Abstract
Diagnostic and therapeutic radionuclides offer an excellent platform for the development of innovative drugs, which enable non-invasive visualization of diseases and complementary targeted treatments. The concept of personalized medicine is realized! This innovation in nuclear medicine together with an increasing demand for high-quality radionuclides and radiopharmaceuticals has triggered the expansion of nuclear medicine as a hospital speciality, together with the development of a new radiotheranostics industry.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
13.1 Introduction
Diagnostic and therapeutic radionuclides offer an excellent platform for the development of innovative drugs, which enable non-invasive visualization of diseases and complementary targeted treatments. The concept of personalized medicine is realized! This innovation in nuclear medicine together with an increasing demand for high-quality radionuclides and radiopharmaceuticals has triggered the expansion of nuclear medicine as a hospital speciality, together with the development of a new radiotheranostics industry.
This chapter describes the successful development of no-carrier-added (n.c.a.) Lutetium-177 and of n.c.a. 177Lu-edotreotide as examples of the successful collaboration between an academic nuclear medicine institution and industry.
13.2 No-Carrier-Added Lutetium-177: The Gold Standard for Radionuclide Treatment
After the introduction of suitable macrocyclic chelators into the targeting molecules, trivalent radiometals such as Yttrium-90 (pure high energy β−-emitter) gained importance for the targeted therapeutic treatment of serious oncological disease [1]. Lutetium-177 in particular, has demonstrated excellent physical properties to enable the precise delivery of cytotoxic dose of beta irradiation to small and large malignant lesions. Furthermore, by emitting soft beta radiation (Eβ 133.6 keV) Lutetium-177 radiolabelled compounds have a favourable safety profile particularly in terms of nephrotoxicity. Small components of photons (112.9 keV, 6% and 208.4 kEv, 10%) enable the visualization and quantitative estimation (dosimetry) of biodistribution by means of SPECT, without having a negative impact on safety (Table 13.1). Starting with Lutetium-177-based treatments of somatostatin receptor-positive tumours in the late 1990s, the use of radionuclide has dramatically increased. A number of novel therapies are being developed for the treatment of serious oncological diseases using tumour receptor targeting, including PSMA and FAP [3, 4].
An important aspect of the successful development of Lutetium-177-based therapies is the availability of a radionuclide with specific activity suitable for the radiolabelling of targeting molecules. The first carrier-added preparations of Lutetium-177 for radiolabelling became commercially available from the early 2000s. Lutetium-177 can be easily produced in a nuclear reactor by the irradiation of the highly enriched stable isotope Lutetium-176 (Fig. 13.1). A high cross-section for the neutron capture reaction enables a reasonable specific activity to be achieved, although the final preparation still consists of a mixture of the stable and radioactive isotopes Lu-176 and Lu-177, respectively. The main drawback of this manufacturing pathway is the co-accumulation of long-lived metastable Lutetium-177 m (half-life 160.44 days). Depending on the irradiation parameters, the fraction of this long-lived radionuclidic impurity varies from 0.2 to 0.7%. The disposal of solid and especially liquid wastes, contaminated with the long-lived impurity, is costly and laborious.
A significant enhancement for the future development of targeted radionuclide therapies was the implementation of no-carrier-added Lutetium-177. If the neutron capture reaction leads to an intermediate β−-unstable nuclide, then a secondary formed radioisotope is an isoton to the target nucleus. In this case the radionuclide can be isolated from the target material chemically in a no-carrier-added form. Thus the irradiation of highly-enriched Ytterbium-176 with neutrons results in short-lived Yb-177, which decays to desired Lu-177 (Fig. 13.2). Furthermore, only the ground state (i.e., Lu-177 g) is generated, providing the highest radionuclidic purity of the preparation free from Lu-177 m contamination.
A challenge in the manufacturing of no-carrier-added Lutetium-177 is the chemical purification of the radioisotope from the massive Ytterbium-176 targets. Two adjacent members of the lanthanide series Yb(III) and Lu(III) are chemically very similar and their chemical separation becomes a difficult scientific task. For production of industrial quantities of no-carrier-added Lutetium-177, gram amounts of Ytterbium-176 must be utilized. In contrast, the accumulated radionuclide corresponds to several micrograms of Lutetium mass.
ITM AG (through its affiliate ITG GmbH) has developed and implemented a unique automated process for the chemical processing of massive irradiated Ytterbium-176 targets and the fast isolation of no-carrier-added Lutetium-177 isotope. In 2007, ITG commenced the first irradiations of enriched Ytterbium-176 for the industrial production of no-carrier-added Lutetium-177 at the Munich research reactor FRMII. Due to the limited operation cycles of research nuclear reactors and in order to secure the weekly production and supply of Lu-177, it was necessary to build up a reactor network with a large number of medium- and high-flux reactors worldwide. Currently, ITM closely cooperates with research reactors in Belgium, France, Netherlands, Poland, South Africa and the USA.
An important characteristic of the no-carrier-added form is the fact that the quality doesn’t depend on the performance among different nuclear reactors, with the highest level of specific activity being ensured for all radionuclide preparations. Furthermore, the specific activity of no-carrier-added Lutetium-177 remains high over the shelf-life of the product. Currently, ITM supplies its European registered product (EndolucinBeta™) worldwide to nuclear medicine departments and to industrial partners for the radiolabelling of tumour-targeting molecules (Fig. 13.2).
13.3 No-Carrier-Added Lutetium-177-Edotreotide for Treatment of Neuroendocrine Tumours
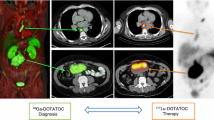
Originally developed in Basel, Switzerland, the synthetic somatostatin analogue edotreotide (or DOTATOC) has been evaluated and clinically used in combination with Yttrium-90 for the therapy of somatostatin receptor-positive tumours. Currently, Ga-68-edotreotide PET imaging agents are approved in Europe and in the USA for visualisation of neuroendocrine tumours (NETs). Excellent pharmacokinetic properties of this peptide combined with the outstanding characteristics of Lutetium-177 make it even more attractive for therapeutic use.
Favourable pharmacokinetics properties of edotreotide were initially reported by comparing the behaviour of 111In-labelled edotreotide with 111In-labelled DOTATATE in the same patients [5]. Particular emphasis was given to kidney uptake and to the tumour-to-kidney ratio. Whereas, the mean absorbed dose to the red marrow was similar, 111In-edotreotide demonstrated a comparably higher tumour-to-kidney absorbed dose ratio. Interestedly, the urinary excretion rate of radiolabelled edotreotide was significantly higher than for DOTATATE, whereas the tumour doses were within the same range. This initial study was performed without a reno-protective amino acid infusion.
Later on, the Bad Berka group investigated the in vivo behaviour of the 177Lu-labeled peptides DOTATATE, DOTANOC, and DOTATOC. The aim of the study was to compare the pharmacokinetics and dosimetry of these three different peptides, considering inter- and intra-patient variability in a large cohort of patients with GEP NETs [6]. This study confirmed the favourable pharmacokinetic properties of radiolabelled edotreotide previously demonstrated by Forrer and colleagues. Edotreotide has a more rapid clearance from healthy organs compared to DOTATATE and DOTANOC providing a high tumour to background ratio and hence a high targeted dose of radiation to the tumour. The authors concluded that of the three peptides, 177Lu-edotreotide results in the highest tumour-to-kidney ratio, indicating that it is and is a very appropriate choice for the therapy of GEP NETs.
The first systematic evaluation of treatment data with no-carrier-added 177Lu-edotreotide in patients with GEP NETs was performed by Professor R. P. Baum at the Zentralklinik Bad Berka, Germany [7]. In this retrospective study, the efficacy and safety of treatment with 177Lu-edotreotide were evaluated in 56 subjects with metastasised, progressive NET (50% gastroenteric, 27% pancreatic, 23% other primaries) who had not received previous PRRT treatment prior to a new diagnosis of progression. Subjects received on average 2.1 (range 1–4) cycles of 177Lu-edotreotide as the sole treatment, administered in median doses of 7.0 GBq, at approximately three-monthly treatment intervals. Forty-three percent (24/56) of the study population underwent only a single 177Lu-edotreotide cycle. Of these, 15 died from progressive disease prior to further PRRT. In total, 26 patients (46%) had died at data-base lock. When stratified for the number of 177Lu-edotreotide cycles received (1 vs. ≥2), subjects treated only once were found to have a significantly lower Karnofsky performance status (KPS) at baseline (70.4 vs. 89.4, p < 0.001), indicating more advanced disease stage. In the total population (A), median progression-free (PFS) and overall survival (OS) were 17.4 and 34.2 months, respectively (Figs. 13.3 and 13.4). In repeatedly treated subjects, PFS was 32.0 months for all (B), 34.5 months for GEP NET (C), and 11.9 months for other NET (D). Objective response rates (ORR) were 33.9%, 40.6%, 54.2%, and 0% for populations A, B, C, and D, respectively. A high number of complete responses (16.1%, 18.8%, and 25.0% for populations A, B, and C) were observed, 78% of which were ongoing at the end of observation. No serious adverse event and only a single case of self-limited grade 3 haematotoxicity was observed (1.8%). No evidence for renal toxicity was found, although 34.4% of subjects had mild renal impairment at baseline. In addition, a long-term safety follow-up of patients included in the retrospective study showed no lasting relevant haematotoxic effects and no long-term renal toxicity for up to 6 years after first PRRT. These data show that 177Lu-edotreotide is an agent for PRRT with the unusual potential to induce objective responses, and lasting disease control in progressive NETS, even when administered in moderate doses. A particularly high therapeutic index is suggested by the observed safety profile which includes subjects with preceding reduced bone marrow or renal function. At the present time, it is standard practice to provide renal protection with a 2.5% lysine/arginine infusion which is given concomitantly with the PRRT infusion.
Kaplan-Meier estimates of PFS in per protocol patients depending on number of 177Lu-edotreotide PRRT cycles [7]
Kaplan-Meier estimates of overall survival in PP patients depending on number of 177Lu-edotreotide PRRT cycles [7]
Patients with G1 and G2 GEP NETS often present late with metastatic disease. Until recently, somatostatin analogues and the molecularly targeted drugs sunitinib and everolimus have provided the mainstays of treatment. These treatments usually result in disease stabilisation for a limited period of time. In the RADIANT-3 trial, everolimus achieved a PFS of 11 months in pancreatic NETS and a similar PFS was achieved in midgut and pulmonary NETS in the RADIANT-4 trial [8, 9]. Peptide receptor radionuclide therapy has recently emerged as a novel treatment option, with a PFS of 28.4 months in G1 and G2 mid-gut NETS being achieved in the NETTER-1 trial [10]. This Phase III study has resulted in the regulatory approval of 177Lu c.a.-DOTATATE for G1 and G2 NETS.
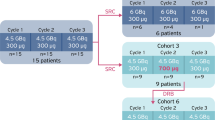
Following on from the Bad Berka study [7], ITM has initiated a Phase III pivotal clinical trial. COMPETE is a prospective, randomised, open-label multi-centre Phase III study to evaluate the safety and efficacy of 177Lu n.c.a.-edotreotide in comparison to everolimus in patients with G1 and G2 GEP NETs. The patients have progressive, somatostatin receptor (SSTR) positive disease on SSTR imaging. Uniquely, patients may be included as first-line therapy. There are 3 sub-studies which focus on 177Lu-edotreotide dosimetry and pharmacokinetics. These sub-studies are of great importance in the development of a personalised, precision therapy approach to the management of patients with PRRT. In addition, 177Lu is uniquely non-carrier-added, which means that it is a pure radionuclide of high specific activity.
The study is ongoing with a target recruitment of 300 patients. A total of 200 patients will receive 4 cycles of 177Lu-edotreotide (7.5 GBq/cycle) every 3 months or until disease progression and 100 patients will receive everolimus 10 mg daily for 24 months or until disease progression. The study duration is 24 months with 5 years follow-up for OS. The primary end-point is PFS as assessed by RECIST 1.1. Key secondary end-points include safety and tolerability, dosimetry, ORR, OS and quality of life (Fig. 13.5). Patients with G3 neuroendocrine neoplasms (Ki-67 > 20%) have more aggressive disease than the G1 and G2 NETs. In 2017, the WHO subdivided G3 NENs into well-differentiated G3 neuroendocrine tumours (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) [11]. A retrospective study of PRRT in G3 NENs has been reported by Professor Baum’s group in Bad Berka [12]. Sixty-nine patients were treated with either 177Lu- or 90Y-labelled somatostatin analogues (DOTATATE or DOTATOC). This was a heterogeneous group of patients both in terms of disease and treatment. Overall, the median PFS was 9.6 months and the median OS was 19.9 months. When the patients were sub-into grouped into NETs with a Ki-67 index of ≤55%, the median PFS was 11 months and the OS 24 months. For NECs with a Ki-67 index of ≥55%, the median PFS was 4 months and the median OS was 7 months. In the patients who had positive SSTR imaging but negative 18F-FDG uptake, the prognosis was dramatically better. Other retrospective studies have also reported beneficial responses to PRRT in G3 NEN, particularly those with a Ki-67 index of ≤55% [13,14,15]. These low-grade G3 NETs are of particular interest for further clinical development. The high-grade G3 NECs (Ki-67 ≥ 55%) respond relatively poorly to PRRT. These tumours might benefit from combination therapies, particularly with DNA repair enzyme inhibitors.
13.4 Conclusion and Acknowledgements
Beyond the radionuclide targeted treatment of GEP NETs, a number of new targeted therapies have been introduced or are under development to treat serious oncological diseases such as castrate-resistant prostate cancer, pancreatic ductal adenocarcinoma and brain tumours. No-carrier-added Lutetium-177 is an excellent platform for development of these innovative treatment options. In addition to the in-house manufacture of radiopharmaceuticals by ITM, the radionuclide is in use in a number of investigational medicinal products, which are currently undergoing clinical trials worldwide.
The relationship that has been developed between ITM and Professor Baum goes back to the early days of ITM’s foundation in 2004. During this time, the company has developed into a world-leading radiotheranostics company with a strong radioisotope manufacturing group and more recently a rapidly expanding clinical oncologics group, dedicated to bringing new radiotheranostics products into the clinic for the benefit of patients. Professor Baum’s legacy will continue, driven by the many nuclear medicine experts who have been mentored by him, together with the many other collaborators in academia, medicine and industry. ITM is continuing to collaborate with Professor Baum’s successors to drive the field of radiotheranostics onwards. Areas of development include the development of 177Lu-zolendonate for osteoblastic bone cancer, novel PSMA targeted therapies for prostate cancer and folate receptor alpha targeted therapies. Professor Baum is one of the early pioneers in radiotheranostics, being one of the key individuals who have developed radiotheranostics into the exciting, rapidly developing field in oncology that it is today. He has been a great collaborative colleague to ITM. That is a great legacy.
References
Fani, Melpomeni; Good, S.; Maecke, Helmut R in Handbook of Nuclear Chemistry: Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences, 2011, Volume 4. Chapter 8. pp. 2143–2178.: Radiometals (non-Tc, non-Re) and Bifunctional Labeling Chemistry.
NuDat 2.8. https://www.nndc.bnl.gov/nudat2/.
Hofaman MS, Violet J, Hicks RJ, Ferdinandus J, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33.
Loktev A, Lindner T, Burger EA, Altmann A, et al. Development of fibroblast activation protein–targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9.
Forrer F, Uusijärvi H, Waldherr C, Cremonesi M, Bernhart P, Mueller-Brand J, Maecke HR. A comparison of 111In-DOTATOC and 111In-DOTATATE: biodistribution and dosimetry in the same patients with metastatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2004;31:1257–62.
Schuchardt C, Kulkarni HR, Prasad V, Zachert C, Müller D, Baum RP. The Bad Berka dose protocol: comparative results of dosimetry in peptide receptor radionuclide therapy using 177Lu-DOTATATE, 177Lu-DOTANOC, and 177Lu-DOTATOC. Resent Res Cancer Res. 2013;194:519–36.
Baum RP, Kluge AW, Kulkarni H, et al. [177Lu-DOTA]0–D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics. 2016;6(4):501–10.
Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIAT-4): a randomised, placebo controlled, phase 3 study. Lancet. 2016;387:968.
Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35.
Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Cancer Research (IACR) and World Health Organisation (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–86.
Zhang J, Kulkarni HR, Singh A, et al. Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: safety and survival analysis in 69 patients. J Nucl Med. 2019;60:377–85.
Carlsen EA, Fazio N, Granberg D, et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: a multicenter cohort study. Endocr Relat Cancer. 2019;26(2):227–39.
Nicolini S, Severi S, Ianniello A, et al. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. 2018;45(6):923–30.
Thang SP, Lung MS, Kong G, et al. Peptide receptor radionuclide therapy (PRRT) in European neuroendocrine tumour society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—a single institution retrospective analysis. Eur J Nucl Med Mol Imaging. 2018;45(2):262–77.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Harris, P., Henkelmann, R., Marx, S., Zhernosekov, K. (2024). The Evolution of n.c.a. 177Lu to n.c.a. 177Lu-Edotreotide for the Treatment of Neuroendocrine Tumours. Sixteen Years of Collaboration Between Zentralklinik Bad Berka and ITM. In: Prasad, V. (eds) Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum. Springer, Cham. https://doi.org/10.1007/978-3-031-33533-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-33533-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33532-7
Online ISBN: 978-3-031-33533-4
eBook Packages: MedicineMedicine (R0)