Abstract
Nowadays, the transformation of biomass into valuable chemicals and fuels through thermochemical, biochemical or even mixed technologies, is becoming increasingly popular and challenging. A promising solution for the near future is the substitution of non-renewable fossil fuels with a sustainable liquid feedstock for biofuel (biodiesel) production. The cost of conventional biodiesel production is higher than that of petroleum-based diesel production since it is produced mostly from expensive high-quality virgin oil. Conventionally, commercial biodiesel is produced via liquid base-catalyzed transesterification of triglycerides components of oil/fat with short-chain alcohols. It is that about 70–80% of the overall biodiesel production cost is associated with the cost of raw materials. Brown grease (with free fatty acid levels > 15%) is created from rendered trap waste and is known as Fats, Oils, and Greases (FOGs), it is a potential source of biodiesel feedstocks and is available at no cost. Many researchers are interested in using low-cost high Free Fatty Acid (FFA) oils as the feedstock for biodiesel production. This paper reviews the effect of feedstock pre-treatment and process parameters on the conversion of FOGs-wastewater to biodiesel by esterification-transesterification process.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Triglyceride (TGs)
- Esterification/transesterification reaction
- Methyl ester
- Heterogeneous base or acid solid catalyst
27.1 Introduction
Sustainable renewable energy production is being intensely disputed worldwide and has many alternative energy resources that exist in varied forms and could be used to substitute the conventional fossil fuels (Fig. 27.1), because gradually fossil fuel resources are declining [1]. One of the promising solutions for the replacement of fossil fuels is substitution with a sustainable biomass feedstock for biofuel (especially biodiesel) production [2]. Biodiesel is a fuel derived from edible and non-edible biomass oils made by chemically reacting lipids such as animal fat (tallow), soybean oil, or some other vegetable oil with an alcohol producing a methyl, ethyl, or propyl ester [3, 4]. It is a well-known process involving the transesterification of oil via homogeneous base catalysis and commercially it is an established method for biodiesel production [5,6,7]. However, homogeneous base catalysts are very sensitive to free fatty acids present in low-quality oil feedstocks, requiring additional acid pre-treatment and neutralization steps which not only cause environmental pollution but also increase the overall cost of biodiesel production. Encouragingly, solid catalysts can provide a green, efficient, and economical pathway for biodiesel production using low-cost oil feedstocks such as waste cooking oil, fats, oils and grease (FOGs) in wastewater [8, 9]. Heterogeneous base or acid solid catalysts have been developed and successfully applied in esterification and transesterification processes of FFAs and triglyceride (TGs) by many researchers [10, 11].
This paper focuses on heterogeneous catalysis in the esterification of high free fatty acid lipid feedstock from wastewater containing fats, oils and greases (FOGs) and their transformation via esterification/transesterification to biodiesel as a promising solution to achieve renewable energy in near future. Esterification/transesterification of FFAs and TGs to alkyl esters in the presence of an acidic or basic catalyst is a route to improving the use of high FFA-TGs oils (e.g., some animal and vegetable oils) in biodiesel production. This work aims to review and understand the parameters that affect the conversion of fatty acids reacted with short chain alcohols to achieve better biodiesel yields.
27.2 Esterification-Transesterification Reaction of FOGs
Biodiesel can be produced by three technologies: 1. Alkaline catalyzed transesterification (suitable for feedstock with low free fatty acid content); 2. Acid catalyzed transesterification/esterification (good for feedstock with high FFA content); and 3. Transesterification two-step process (good for feedstock with high FFA content) [12, 13]. In general, alkaline metal hydroxides or methoxides are very effective catalysts for transesterification. The rate of alkaline catalyzed transesterification is about 4000 times faster than acid catalyzed transesterification, but its drawback is that FFA cannot be converted to ester. The FFAs are only neutralized to fatty soap, which further complicates the separation causing an additional loss of biodiesel in the separation step. Acid is a good catalyst for both esterification and transesterification, however the rate of transesterification over acid catalyst is very much slower than that of esterification [5]. This is the reason why some researchers choose the two-step process for high FFA feedstock (esterification of FFA with acid catalyst followed by alkaline catalyzed transesterification). Total reaction times are still shorter than those experienced in the one step acid catalysis.
Fat/vegetable oil is primarily a triglyceride (glycerol ester of fatty acids), whereas biodiesel is the mono-methyl ester of fatty acids. For this reason, biodiesel production process is a transesterification (see Scheme 1) process which is carried out by substituting glycerol groups by methyl groups in the presence of sodium methoxide as catalyst with the glycerol obtained as a side product. It is this trans-esterification process which is used often in technology today, but, instead of this single step process, sometimes the vegetable oil is hydrolyzed in a first step by, for example, enzymatic hydrolysis or water vapor hydrolysis at high temperature and high pressure to free the fatty acids which are then converted to biodiesel by the esterification (see Scheme 1) reaction with methyl alcohol. However, this method is not generally preferred by industry.
One of the holistic effective ways for FOG management is biodiesel production by esterification/transesterification of fats. Since FOGs is rich in lipids, it is suggested as a cost-effective feedstock for biodiesel, which overcomes many economic disadvantages associated with the utilization of other feedstocks. FOG possesses various ranges of lipids and FFAs, with different biodiesel conversion technologies showing specificity towards the type of raw material for effective conversion. Thus, not all FOG constituents can be effectively converted into biodiesel using a single technology. For instance, only TGs are highly preferred raw material for conventional transesterification to attain the maximum biodiesel yield. However, some sources of FOG may contain up to 90% of FFAs which hinders the transesterification reaction [14,15,16,17].
27.3 Literature Review of Esterification-Transesterification Reaction of FOGs
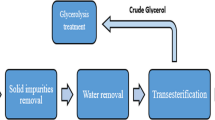
In this review we mostly focused on conversion of FFAs in fats, oils and grease (FOG) of wastewater, very little was known about FOG discharged at household level. To address this shortcoming, following a year-long monthly collection of household waste, FOG production was calculated at 2.3 kg/year per household, equivalent to 0.8 kg/year per capita in the United Kingdom, these numbers translate to an annual estimated household FOG production of 62,380 tonnes. Physico-chemical characterization of household FOG showed promising results for biodiesel production [14]. It can be summarized that the use of FOGs for biodiesel production also resolves the problems related to their discharge and complex contamination to the environment. Unfortunately, as discussed above FOGs normally contain large amounts of free fatty acids (FFA), which readily react with alkaline catalysts via saponification, thus lowering the biodiesel yield. Usually, a pre-esterification step is carried out to firstly convert FFAs to FAME with a homogeneous acid catalyst, and then transesterification is performed with alkaline catalyst. However, direct in situ transesterification refers to simultaneous conversion of FOG into biodiesel that was recently discussed as an alternative route to overcome the two-step conversion (See Fig. 27.2). The simultaneous conversion involves the reagents, catalyst and oil mixed directly without prior extraction [18, 19].
The skipping of the extraction step results in significant reduction in the energy consumption and total cost, as well as reduction of physical footprint [20]. Few studies have been performed to explore the feasibility of biodiesel production by the application of in situ transesterification [21, 22]. For example, the work done by Dehghani and Haghighi is shown in Fig. 27.3a, which summarizes the esterification and transesterification reaction of FOGs constituents for FAMEs formation. Si/Ce was used as a nano-catalyst and enhanced the conversion rate of the waste cooking oil into biodiesel significantly (Fig. 27.3b) [15] to about 94.3%, at the end of the seventh cycle the biodiesel conversion dropped to 88.7% conversion suggesting that the nano-catalyst could be re-used [15]. While, Fig. 27.3b illustrated that biodiesel conversion rate is increased extraordinarily as a consequence of Ce introduction into support structure. By increasing the Ce amount (decreasing Si/Ce ratio) to Si/Ce ratio of 10, the conversion increases steadily but for smaller Si/Ce ratios the conversion rate decreases significantly. So, among the synthesized catalysts, the best catalyst for biodiesel production is Mg/CeMCM-41 (Si/Ce = 10), with conversion percentage of 94.3%. According to an early investigation conducted by Tu et al. [22], the optimum operating conditions for in situ transesterification of FOG were 20% H2SO4 and 10:1 methanol: FOG at 65 °C for 7 h, whereby 85.43% of FOG in the raw sewer grease was converted to biodiesel.
The esterification and transesterification reaction for fat, oil, and grease (FOG) conversion into biodiesel (a) and conversion efficiency of yellow grease into biodiesel using different molar ratios of Si/Ce (0, 5, 10, 25, and 50) (b) [15]
In addition, Abbaszaadeh et al. [16] estimated the effectiveness of the thermally induced simultaneous esterification/transesterification of FOG samples to FAMEs through typical homogeneous acid (e.g., H2SO4) catalyzed reactions. Conventional H2SO4 catalyzed reaction produced FAMEs with 27.7% (from FOG-high) and 9.2% (from FOG-low) yields. These results indicated that it was difficult to convert the FOG to FAMEs by the conventional catalyzed methods [16]. Lee et al. also reported the thermally induced esterification/transesterification reaction at 340 °C for samples derived from FOG-high and FOG-low sources by conventional synthesis of FAMEs over an acidic homogeneous H2SO4 catalyst. The highest total FAME yield for FOG-high reached 83.4% at 380 °C at a methanol/feedstock ratio of 20 and H2SO4 to feedstock molar ratio was 1.3. A further increase in temperature from 380 to 390 °C led to a decrease in the yield from 83.4 to 78.7%. In contrast the highest FAME yield for FOG-low was 74.1% at 350 °C at the same methanol/feedstock ratio of 20. Again, an increase in temperature from 350 to 390 °C led to a decrease in the yield from 74.1 to 59.3%. They suggested that only 83.4% of the initial masses of FOG-high and 74.1% of FOG-low could be converted into FAMEs. FOG-high contains lipids (85.2 wt.%), FFAs (11.6 wt.%), and impurities (3.2 wt.%), and FOG-low contains lipids (76 wt.%), FFAs (9.9 wt.%), and impurities (14.1 wt.%). Impurities are not converted into FAMEs, meaning that they remain after the thermally induced simultaneous esterification/transesterification process. Taking into account the number of impurities in the feedstock, the total FAME yield from FOG-high and FOG-low would be 86.2% and 86.3%, respectively. Their observation suggested that thermally induced FAME production can be achieved via a single step by combining esterification and transesterification without removing impurities in FOGs [18].
The high lipid content contained in waste spent coffee grounds (SCG) was converted to biodiesel through an in-situ transesterification method by Tarigan et al. [3]. A new approach reactive extraction soxhlet (RES) method of simultaneously extracting and converting lipid from wet SCG biomass to biodiesel in a single-step process at a mild reaction temperature and short reaction time was proposed. Homogeneous sulphuric acid or sodium hydroxide with a concentration of 0.75 M were used as catalysts. The FA to FAME conversion efficiency was more than 90% using sodium hydroxide in methanol with hexane as co-solvent and a ratio of 1:2, and 30-min reaction time. The FA extraction efficiencies averaged 58.1 mol% ranging from 48.6 to 78.1 mol% [3]. The new approach of situ transesterification of wet SCG using RES method resulted in lower energy consumption and reaction time compared to the two-step method which requires a separate extraction and transesterification process [3]. In addition, Suryani et al. [23] developed an in-situ biodiesel transesterification production process using the residual oil from spent bleaching earth (SBE). The stirring speeds applied were 650 rpm and 730 rpm, and the reaction time varied from 60, 90 and 120 min. The combination of 730 rpm stirring speed for 90 min transesterification resulted in the best biodiesel characteristics with the yield of 85%, a specific energy of 6738 kJ/kg and a heater efficiency of 48% [23]. Endalew et al. [24] investigated mixtures of solid base (CaO and Li-CaO) and acid ((Fe2(SO4)3) heterogeneous catalyst for single-step simultaneous esterification and transesterification of high content free fatty acid (FFA) containing Jatropha curcas oil (JCO).
The reaction conditions used were: 60 °C reaction temperature, 3 h of reaction time, 6:1 molar-based alcohol to oil ratio, 5 wt.% catalyst (based on the amount of oil) and an agitation speed of 300 revolutions per min (rpm). Adjusting the CaO:Fe2(SO4)3 weight ratio to 3:1, the FAME yield was 93.37%, while for the Li-CaO catalyst gave a FAME yield of 96% with the same ratio [24]. Mixture of solid base catalysts (CaO and Li-CaO) and solid acid catalyst (Fe2(SO4)3) were found to give complete conversion to biodiesel in a single-step simultaneous esterification and transesterification process. Later, a new method for waste grease extraction (WGE) was developed, where yellow grease was mixed with raw sewer grease (3.15:1, w/w) at 70 °C for 240 min [25]. During the process, 100% of the FOG in the sewer grease was dissolved/extracted into the liquid yellow grease phase, which separated into two phases with the upper layer containing the FOG (see Fig. 27.4). This extraction method resulted in FFAs content increasing from 2.68 wt.% in the yellow grease to 8.48 wt.% in the extracted FOG, which can be converted directly into biodiesel by in situ transesterification. Using WGE for in situ conversion of FOG into biodiesel has several advantages comparing to the conventional methods. WGE avoids the drying of raw sewer grease that is necessary for many other conversion techniques including in situ transesterification. In addition, using yellow grease for WGE is cost-effective when compared with other techniques used for FOG separation from sewer grease, such as centrifugation. In situ transesterification contains fewer steps compared to other conversion methods and can achieve satisfactory results with FFAs-rich feedstocks. Therefore, it might reduce the complexity and capital investment of FOG conversion. However, methanol and H2SO4 inputs are significantly higher for in situ transesterification due to mass transfer limits, even though most of the methanol is recovered after conversion. Therefore, future research is needed in order to improve WGE and in situ transesterification through enhancement of extraction and conversion rates, respectively [25].
Moreover, Harvianto and Ulfasha [26] firstly performed the esterification (pre-treatment) using a volume ratio of methanol:FOG ratio of 0.09, at 70 °C for 180 min with 1.2 ml of 98% H2SO4 (10 wt.% of FFA). The amount of conversion in the esterification reaction was shown by the acid value at the end of the reaction. The lower the acid value the greater the conversion with the authors achieving a low value of 0.68 mg KOH/g acid. This was then followed by the transesterification reaction which was carried out with a methanol: FOG ratio of 0.26(v/v) at 70 °C for 30 min with 2.55 g of KOH catalyst [26]. The current study proposed a new approach for biodiesel synthesis from wet spent coffee grounds (SCGs) using 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) as both a green solvent and catalyst. The maximum biodiesel yield was 97.18%, with reaction condition a methanol amount of 6.25 mL/g of wet SCGs, DBU amount of 14.46 mL/g of wet SCGs, temperature of 60.2 °C, and reaction time of 28.65 min through response surface methodology (RSM) [27]. Author reported that DBU-catalyzed direct transesterification could be an economically feasible method for biodiesel synthesis from SCGs. This is due to reusability of DBU for 10 cycles at a lower temperature (60.2 °C) than does the conventional process (95 °C) [28].
Furthermore, this process is eco-friendly because it eliminates the use of harmful solvents and catalyst. Furthermore, an extensive literature review has been carried out in order to assess the advantages and disadvantages of the different methodologies in biodiesel production via catalytic esterification and transesterification (see Table 27.1). A summary of work performed so far shows that catalyst structure, morphology, texture, optimization and reaction parameters such as temperature, catalyst concentration, reaction time, alcohol to substrate molar ratio, type of alcohol have a significant influence on catalytic activity in biodiesel production. Despite a large number of studies carried out on the heterogeneous solid acid or base catalytic esterification/transesterification, there are still a number of drawbacks that hinder industrial application. Therefore, there is a need to develop cheaper more efficient solid base catalysts that are less energy demanding in terms of their process conditions and that have optimal lifetime stability.
27.4 Conclusions
This review has shown that the esterification/transesterification of high FFAs-lipid feedstocks from wastewater containing FOGs is a possible alternative route to biodiesel production as a renewable energy. Based on this literature review, a number of studies have been done on the esterification/transesterifications of FFAs and their transformation into fatty acid methyl esters (FAMEs) which is the main constituent of biodiesel. The yield of biodiesel depends on a number of parameters; such as catalyst concentration, catalyst type, and molar ratio of reactants, reaction temperature, and reaction time and optimization of these reaction conditions. The optimal temperature ranged between 60 and 70 °C, depending on the amount of free fatty acids that the oil contains and the molar ratio of alcohol to feedstock should be increased to between 6:1 up to 20:1 with the use of an acidic catalyst with concentration about 6 wt.% up to 10 wt.% for heterogeneous solid acidic catalysts and between 3 to 5%v/v for H2SO4, which is the most commonly used catalyst. Therefore, all reaction parameters are co-related to each other and all of them have significant influence on the reaction, therefore all parameters have to be optimized.
If heterogeneous acid catalysts could be as efficient in the esterification/transesterification of FOGs as it is with fresh oil such as vegetable oil, this would motivate its use in industry, even if catalyst production increases some costs but, at the same time, decreases the associated costs of catalyst separation and purification after the reaction and indeed can enable catalyst re-use. Future steps should include an analysis of heterogeneous catalyst usage in the transformation of FOGs into a biofuel.
References
W. Nabgan, A.A. Jalil, B. Nabgan, A.H. Jadhav, M. Ikram, A. Ul-Hamid, M.W. Ail, N.S. Hassan RSC, Sustainable biodiesel generation through catalytic transesterification of waste sources: a literature review and bibliometric survey RSC Adv. 12, 1604 (2022)
S.R. Medipally, F.M. Yusoff, S. Banerjee, M. Shariff, Microalgae as sustainable renewable energy feedstock for biofuel production. Biomed. Res. Int. 3, 519513 (2015)
J.B. Tarigan, M. Ginting, S.N. Mubarokah, F. Sebayang, J. Karo-karo, T.T. Nguyen, J. Ginting, E.K. Sitepu, Direct biodiesel production from wet spent coffee grounds. RSC Adv. 9, 35109 (2019)
S. Semwal, A.K. Arora, R.P. Badoni, D.K. Tuli, Review Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 102, 2151–2161 (2011)
M.K. Lam, K.T. Lee, A.R. Mohamed, Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 28, 500–518 (2010)
D. Samios, F. Pedrotti, A. Nicolau, Q.B. Reiznautt, D.D. Martini, F.M. Dalcin, A transesterification double step process-TDSP for biodiesel preparation from fatty acids triglycerides. Fuel Process. Technol. 90, 599–605 (2009)
C. Urrutia, N. Sangaletti-Gerhard, M. Cea, A. Suazo, A. Aliberti, R. Navia, Short communication: two-step esterification–transesterification process of wet greasy sewage sludge for biodiesel production. Bioresour. Technol. 200, 1044–1049 (2016)
A. Munyentwali, H. Li, Q. Qihua Yang, Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 633, 118525 (2022)
I. Istadi, S.A. Prasetyo, T.S. Nugroho, Characterization of K2O/CaO-ZnO Catalyst for Transesterification of Soybean Oil to Biodiesel. Procedia Environ. Sci. 23, 394–399 (2015)
M. Prabu, M. Manikandan, P. Kandasamy, P.R. Kalaivani, N. Rajendiran, T. Raja, Synthesis of biodiesel using the Mg/Al/Zn hydrotalcite/SBA-15 nanocomposite catalyst. ACS Omega 4, 3500–3507 (2019)
G. Lawer-Yolar, B. Dawson-Andoh, E. Atta-Obeng, Synthesis of biodiesel from tall oil fatty acids by homogeneous and heterogeneous catalysis. Sustain. Chem. 2(1), 206–221 (2021)
J.M. Marchetti, A.F. Errazu, Short communication esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides, Biomass Bioenergy 32, 892–895 (2008)
Z. Khan, F. Javed, Z. Shamair, A. Hafeez, T. Fazal, A. Aslam, W.B. Zimmerman, F. Rehman, Current developments in esterification reaction: a review on process and parameters, J. Ind. Eng. Chem. 103, 80–101 (2021)
T. Collin, R. Cunningham, M. Deb, R. Villa, J. MacAdam, B. Jefferson, Energy potential of household fats, oils and grease waste, Water Environ. J. , 36, 1–8 (2022). Retreived from https://doi.org/10.1111/wej.12744
S. Dehghani, M. Haghighi, Sono-dispersed MgO over cerium-doped MCM-41 nanocatalyst for biodiesel production from acidic sunflower oil: Surface evolution by altering Si/Ce molar ratios. Waste Manag. 95, 584–592 (2019)
A. Abbaszaaseh, B. Ghobadian, M.E. Omidkhah, G. Najafi, Current biodiesel production technologies: a comparative review, Energy Convers. Manag. 63, 138–148 (2012)
R.A. Ahmed, S. Rashid, K. Huddersman, Esterification of stearic acid using novel protonated and crosslinked amidoximated polyacrylonitrile ion exchange fibres. J. Ind. Eng. Chem. 119, 550–573 (2023)
J. Lee, J.-M. Jung, C. Park, B.-H. Jeon, C.-H. Wang, S.-R. Lee, E.E. Kwon, Rapid conversion of fat, oil and grease (FOG) into biodiesel without pre-treatment of FOG. J. Cleaner Prod. 168, 1211–1216 (2017)
R. Ahmed, K. Huddersman, Review of biodiesel production by the esterification of wastewater containing fats oils and grease (FOGs). J. Ind. Eng. Chem. 110, 1–14 (2022)
O. Aboelazayem, M. Gadalla, B. Saha, Biodiesel production from waste cooking oil via supercritical methanol: Optimisation and reactor simulation. Renew. Energy 124, 144–154 (2018)
O.K. Choi, J.S. Song, D.K. Cha, J.W. Lee, Biodiesel production from wet municipal sludge: Evaluation of in situ transesterification using xylene as a cosolvent. Bioresour. Technol. 166, 51–56 (2014)
Q. Tu, University of Cincinnati, 2015. https://doi.org/oai:etd.ohiolink.edu: ucin1448
A. Suryani, Z. Mubarok Suprihatin, M. Romli, E.N. Yunira, Process design of in situ esterification-transesterification for biodiesel production from residual oil of spent bleaching earth (SBE). IOP Conf. Series: Earth. Environ. Sci. 65, 012040 (2017)
A.K. Endalew, Y. Kiros, R. Zanzi, Heterogeneous catalysis for biodiesel production from Jatropha curcas oil (JCO). Energy 36(5), 2693–2700 (2011)
Q. Tu, J. Wang, M. Lu, A. Brougham, T. Lu, A solvent-free approach to extract the lipid fraction from sewer grease for biodiesel production, Waste Manag. 54, 126–130 (2016)
G.R. Harvianto, H.N. Ulfasha, Utilization of Fats, Oils, and Greases (FOG) from Restaurant Waste for Biodiesel Production. Int. J. Environ. Bioenergy 3(1), 56–66 (2012)
H.C. Nguyen, M.L. Nguyen, F.-M. Wang, H.-Y. Juan, C.-H. Sub, Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Bioresour. Technol. 296, 122334 (2020)
J. Park, B. Kim, J.W. Lee, In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresour. Technol. 221, 55–60 (2016)
I. Thushari, S. Babel, Short communication sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour. Technol. 248, 199–203 (2018)
E. Babayigit, A.D. Atik, A. Erdincler, Direct liquid–liquid lipid extraction method for biodiesel production from sewage and petrochemical industry sludges. Waste Biomass Vaporization 9, 2471–2479 (2018)
J. Qi, F. Zhu, X. Wei, L. Zhao, Y. Xiong, X. Wu, F. Yan, Comparison of biodiesel production from sewage sludge obtained from the A2 /O and MBR processes by in situ transesterification. Waste Manag. 49, 212–220 (2016)
M. Olkiewicz, A. Fortuny, F. Stüber, A. Fabregat, J. Font, C. Bengoaa. Evaluation of different sludges from WWTP as a potential source for biodiesel production. Procedia Eng. 42, 634–643 (2012)
A. Mondala, K. Liang, H. Toghiani, R. Hernandez, T. French, Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour. Technol. 100, 1203–1210 (2009)
C. Pastore, A. Lopez, V. Lotito, G. Mascolo, Biodiesel from dewatered wastewater sludge: A two-step process for a more advantageous production. Chemosphere 92(6), 667–673 (2013)
N. Tran, P. Ho, P. Hall, E. McMurchie, J. Ngothai, Extraction of fats, oil and grease from grease trap waste for biodiesel production. Multidisc. Eng. Sci. Stud. 3, 1853–1859 (2017)
Acknowledgements
This work is supported by funding from the co-sponsors Daphne Jackson Trust, Society of Chemists in Industry (SCI) and Royal Society of Chemistry (RSC), and hosted at De Montfort University, Leicester.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Ahmed, R.A., Huddersman, K. (2023). Short Review of Biodiesel Production by the Esterification/Transesterification of Wastewater Containing Fats Oils and Grease (FOGs). In: Nixon, J.D., Al-Habaibeh, A., Vukovic, V., Asthana, A. (eds) Energy and Sustainable Futures: Proceedings of the 3rd ICESF, 2022. ICESF 2022. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-031-30960-1_27
Download citation
DOI: https://doi.org/10.1007/978-3-031-30960-1_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-30959-5
Online ISBN: 978-3-031-30960-1
eBook Packages: EnergyEnergy (R0)