 2013; Jarnemo et al. 2014), red deer movement patterns (Allen et al. 2014), and habitat and crop selection (Månsson et al. 2021). We estimated total and core area home range sizes on two temporal scales: annual and for five distinct seasons pre-defined on the basis of external factors and of the annual cycle of red deer ecology: (1) calving, (2) summer, (3) rut, (4) hunt, and (5) winter-spring. We compare our results with reviewed data on red deer home range sizes in Europe and discuss implications for management.
Methods
Study area
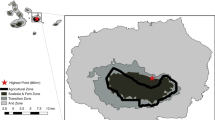
The study was conducted in two different regions in Sweden: Skåne and Kolmården (Fig. 1). Skåne is the southernmost region in Sweden (N55°65E13°50). The region is dominated by agricultural land 45%, whereas forest cover 35%. The productive forest area consists of broadleaved 37%, spruce 36%, pine 10%, mixed conifer/broadleaved 7%, and mixed conifer 5% (Nilsson and Cory 2011). The mean annual temperature is 6.5 °C with mean annual precipitation of 800 mm, and the average number of snow days per year was 40 with a mean max depth of 10 cm (https://www.smhi.se/).
Map showing the locations of the two study areas in Sweden (left) and the habitat composition in the Kolmården study area (top right) and Skåne study area (bottom right). From Månsson et al. 2021
Kolmården is situated in south-central Sweden (N58°75E16°40). Kolmården has no exact borders but is an old name of an area of deep and inaccessible forests and rocky terrain, stretching over the border between the counties of Södermanland and Östergötland. The Södermanland County has an agricultural cover of 20% and a forest cover of 55%. Scots pine (32%), Norway spruce (28%), and mixed conifer forests (18%) are the most common forest types (Nilsson and Cory 2011). The mean annual temperature is 5.5 °C with a mean annual precipitation of 787 mm, and the average number of snow days per year is 80 with a mean max depth of 35 cm (https://www.smhi.se/). In Kolmården, red deer were marked (see Jarnemo & Wikenros 2014 for further details) on the two bordering estates, Stavsjö and Virå, and on the estate Valinge. Stavsjö-Virå consists of 84% forest, 8% mire, 6% bedrock, 1% agricultural land, and 1% buildings etc., whereas Valinge consists of 64% forest, 2% mire, 8% bedrock, 22% agricultural land, and 5% buildings etc.
The study area in Skåne is situated in a coordinated management area of 260,000 ha in the south-eastern part of the county, where harvest is regulated in a license system. Outside the license area, there is an open hunting season in the rest of Skåne on all animals (calves, hinds, stags). The hunting season lasts from the second Monday in October to 31 January, i.e. after the rut that in Sweden takes place from the last week of August until the beginning of October (Jarnemo 2011; Jarnemo et al. 2017). In the license area there are approximately 180 hunting units, varying in size from 200 to 10,000 ha, that apply annually for a license to harvest red deer. Based on size of hunting unit, local red deer abundance, type of area (female-calf or male area (Jarnemo 2008)), habitat composition, and damage situation, applying units are given a license of a specified number of deer in the categories calf, female, male with maximum five tines, male with maximum eight tines, and deer free of choice. The 2006 harvest in the license area was on average 2.3 deer/1000 ha for the license applying hunting units, but with large variations between units. For the estates where deer were captured the average harvest was 5.9 deer/1000 ha.
In the rest of Sweden, north of Skåne, there are two hunting regimes. In order to be allowed to hunt adult red deer, a hunting unit has to be a member of a red deer management area. Areas that are not registered to a red deer management area are only allowed to harvest calves. In red deer management areas, the hunters make 3-year culling plans that must be approved by the county administrative board. The general hunting season starts post-rut on the 2nd Monday in October and lasts until 31 January. However, during 16th of August to the 2nd Monday in October, culling females and calves are allowed in red deer management areas, but only by using sit-and-wait or stalking hunting methods. In the Kolmården study area there were six management areas ranging in size from 2800 to 10,000 ha, during the study period. The 2006 harvest in these six areas was 12.1 deer/1000 ha.
In both study areas, wolves (Canis lupus) only made rare visits during the years of study. The same applies for lynx (Lynx lynx) in the Skåne area, whereas in Kolmården the occurrence of lynx probably was continuous, although sparse.
GPS locations
Adult red deer were tranquilised at supplemental feeding stations in February–March and fitted with a Vectronic Global Positioning System (GPS) collar and plastic ear-tags (Allen et al. 2014; Jarnemo and Wikenros 2014). Females were at least 2 years old, and only males estimated to be at least 5–6 years old were chosen. GPS locations (n = 97,486) from 39 tagged deer (12 females and 4 males in Skåne, and 13 females and 10 males in Kolmården) during 2006–2013 was used. The collars were mostly set to record 5–6 GPS fixes per 24 h. The pre-defined seasons used were set according to external factors such as climate and hunting season, and to distinct periods in red deer ecology such as calving and rut: calving (15 April–31 May), summer (1 June–20 August), rut (21 August–7 October), hunt (8 October–31 January), and winter-spring (1 February–14 April). An annual cycle was defined as 1st of February to 31st of January, to follow the defined seasons.
Home range size and habitat composition
We estimated annual and seasonal utilization distributions for all individual red deer by using the Kernel method. The Kernel method asses a probability density function based on the included locations, and thus gives a probability of the individual animal being at any given location within the defined home range (Worton 1989). We assessed both annual and seasonal home ranges using the 95% to estimate the total areas used (i.e. excluding very rare excursions), and 50% isopleth to estimate the core area of use. We used the standard reference bandwidth, after visual inspection of varying bandwidth in relation to the focal locations (e.g. minimizing separation between activity areas, as recommended in Kie (2013)). We only calculated annual and seasonal home range sizes when ≥ 30 locations per individual were available. We derived the habitat composition within the home ranges from the Swedish land cover data (0.1 × 0.1 km; Swedish Environmental Protection Agency) and thereafter used the proportion of forest as an index for habitat composition (i.e. to avoid collinearity). Kernel calculations was conducted using the package ‘adehabitatHR’(Calenge 2020) in R version 3.6.3 (R Core Team 2021) and visual inspection of home ranges and assessment of habitat composition in ArcMap (version 10.7). We compared the home range sizes with reviewed data on red deer home range sizes in Europe (Table 1).
Analyses
We included all females in the analyses but excluded males (except for descriptive calculations of annual home range size) due to the small sample size of males in Skåne. We conducted analyses in R using linear mixed models (LMMs) in the lme4 package (Bates et al. 2015). First, we used annual home range size (first with 95% Kernel estimates than repeated with 50% Kernel estimates) as response variables and the proportion of forest as explanatory variable. Second, we used seasonal home range size (first with 95% Kernel estimates than repeated with 50% Kernel estimates) as response variables and proportion of forest, season (calving, summer, rut, hunt, and winter-spring), and the interaction between proportion of forest and season as explanatory variables. Female-ID was included as random variable in all analyses to account for repeated observations of the same individual, and study area (Skåne or Kolmården) was included as random factor to account for additional unexplained variation due to the location of the study areas. In order to meet the assumption of normally distributed residuals, the response variables were transformed by ln(x) or ln(x + 1) (for seasonal 50% Kernel estimates). We compared candidate models using the sample-size corrected Akaike information criterion (AICc) and AICc weights (wi) from the ‘MuMIn’ package (Bartón 2013) in R. Model estimates were derived from the top model and back-transformed in the figures.
Results
Annual home range size
Females in Skåne had on average larger annual home ranges than females in Kolmården (Table 2). For both 95% and 50% estimates of home range size, the top model included the proportion of forest and the two random variables (Table 3). Annual home range size decreased with increasing proportion of forest both for 95% (Fig. 2) and 50% Kernel (Fig. 3) estimates.
Annual home range size (ha, n = 59) of red deer females (n = 25) in relation to proportion of forest within the home ranges in Skåne (blue points) and Kolmården (red points), Sweden during 2006–2011 estimated with 95% (left) and 50% (right) Kernel. The lines show back-transformed parameter estimates from the model (see Table 3) and 95% confidence intervals. Note the different scales of the y-axis
Seasonal home range size with 95% Kernel estimates (top figures) and 50% Kernel estimates (bottom figures) of female red deer (n = 25) in relation to proportion of forest within the home ranges in Skåne (blue points) and Kolmården (red points), Sweden during 2006–2011. Seasons are classified according to calving (15 April–31 May), summer (1 June–20 August), rut (21 August–7 October), hunt (8 October–31 January), and winter-spring (1 February–14 April). The lines show back-transformed parameter estimates from the model (see Table 4). Four data points (29,694 ha during calving and 37,306 ha during winter-spring with 95% Kernel estimates, and 5653 ha during calving and 7917 during winter-spring with 50% Kernel estimates) are not visualized
Annual home range size (n = 8) of the males in Skåne (n = 4) averaged 3690 ha (median = 1480, range 1200–11940) and 750 ha (median = 340, range 280–2180) for 95% and 50% Kernel, respectively. Corresponding annual home range sizes (n = 16) of the males in Kolmården (n = 10) averaged 5750 ha (median = 4090, range 1770–20,450) and 1280 ha (median = 930, range 310–4950).
The females in Kolmården were all resident in their home ranges. In Skåne, one female displayed a seasonal migration between an area where she spent autumn and winter, and an area where she spent spring, summer, and rut. The distance between the two areas was 10 km. Two females in Skåne displayed a pattern where they more irregularly shifted, or commuted, between different areas. One between two areas 7 km apart, and one between three areas where the distance from the marking area was 10 and 26 km, respectively.
In Kolmården, seven males made a seasonal migration between a distinct rutting area and the area where they spent the rest of the year. The average distance in-between was 11 km (centre-centre, range 6–18 km). Of the four males in Skåne, one performed a rutting migration of 19 km, whereas the three remaining had rutting areas bordering the areas where they spent the rest of the year.
Home range size in different seasons
The number of individual seasonal home ranges used in the analyses constituted 96.7% (264 out of 273) of the total number of seasons for females with functioning collars during the study period. The best model explaining variation in seasonal home range size (both for 95% and 50% Kernel estimates) included proportion of forest, season and the interaction between proportion of forest and season (Table 4). Seasonal home range size decreased with increasing proportion of forest during the calving, hunting, and winter-spring seasons both with 95% and 50% Kernel estimates but not during the summer and rut seasons (Fig. 3). In Skåne, the seasonal home ranges were larger during the calving, hunting, and winter-spring seasons than during the summer and rut seasons, and during these seasons they were also larger than during the correspondent seasons in Kolmården. In Kolmården, home range size was similar for all seasons (Table 2).
Discussion
We found an effect of habitat composition on red deer home range sizes, as annual home range size, as well as home range sizes during calving, hunting season, and winter-spring, were negatively related to proportion forest in the home range. Comparing our study with other studies in Europe (Table 1), the home range sizes in our study areas (Table 2) are in the mid to upper end of the range of resident deer, but below deer performing altitudinal migrations. Annual home ranges of the red deer females were on average three times larger in the mixed forest agricultural landscape compared to the forest landscape. The core area use (Samuel et al. 1985; Powell 2000; Kernohan et al. 2001) was also larger in the mixed landscape than in the forest landscape, and the females in the mixed landscape also showed a larger variation in home range size between seasons than in the forest landscape. The seasonal home ranges were larger in the mixed landscape than the forest landscape during calving, hunting, and winter-spring, but were similar sized during summer and rut. Contrary to the females, the average home range size was larger for the males in the forest landscape than the males in the mixed landscape. However, as we only had four marked males in the mixed landscape, this result should be interpreted with caution. There was also a large overlap in male home range sizes between the areas.
Female annual home range sizes
Annual home range size decreased with increasing proportion forest, a pattern shown by females both in Skåne and in Kolmården (Figs. 2 and 3). As expected, the females in Kolmården had a higher proportion forest in their annual home ranges than the females in Skåne (Figs. 2 and 3). The home ranges in Kolmården were thus generally smaller than in Skåne, both regarding the 95% and the 50% Kernels (Figs. 2 and 3; Table 2). Other studies have shown that red deer home range sizes are smaller in forest dominated landscapes compared to mixed forest-agricultural landscapes (Szemethy et al. 1998, 2003; Náhlik et al. 2009; Reinecke et al. 2014)—note, however, that Kamler et al. (2008), Kropil et al. (2015), and Zlatanova et al. (2019) observed among the largest home range sizes for red deer in Europe, and did so in forest dominated landscapes, a pattern that is suggested to be explained by the presence of large carnivores and a dominance of broad-leaved forests. The relationship of home range sizes decreasing with increasing proportion forest can be affected by several factors, e.g. landscape structure, dispersion of resources, and sensitivity to disturbance. Increases in spatial heterogeneity can increase home range size (Kie et al. 2002; Anderson et al. 2005). Fragmentation leading to fewer, smaller, and more scattered forest patches providing shelter for the deer, could induce longer movements for the deer between different forest patches, and thus also larger home range sizes. Bevanda et al. (2015) found that red deer displayed a hump-shaped pattern where home range size was largest at intermediate patch aggregation. Moreover, the sensitivity to human disturbance through hunting (Jeppesen 1987; Sunde et al. 2009; Jarnemo and Wikenros 2014) and recreation (Sibbald et al. 2011; Coppes et al. 2017; Scholten et al. 2018) (something that also might be affected by sex (Laguna et al. 2021)) should increase with decreasing forest patch size, further inducing deer movements and increasing home range size. Gillich et al. (2021) though found smaller home range sizes in an area with high human disturbance than in an area with low disturbance, possibly due to constrained movement possibilities and/or few alternative safe areas. However, in an open landscape with small and scattered forest patches, human disturbance may result in enlarged home range sizes as the deer have to move large distances between places offering security cover. Moreover, sensitivity to disturbance should be more pronounced during the leafless period, and this effect is likely to be especially strong in the nemoral zone in Skåne, where broadleaved forests and trees are more abundant (Nilsson and Cory 2011).
In accordance with the inverse relationship between food abundance and home range size (Ford 1983; O’Neill et al. 1988; Anderson et al. 2005), Schmidt (1993) and Reinecke et al. (2014) found that red deer home range sizes were smaller in areas with supplemental feeding. Mysterud et al. (2023), however, did not see an effect of supplemental feeding on size of red deer core areas or home range sizes. We did not have detailed data on supplemental feeding, but it was extensive in both regions during the hunting and winter-spring seasons. However, there are differences between Skåne and Kolmården regarding abundance and distribution of forage that probably are of significance for the variations in home range size and movements. An earlier study in the two areas found that the forests in Kolmården were rich in vegetation in field and bush layers, as opposed to Skåne where vegetation was sparse inside the forests. This study could then show a strong negative relationship between availability of forage in the forests and level of deer damage on trees (Jarnemo et al. 2014). The deer in Kolmården can thus forage inside forests, whereas the deer in Skåne find little to eat, except for bark, in the forests, especially from late autumn until spring. Instead, the deer in Skåne must to a large extent rely on foraging on crops (Månsson et al. 2021). The modern and strongly rationalized agriculture in this area has single fields that may be up to 100 ha, meaning that if the deer want to vary food intake, they may have to move over large areas. Allen et al. (2014) found that the red deer in the mixed forest-agricultural landscape in Skåne in winter (January–March) used more than twice as large areas during the night-time feeding as compared to the deer in the forest dominated landscape in Kolmården. Location of attractive crops does also change as different crops mature and vary in palatability with season. Deer feeding on crops will also experience that an abundant attractive food source can disappear from 1 day to another during harvest and ploughing, forcing the deer to shift area for foraging. The large home range sizes shown for red deer in mixed forest-agricultural landscapes (Szemethy et al. 1998; Náhlik et al. 2009; Reinecke et al. 2014) might thus not only be an effect of fragmented and scattered forest patches, but also a consequence of that deer foraging on crops may need to move over large areas to vary their diet, and may have to adapt to seasonal changes in availability of crops and of preferable combinations of attractive crops and secure daytime resting sites. When the crop land decreased due to a replacement with natural vegetation in DeSoto National Wildlife Refuge in Nebraska, USA, Walter et al. (2009) observed that mean annual home range size of white-tailed deer was halved.
Females in Kolmården seemed to conform to a stationary behaviour, as they did not display any distinct movements between different areas, a finding that can be expected in a homogenous landscape with small altitudinal variations. However, Szemethy et al. (2003) and Prokešová (2004) showed that red deer females during the summer translocated from forested areas into agricultural areas, and one female in Skåne followed this pattern. Another female in Skåne performed a distinct seasonal migration between an autumn–winter area and a spring–summer-rut area. Both her areas offered forest cover, although the autumn–winter area had (for the region) a larger homogeneous coniferous forest that might be of benefit during snow cover and low temperatures. Interestingly her departure from the rut area coincided with the start of the hunting season, a behaviour that has been observed previously in red deer (Jarnemo and Wikenros 2014; Rivrud et al. 2016). The more irregular movements observed for the two shifters might at least partly be explained by hunting disturbance (Jeppesen 1987; Sunde et al. 2009; Jarnemo and Wikenros 2014), but the movements were made also outside the hunting season. They could be triggered by other types of human disturbance (Sibbald et al. 2011; Coppes et al. 2017; Scholten et al. 2018), but another possible explanation is that these females sought places where they had favourable combinations of attractive crops and safe daytime cover (i.e. dense forest plantations). With crop rotation, different crops varying in attractiveness during the year, and cover in forests changing with season (i.e. leafless or foliated), the locations of preferable forage-cover combinations vary.
Male home range sizes
Our results indicated that males in general had larger home ranges than females, a pattern that seems common for red deer (see Table 1). The average of the annual home range sizes in Table 1 (Kernels and MCPs mixed) is 1223 ha for females and 3175 ha for males. Of 18 studies in Table 1 where annual home range size was studied for both females and males, the average male/female ratio in home range size was 2.6 with a range from 0.6 to 6.3, with Clutton-Brock et al. (1982) as the only example of smaller home range size for males. In our study, the male/female ratio of annual home range sizes was 1.9 in Skåne and 8.1 in Kolmården, keeping in mind that we only had four marked males in Skåne. Seven out of ten males in Kolmården made a rut migration similar to what earlier has been observed for red deer males in Skåne (Jarnemo 2008). A plausible explanation for this migratory behaviour is offered by the sexual segregation theory (Main et al. 1996; Ruckstuhl and Neuhaus 2000, 2005). The Kolmården males left the rutting areas and returned to their winter-summer areas within 1 week after the start of the hunting season, their departure triggered by hunting activities (Jarnemo and Wikenros 2014), a behaviour also noted by Rivrud et al. (2016).
Seasonal home range sizes
As for annual home range sizes, there is a large variation between studies regarding sizes of seasonal home ranges. For males average seasonal home range sizes stretch between 30 and 5310 ha, and for females between 30 and 2570 ha (Table 1). The studies, however, also show divergent patterns concerning the size relationship between different seasons, but also that individual strategies and inter-annual climatic variations have an impact. Bocci et al. (2010) found that resident hinds had smaller home range sizes in summer than in winter, whereas individuals with separated summer and winter ranges had larger home range sizes in summer, but they also found that this pattern was not true for all years. Winter severity is one example of a factor that can affect home range size and winters with more snow can restrict deer movements, and thus, perhaps in the combination with supplemental feeding, result in smaller winter ranges (Georgii and Schröder 1983; Schmidt 1993; Koubek and Hrabe 1996). On the other hand, larger winter home range sizes (Kamler et al. 2008; Náhlik et al. 2009; Bojarska et al. 2020) may reflect a need to roam over larger areas for foraging. Inter-seasonal variations in home range size clearly can be affected by several factors such as type of landscape, winter severity, hunting, as well as the capability of various habitats to offer food and shelter during different seasons (see e.g. Kamler et al. 2008; Bocci et al. 2010; Bevanda et al. 2015; Bojarska et al. 2020; Laguna et al. 2021).
In our study seasonal home range sizes decreased with increasing proportion forest, but this effect was only evident during calving, hunting, and winter-spring. The largest home range sizes in our study were observed during winter-spring, and secondly, during the hunting season and the calving season. Hunting activities can induce flight distances over several kilometres, sometimes even tens of kilometres, and the effect of this type of disturbance is stronger in more open landscapes (Sunde et al. 2009; Jarnemo and Wikenros 2014).
We found that home range sizes were smallest during summer, a pattern also found in other studies (e.g. Kamler et al. 2008; Náhlik et al. 2009; Gillich et al. 2021), and that may reflect a higher food abundance. However, during summer, the deer can find cover also in the open landscape. Hedgerows and coppices can now offer daytime shelter as opposed to when it is leafless. As crops grow taller, they may also provide cover for daytime resting (Månsson et al. 2021). This means that the deer can spend daytime resting in the night-time foraging habitats, and that the spatial heterogeneity of different resource patches is reduced.
In both study areas, the general rutting system seems to be one where dominant harem holders defend mobile harems (Jarnemo 2011, 2014; Jarnemo et al. 2017). Although deer in both areas commonly seem to move between daytime resting in forests and night-time feeding in open terrain (Allen et al. 2014), female movements may be restricted as they join the harems of dominant males in order to avoid harassment from younger males (Carranza and Valencia 1999). Whereas males can visit different rutting grounds within a rutting season (Jarnemo 2011), females seem to be more resident. However, there were movements indicating that females could conduct rut excursions (Stopher et al. 2011). One interesting example was when three females in Kolmården, that spent the rut in the same rutting ground, one after the other in the 4th, 5–6th, and 8th of September 2008, left this rutting ground and moved nearly 4 km to another ground held by strong, dominant 10-year-old stag (harvested after the rut and aged by cementum annuli on incisors). They all returned to their original rutting ground after approximately 24 h. For males, the size of the home range during the rut may be affected by the status of the male. Dominant harem holders should be more confined to a single rutting ground, whereas subordinate males can move over longer distances visiting different rutting grounds (Jarnemo 2011).
The smallest seasonal home range was 2 ha (Kernel 95) and was found for a female during the rut 2009. In 2008, the same female had a home range during the rut of 1755 ha. During the rut of 2009 she had used a 2.5 ha woodlot for daytime bedding, and a bordering field with autumn sown rapeseed for night-time feeding. In March 2010, her transmitter turned silent and in November 2010 her remains were found. She was then aged (by cementum annuli on incisors) to 23–25 years. Decreases in home range size of old individuals have been found to be predictive of mortality within the following year (Froy et al. 2018).
Management implications
The six red deer management areas in the Kolmården study area had an average size of 5300 ha (range 2800–10,000). The females in Kolmården were resident and had annual home ranges with an average size of 810 ha (95% Kernel, range 154–3914; Table 2). A single red deer management area should thus be large enough to more or less manage its own females.
In Skåne, home range sizes were larger, and there were also females moving between different separated areas. In the hunting season 2010/2011, 180 license units in Skåne had an average size of 700 ha and varied between 200 and 10,200 ha. However, one license unit can contain several estates, and it is common that especially smaller estates join in a common license application. With larger home range sizes and a large variation in size of hunting and license units, it is probably common that red deer females cover several hunting and license units in their movements and home ranges. That females, and perhaps also herds of females, may move between separated areas, covering different estates and hunting units, do also complicate damage mitigation, especially if damage occur outside the hunting season in areas where the deer spend less time during the hunting season. The coordinated management in the license area, should be expedient, and perhaps even necessary, in order to reach and make trade-offs between different goals of conservation, game management, and damage mitigation.
Annual and seasonal home ranges estimated with Kernel 50% isopleth had a size that were approximately 1/5 (18–22%) of the Kernel 95% home ranges, regardless of habitat composition, sex, or region (Table 2). Zlatanova et al. (2019) had similar results with an average core area size that were 19% of total home range size (variation 9 to 27%). Knowledge of core areas can be relevant in order to identify critical resources, and be of help for the understanding and estimation of damage risk, as well as for damage mitigation. One example is damage to forest plantations through browsing and bark stripping where damage risk increase with human disturbance as the deer get restricted in their movements and are forced to seek cover in the dense forest stands during daytime, resulting in decreased daily home range sizes and high damage rates (Náhlik et al. 2009). Further studies could focus on differences in habitat selection between core area and total home range as well as investigating differences between diurnal and nocturnal spatial use.
In Kolmården, seven of ten males seasonally migrated between rutting areas and winter-summer areas in a similar pattern as have been previously shown for males in Skåne (Jarnemo 2008). Six of the migrating Kolmården males were gathered in the same 2800 ha (MCP) area during the rut, but were dispersed over approximately 32,000 ha (MCP) during the rest of the year. The seasonal migration may increase the risk of overharvesting of males as they are hunted both in rutting areas in the beginning of the hunting season and in the winter grounds later on in the season (Jarnemo 2008; Sibbald et al. 2011; Jarnemo and Wikenros 2014). Furthermore, the male seasonal migrations need also to be taken into consideration in monitoring. When an aerial survey was conducted in Kolmården in February 2006, it was concluded that male ratio was relatively high in the western part (Östergötland County), whereas the sex ratio was highly skewed towards females in the eastern part (Södermanland county). However, during the research project it was discovered that males rutting in the eastern part could spend the winter in the western part, i.e. deer management should not be separated in an eastern and western area, but coordinated despite being separated by an administrative county border. Whereas, the license area in Skåne covers both rutting areas and male winter-summer areas, allowing a coordination of male harvest, the red deer management areas in Kolmården are too small to encompass the male movements. In accordance with earlier studies (Jarnemo 2008; Kropil et al. 2015; Meisingset et al. 2018; Fattorini et al. 2020), we also recommend that local red deer management units should be coordinated over larger areas to match spatial use by seasonally migrating males.
Availability of data and material
The datasets generated during and/or analysed during the current study are available in the Dryad repository, https://doi.org/10.5061/dryad.qrfj6q5jk.
References
Allen AM, Månsson J, Jarnemo A, Bunnefeld N (2014) The impacts of landscape structure on the winter movements and habitat selection of female red deer. Eur J Wildl Res 60:411–421. https://doi.org/10.1007/s10344-014-0797-0
Anderson D, Forester J, Turner M et al (2005) Factors influencing female home range sizes in elk (Cervus elaphus) in North American landscapes. Landsc Ecol 20:257–271
Apollonio M, Andersen R, Putman R (2010) European ungulates and their management in the 21st century. Cambridge University Press, Cambridge
Apollonio M, Belkin VV, Borkowski J et al (2017) Challenges and science-based implications for modern management and conservation of European ungulate populations. Mammal Res 62:209–217. https://doi.org/10.1007/s13364-017-0321-5
Bartón K (2013) MuMIn: Multi-model inference. https://cran.r-project.org/web/packages/MuMIn/index.html
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Beddington JR (1974) Age structure, sex ratio and population density in the harvesting of natural animal populations. J Appl Ecol 11:915–924
Beier P, McCullough D (1990) Factors influencing white-tailed deer activity patterns and habitat use. Wildl Monogr 109:1–51
Bevanda M, Fronhofer EA, Heurich M et al (2015) Landscape configuration is a major determinant of home range size variation. Ecosphere 6:1–12. https://doi.org/10.1890/ES15-00154.1
Bobek B, Merta D, Furtek J (2016) Winter food and cover refuges of large ungulates in lowland forests of south-western Poland. For Ecol Manage 359:247–255
Bocci A, Monaco A, Brambilla P et al (2010) Alternative strategies of space use of female red deer in a mountainous habitat. Ann Zool Fennici 47:57–66. https://doi.org/10.5735/086.047.0106
Bojarska K, Kurek K, Śnieżko S et al (2020) Winter severity and anthropogenic factors affect spatial behaviour of red deer in the Carpathians. Mammal Res 65:815–823. https://doi.org/10.1007/s13364-020-00520-z
Borkowski J, Ukalska J (2008) Winter habitat use by red and roe deer in pine-dominated forest. For Ecol Manage 255:468–475
Borkowski J, Ukalska J, Jurkiewicz J, Chećko E (2016) Living on the boundary of a post-disturbance forest area: the negative influence of security cover on red deer home range size. For Ecol Manage 381:247–257. https://doi.org/10.1016/j.foreco.2016.09.009
Buckland S, Ahmadi S, Staines B et al (1996) Estimating the minimum popualtion size that allows a given annual number of mature red deer stags to be culled sustainably. J Appl Ecol 33:118–130
Calenge C (2020) R package ‘adehabitatHR’. https://cran.r-project.org/web/packages/adehabitatHR/index.html
Carpio AJ, Apollonio M, Acevedo P (2021) Wild ungulate overabundance in Europe: contexts, causes, monitoring and management recommendations. Mamm Rev 51:95–108. https://doi.org/10.1111/mam.12221
Carranza J, Valencia J (1999) Red deer females collect on male clumps at mating areas. Behav Ecol 10:525–532
Caughley G (1977) Analysis of vertebrate populations. Wiley, London
Clutton-Brock T, Albon S (1989) Red deer in the Highlands. Wiley-Blackwell, Oxford
Clutton-Brock T, Harvey P (1978) Mammals, resources and reproductive strategies. Nature 273:191–195
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer: behaviour and ecology of two sexes. Edinburgh University Press, Edinburgh
Clutton-Brock TH, Coulson TN, Milner-Guiland EJ et al (2002) Sex differences in emigration and mortality affect optimal management of deer populations. Nature 415:633–637. https://doi.org/10.1038/415633a
Coppes J, Burghardt F, Hagen R et al (2017) Human recreation affects spatio-temporal habitat use patterns in red deer (Cervus elaphus). PLoS One 12:1–19. https://doi.org/10.1371/journal.pone.0175134
Coulon A, Morellet N, Goulard M et al (2008) Inferring the effects of landscape structure on roe deer (Capreolus capreolus) movements using a step selection function. Landsc Ecol 23:603–614. https://doi.org/10.1007/s10980-008-9220-0
De Vires MF (1995) Large herbivores and the design of large-scale nature reserves in Western Europe. Conserv Biol 9:25–33. https://doi.org/10.1046/j.1523-1739.1995.09010025.x
Fattorini N, Lovari S, Watson P, Putman R (2020) The scale-dependent effectiveness of wildlife management: a case study on British deer. J Environ Manage 276. https://doi.org/10.1016/j.jenvman.2020.111303
Ford R (1983) Home range in a patchy environment: optimal foraging predictions. Am Zool 23:315–326
Frair JL, Merrill EH, Visscher DR et al (2005) Scales of movement by elk (Cervus elaphus) in response to heterogeneity in forage resources and predation risk. Landsc Ecol 20:273–287. https://doi.org/10.1007/s10980-005-2075-8
Froy H, Börger L, Regan CE et al (2018) Declining home range area predicts reduced late-life survival in two wild ungulate populations. Ecol Lett 21:1001–1009. https://doi.org/10.1111/ele.12965
Gazzola A, Bertelli I, Avanzinelli E et al (2005) Predation by wolves (Canis lupus) on wild and domestic ungulates of the western Alps, Italy. J Zool 266:205–213. https://doi.org/10.1017/S0952836905006801
Georgii B, Schröder W (1983) Home range and activity patterns of male red deer (Cervus elaphus L.) in the Alps. Oecologia 58:238–248
Gerhardt P, Arnold JM, Hackländer K, Hochbichler E (2013) Determinants of deer impact in European forests - a systematic literature analysis. For Ecol Manage 310:173–186. https://doi.org/10.1016/j.foreco.2013.08.030
Gill R (1992) A review of damage by mammals in north temperate forests: 1. Deer Forestry 65:145–169
Gillich B, Michler FU, Stolter C, Rieger S (2021) Differences in social-space–time behaviour of two red deer herds (Cervus elaphus). Acta Ethol. https://doi.org/10.1007/s10211-021-00375-w
Ginsberg JR, Milner-Gulland EJ (1994) Sex-biased harvesting in dynamics population for implications ungulates : use and sustainable conservation. Conserv Biol 8:157–166
Hewison A, Vincent J, Reby D (1998) Social organisation of European roe deer. In: Andersen R, Duncan P, Linnell J (eds) The European roe deer: the biology of success 189–219
Jarnemo A (2008) Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management. Eur J Wildl Res 54:327–333
Jarnemo A (2011) Male red deer (Cervus elaphus) dispersal during the breeding season. J Ethol 29:329–336
Jarnemo A (2014) Kronviltprojektet 2005–2013. Final report The red deer project 2005-2013. Grimsö Wildlife Research Station, SLU, Riddarhyttan
Jarnemo A, Jansson G, Månsson J (2017) Temporal variations in activity patterns during rut – implications for survey techniques of red deer, Cervus elaphus. Wildl Res 44:106–113
Jarnemo A, Minderman J, Bunnefeld N, et al (2014) Managing landscapes for multiple objectives: alternative forage can reduce the conflict between deer and forestry. Ecosphere 5. https://doi.org/10.1890/ES14-00106.1
Jarnemo A, Widén A, Månsson J, Felton AM (2022) The proximity of rapeseed fields influences levels of forest damage by red deer. Ecol Solut Evid 3:1–12. https://doi.org/10.1002/2688-8319.12156
Jarnemo A, Wikenros C (2014) Movement pattern of red deer during drive hunts in Sweden. Eur J Wildl Res 60. https://doi.org/10.1007/s10344-013-0753-4
Jȩdrzejewski W, Schmidt K, Theuerkauf J et al (2002) Kill rates and predation by wolves on ungulate populations in Białowieża primeval forest (Poland). Ecology 83:1341–1356. https://doi.org/10.1890/0012-9658(2002)083[1341:KRAPBW]2.0.CO;2
Jeppesen J (1987) Impact of human disturbance on home range, movements and activity of red deer (Cervus elaphus) in a Danish environment. Danish Rev Game Biol 13:1–38
Jerina K (2012) Roads and supplemental feeding affect home-range size of Slovenian red deer more than natural factors. J Mammal 93:1139–1148. https://doi.org/10.1644/11-MAMM-A-136.1
Kamler JF, Jȩdrzejewski W, Jȩdrzejewska B (2008) Home ranges of red deer in a European old-growth forest. Am Midl Nat 159:75–82. https://doi.org/10.1674/0003-0031(2008)159[75:HRORDI]2.0.CO;2
Kernohan B, Gitzen R, Millspaugh J (2001) Analysis of animal space use and movement. In: Millspaugh J, Marzluff J (eds) Radio tracking and animal populations. Academic Press, San Diego, pp 125–166
Kie J, Bowyer R, Nicholson M et al (2002) Landscape heterogeneity at different scales: effects on spatial distribution of mule deer. Ecology 83:530–544
Kie JG (2013) A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Anim Biotelemetry 1:13. https://doi.org/10.1186/2050-3385-1-13
Kie JG, Ager AA, Bowyer RT (2005) Landscape-level movements of North American elk (Cervus elaphus): effects of habitat patch structure and topography. Landsc Ecol 20:289–300. https://doi.org/10.1007/s10980-005-3165-3
Koubek P, Hrabe V (1996) Home range dynamics in the red deer (Cervus elaphus) in a mountain forest in central Europe. Folia Zool 45:219–222
Kropil R, Smolko P, Garaj P (2015) Home range and migration patterns of male red deer Cervus elaphus in Western Carpathians. Eur J Wildl Res 61:63–72
Kuijper DPJ (2011) Lack of natural control mechanisms increases wildlife-forestry conflict in managed temperate European forest systems. Eur J for Res 130:895–909
Kuijper DPJ, Jedrzejewska B, Brzeziecki B et al (2010) Fluctuating ungulate density shapes tree recruitment in natural stands of the Białowieza Primeval Forest, Poland. J Veg Sci 21:1082–1098. https://doi.org/10.1111/j.1654-1103.2010.01217.x
Laguna E, Carpio AJ, Vicente J, et al (2021) The spatial ecology of red deer under different land use and management scenarios: protected areas, mixed farms and fenced hunting estates. Sci Total Environ 786:147124. https://doi.org/10.1016/j.scitotenv.2021.147124
Langvatn R, Loison A (1999) Consequences of harvesting on age structure, sex ratio and population dynamics of red deer Cervus elaphus in central Norway. Wildlife Biol 5:213–223. https://doi.org/10.2981/wlb.1999.026
Linnell JDC, Cretois B, Nilsen EB, et al (2020) The challenges and opportunities of coexisting with wild ungulates in the human-dominated landscapes of Europe’s Anthropocene. Biol Conserv 244:108500. https://doi.org/10.1016/j.biocon.2020.108500
Lovari S, Cuccus P, Murgia A et al (2007) Space use, habitat selection and browsing effects of red deer in Sardinia. Ital J Zool 74:179–189. https://doi.org/10.1080/11250000701249777
Luccarini S, Mauri L, Ciuti S et al (2006) Red deer (Cervus elaphus) spatial use in the Italian alps: home range patterns, seasonal migrations, and effects of snow and winter feeding. Ethol Ecol Evol 18:127–145. https://doi.org/10.1080/08927014.2006.9522718
Main MB, Weckerly FW, Bleich VC (1996) Sexual segregation in ungulates: new directions for research. J Mammal 77:449–461. https://doi.org/10.2307/1382821
Månsson J, Jarnemo A (2013) Bark-stripping on Norway spruce by red deer in Sweden: level of damage and relation to tree characteristics. Scand J for Res 28:117–125. https://doi.org/10.1080/02827581.2012.701323
Månsson J, Nilsson L, Felton AM, Jarnemo A (2021) Habitat and crop selection by red deer in two different landscape types. Agric Ecosyst Environ 318:107483. https://doi.org/10.1016/j.agee.2021.107483
Meisingset EL, Loe LE, Brekkum Ø et al (2018) Spatial mismatch between management units and movement ecology of a partially migratory ungulate. J Appl Ecol 55:745–753. https://doi.org/10.1111/1365-2664.13003
Milner-Gulland E, Coulson T, Clutton-Brock T (2004) Sex differences and data quality as determinants of income from hunting red deer Cervus elaphus. Wildlife Biol 10:187–201
Milner J, Bonenfant C, Mysterud A et al (2006) Temporal and spatial development of red deer harvesting in Europe: biological and cultural factors. J Appl Ecol 43:721–734
Mysterud A, Coulson T, Stenseth N (2002) The role of males in the dynamics of ungulate populations. J Anim Ecol 71:907–915
Mysterud A, Rivrud IM, Brekkum Ø, Meisingset EL (2023) Effect of legal regulation of supplemental feeding on space use of red deer in an area with chronic wasting disease. Eur J Wildl Res 69:1–10. https://doi.org/10.1007/s10344-022-01630-6
Náhlik A, Sándor G, Tari T, Király G (2009) Space use and activity patterns of red deer in a highly forested and in a patchy forest-agricultural habitat. Acta Silv Lignaria Hungarica 5:109–118
Naugle D, Jenks J, Kernohan B, Johnson R (1997) Effects of hunting and loss of escape cover on movements and activity of female white-tailed deer, Odocoileus virginianus. Can Field-Naturalist 111:595–600
Nilsson P, Cory N (2011) Forestry Statistics 2011. Official statistics of Sweden. SLU, Umeå
Nowak S, Mysłajek RW, Jędrzejewska B (2005) Patterns of wolf Canis lupus predation on wild and domestic ungulates in the Western Carpathian Mountains (S Poland). Acta Theriol (warsz) 50:263–276. https://doi.org/10.1007/BF03194489
O’Neill R, Milne B, Turner M, Gardner R (1988) Resource utilization scales and landscape pattern. Landsc Ecol 2:3–69
Powell R (2000) Animal home ranges and territories and home range estimators. In: Boitani L, Fuller T (eds) Research techniques in animal ecology: controversies and consequences. Colombia University Press, New York, pp 65–110
Prokešová J (2004) Red deer in the floodplain forest: the browse specialist? Folia Zool 53:293–302
Putman RJ (2012) Effects of heavy localised culling on population distribution of red deer at a landscape scale: an analytical modelling approach. Eur J Wildl Res 58:781–796. https://doi.org/10.1007/s10344-012-0624-4
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Reimoser F (2003) Steering the impacts of ungulates on temperate forests. J Nat Conserv 10:243–252
Reinecke H, Leinen L, Thißen I et al (2014) Home range size estimates of red deer in Germany: environmental, individual and methodological correlates. Eur J Wildl Res 60:237–247. https://doi.org/10.1007/s10344-013-0772-1
Richard E, Said S, Hamann JL, Gaillard JM (2011) Toward an identification of resources influencing habitat use in a multi-specific context. PLoS ONE 6:1–9. https://doi.org/10.1371/journal.pone.0029048
Rivrud I, Bischof R, Meisingset E et al (2016) Leave before it’s to late: anthropogenic and environmental triggers of autumn migration in a hunted ungulate population. Ecology 97:1058–1068
Rivrud I, Loe L, Mysterud A (2010) How does local weather predict red deer home range size at different temporal scales? J Anim Ecol 79:1280–1295
Ruckstuhl K, Neuhaus P (2000) Sexual segregation in ungulatese: a new approach. Behaviour 137:361–377
Ruckstuhl K, Neuhaus P (2005) Sexual segregation in vertebrates. Cambridge University Press, Cambridge, Ecology of the two sexes
Saïd S, Servanty S (2005) The influence of landscape structure on female roe deer home-range size. Landsc Ecol 20:1003–1012. https://doi.org/10.1007/s10980-005-7518-8
Samuel M, Pierce D, Garton E (1985) Identifying areas of concentrated use within the home range. J Anim Ecol 54:711–719
Schmidt K (1993) Winter ecology of nonmigratory Alpine red deer. Oecologia 95:226–233. https://doi.org/10.1007/BF00323494
Scholten J, Moe SR, Hegland SJ (2018) Red deer (Cervus elaphus) avoid mountain biking trails. Eur J Wildl Res 64. https://doi.org/10.1007/s10344-018-1169-y
Sibbald AM, Hooper RJ, McLeod JE, Gordon IJ (2011) Responses of red deer (Cervus elaphus) to regular disturbance by hill walkers. Eur J Wildl Res 57:817–825. https://doi.org/10.1007/s10344-011-0493-2
Sorensen AA, van Beest FM, Brook RK (2015) Quantifying overlap in crop selection patterns among three sympatric ungulates in an agricultural landscape. Basic Appl Ecol 16:601–609. https://doi.org/10.1016/j.baae.2015.05.001
Spake R, Bellamy C, Gill R et al (2020) Forest damage by deer depends on cross-scale interactions between climate, deer density and landscape structure. J Appl Ecol 57:1376–1390. https://doi.org/10.1111/1365-2664.13622
Stopher K, Nussey D, Clutton-Brock T et al (2011) The red deer rut revisited: female excursions but no evidence females move to mate with preferred males. Behav Ecol 22:808–818
Sunde P, Olesen CR, Madsen TL, Haugaard L (2009) Behavioural responses of GPS-collared female red deer Cervus elaphus to driven hunts. Wildlife Biol 15:454–460. https://doi.org/10.2981/09-012
Svenning JC (2002) A review of natural vegetation openness in north-western Europe. Biol Conserv 104:133–148. https://doi.org/10.1016/S0006-3207(01)00162-8
Szemethy L, Heltai M, Matrai K, Peto Z (1998) Home ranges and habitat selection of red deer (Cervus elaphus) on a lowland area. Gibier Faune Sauvage 15:607–615
Szemethy L, Mátrai K, Katona K, Orosz S (2003) Seasonal home range shift of red deer hinds, Cervus elaphus: are there feeding reasons? Folia Zool 52:249–258
Takarabe K, Iijima H (2020) Abundant artificial grasslands around forests increase the deer impact on forest vegetation. Eur J for Res 139:473–482. https://doi.org/10.1007/s10342-020-01262-y
Torres-Porras J, Carranza J, Pérez-González J et al (2014) The tragedy of the commons: unsustainable population structure of Iberian red deer in hunting estates. Eur J Wildl Res 60:351–357. https://doi.org/10.1007/s10344-013-0793-9
Valente AM, Acevedo P, Figueiredo AM et al (2020) Overabundant wild ungulate populations in Europe: management with consideration of socio-ecological consequences. Mamm Rev 50:353–366. https://doi.org/10.1111/mam.12202
Van Beest FM, Rivrud IM, Loe LE et al (2011) What determines variation in home range size across spatiotemporal scales in a large browsing herbivore? J Anim Ecol 80:771–785. https://doi.org/10.1111/j.1365-2656.2011.01829.x
van Beest FM, Van Moorter B, Milner JM (2012) Temperature-mediated habitat use and selection by a heat-sensitive northern ungulate. Anim Behav 84:723–735. https://doi.org/10.1016/j.anbehav.2012.06.032
Verheyden H, Ballon P, Bernard V, Saint-andrieux C (2006) Variations in bark-stripping by red deer Cervus elaphus across Europe. Mamm Rev 36:217–234. https://doi.org/10.1111/j.1365-2907.2006.00085.x
Walter WD, Vercauteren KC, Gilsdorf JM, Hygnstrom SE (2009) Crop, native vegetation, and biofuels: response of white-tailed deer to changing management priorities. J Wildl Manage 73:339–344. https://doi.org/10.2193/2008-162
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164. https://doi.org/10.2307/1938423
Zlatanova D, Popova E, Ahmed A, et al (2019) Red deer on the move: home range size and mobility in Bulgaria. Ecol Montenegrina 23:47–59. https://doi.org/10.37828/EM.2019.23.7
Acknowledgements
We are grateful to the landowners, game managers, and hunters on the participating estates. We thank Ove Fransson, Per Grängstedt, Andreas Jonsson, Peter Jonzon, Douglas Klingspor, John Källström, Gustaf Lannek, Per Larsson, Håkan Lindgren, Jonas Malmsten, Henrik Negendanck, Mathias Nilsson, Daniel Nilsson, Stefan Nyman, Johan Palmgren, Frederik Pedersen, Bengt Röken, Kent Sköld, Håkan Svensson, Bosse Söderberg, Henning Sörensen for field assistance, and Henrike Hensel, Jenny Mattisson, Johanna Månsson Wikland, and Johan Månsson for administration of transmitters. Thanks to Manisha Bhardwaj for support in R.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. The study was financed by Svenska Jägareförbundet, Naturvårdsverket, Region Skåne, Stiftelsen Skånska Landskap, Carl Piper, Högestads & Christinehofs Fideikommiss, Holmen Skog AB, Johan Hansen och Ittur Jakt AB, Caesar Åfors och Virå Bruk AB, Sveaskog, Karl-Erik Önnesjös stiftelse för vetenskaplig forskning och utveckling, Marie-Claire Cronstedts Stiftelse, Stiftelsen Oscar och Lili Lamms Minne, Ericsbergs Fideikommiss AB, Helge Ax:son Johnsons stiftelse, Ågerups & Elsagårdens Säteri AB, Håkan Wikholm Assmåsa Gods AB, and samt Kolmårdens insamlingsstiftelse/Tåby Allmänning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures including capture, handling, and collaring of red deer were examined by the animal ethics committee for central Sweden and fulfilled the ethical requirements for research on wild animals (decisions M258-06 and 50–06).
Conflict of interest
The authors declare no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jarnemo, A., Nilsson, L. & Wikenros, C. Home range sizes of red deer in relation to habitat composition: a review and implications for management in Sweden. Eur J Wildl Res 69, 92 (2023). https://doi.org/10.1007/s10344-023-01719-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01719-6