Abstract
Novel insights in the development of polymer coating of mild steel using functionalization of rice husk ash nanoparticles by activated orange juice were investigated. For the potentiostat/galvanostat corrosion performance of the coated materials, 5 wt% potassium sulfate (K2SO4(aq), 5 wt% sodium chloride (NaCl(aq), and 1 M sulfuric acid (H2SO4(aq) were used. 78.81, 71.86, and 55.11% corrosion resistance of the samples of K2SO4, H2SO4, and NaCl. It was concluded that orange juice was able to enhance the dispersion of RHnp in the epoxy coating. The presence of citrate ions in the orange juice acts as a stabilizer and reducing agent, which was attributed to the fine grain size and good corrosion resistance of the composite coating. The work has established that rice husk ash nanoparticles by activated orange juice can be used in the development of composites coating mild steel.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The epoxy coating of mild steel has been used in recent times to combat the high steel in concrete [1]. The good corrosion resistance, poor electrical conductivity, and ease of application are the reasons behind the usage of epoxy coating in concrete [2]. However, the properties of the coating may not withstand service conditions such as wear, marine, or seawater corrosion, as a result of the hydrophilic feature of epoxy, which weakens the coating [3, 4]. Hence, an effort has been made by the researcher to add second phase nanoparticles to overcome this great problem. Tianhao Ge et al. [5] reported on the microstructural and electrochemical behavior of an epoxy-grapheme-zinc multilayer coating. Xianming et al. [6] reported on the corrosion resistance and mechanical properties of an epoxy coating of mild steel with Fe2O3, SiO2, Zn, and clay nanoparticles. They all concluded that incorporation of nanoparticles into epoxy results in clustering and agglomeration of particles [7] and that functionalization and blending treatment of the nanoparticles can reduce the limitations [8]. For example, organic and inorganic compounds such as polyaniline emeraldine salt and aminopropyltriethoxysilane have been used to functionalize nanowhiskers/epoxy coating of mild steel by Cleide Borsoia et al. [9].
The researcher has shown that the inorganic and organic compounds are not eco-friendly and costly, which hindered the wide usage of these compounds in the functionalization of nanoparticles for mild steel. Effort was made in this work to develop a reducing agent using plant extract (orange juice) [10] because it has been reported by Aigbodion et al. [11] that orange juice contains phenolic compounds (ferulic, hydroxybenzoic, hesperidin, hydroxycinnamic, ascorbic, and citric) that can be used as functionalization of nanoparticles for epoxy coating of mild steel [12]. This work used 5 wt% potassium sulfate (K2SO4(aq), 5 wt% sodium chloride (NaCl(aq), and 1 M sulfuric acid (H2SO4(aq) for corrosion performance, as a continuation of the author’s and co-worker’s work.
2 Materials and Method
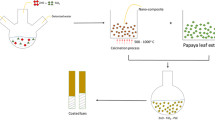
Mild steel of 0.15%C composition was grounded with a series of grit papers (240–1200PC) and inserted in a 2.9 wt.% hexafluorozircoic acid solution for a period of 1 h. The orange fruit was washed, cleaned, and pasteurized for 2 h at a temperature of 85oC, and the orange juice was then activated in steam at 65oC [12]. The sol-gel method was used in the production of the rice husk nanoparticles (RHnp) using a 35% sodium hydroxide solution. Details of the production procedure are discussed elsewhere [12]. LY556 (HERENBA BRAND) Epoxy and HY951 Hardener were used in the production of the epoxy coating. The modification of the epoxy was done using fluorinated poly (aryl ether ketone) (FPEK). The modified epoxy: hardener ratio of 2: 1 and 2%RHnp was used in the production of the epoxy composite coating. The epoxy was then mixed with 10ml of orange juice to help disperse the RHNP. A spray pyrolysis machine operating at 250rpm was used to deposit the mixture on the mild steel surface. The coated sample was cured on the laboratory floor for 24 h. The particle size of the RHnp was determined using the transmission electron microscope model (JEOL JSM840A). The microstructure was done using TESCAN SEM. A new corrosion tester model CHI604E was used for the electrochemical analysis at potentials of −1.5V to 1.5V and 0.0012V s-1. The chloride-based road salt, heavy industrial pollution conditions, and heavy rain conditions were used in this study with 5 wt% potassium sulfate (K2SO4(aq), 5 wt% sodium chloride (NaCl(aq), and 1 M sulfuric acid (H2SO4(aq). The polarization resistance was computed using Eq. 1 [2].
βa = Tafel anodic constant, βc = cathodic Tafel constant
3 Results and Discussion
Figure 1 displays the TEM image of the RHnp. It was observed that segregation and particle clustering were not seen in the TEM image. However, random distribution of fine particles was observed these were attributed to the reducing ability of the orange juice that prevents particle clusters. A similar observation was obtained in the work of [4]. An average particle size of 31.94–70.99 nm was obtained.
Figure 2 shows the results of the corrosion analysis. It was observed that the coated samples shifted the potential to a more positive one. There is a very significant difference in the Tafel plot of the coated and that of the mild steel samples. The coated samples’ potentials were shifted to higher potentials and lower current density. The coated 5% NaCl sample has a higher potential and hence a lower corrosion rate. The mild steel samples have the lowest potential of all the samples. It was observed that the %wt%K2SO4 (aq) has a higher tendency of corrosion as a result of the lower potential, but the developed composite has a higher potential and shifted the Tafel curves to the right in the three media under investigation. The higher rate of corrosion experience for the mild steel sample could be attributed to the fact that iron oxidation and change in pH values are the major factors affecting the corrosion behavior of this cast iron. At work, corrosion rates of 3200, 2015 and 1016 mpy were obtained for the substrate, while the coated sample corrosion rates of 678, 567 and 456 mpy were obtained for K2SO4, H2SO4 and NaCl, which corresponded to 78.81, 71.86 and 55.11%.
Figure 3 displays the corroded surfaces of the samples. There was a great difference in the corroded surface of the mild steel as compared with that of the coated samples. The formation of passive films was noticed in coated samples. The passive films covered the sample surface and increased the potential of the composite sample to lower degradation by corrosion. However, the composite sample in 5wt%NaCl has the higher passive film. This is the main reason the composite sample in 5wt%NaCl has the higher corrosion resistance due to the high amount of corrosion product in the form of nodules as evidenced.
4 Conclusions
New insights in using orange juice as a reducing agent to functionalize the rice husk ash nanoparticle for epoxy coating of mild steel have been experimentally determined in this work. It was concluded that orange juice was able to enhance the dispersion of RHnp in the epoxy coating. 78.81, 71.86, and 55.11% corrosion resistance of the samples of K2SO4, H2SO4, and NaCl 2wt%. The work finds out that the formation of nanostructure and functionalization with orange juice helped increase the purity of the silica content of the ramp. The presence of citrate ions in the orange juice acts as a stabilizer and reducing agent, which was attributed to the fine grain size and good corrosion resistance of the composite coating.
References
Ramezanzadeh, B., Arman, S.Y., Mehdipour, M.: Anticorrosion properties of an epoxy zinc-rich composite coating reinforced with zinc, aluminum, and iron oxide pigments. J. Coatings Technol. Res. 11(5), 727–737 (2014)
El Sockary, M.A., et al.: A new double layer epoxy coating for corrosion protection of petroleum equipments. Int. J. Eng. Res. Appl. 65–73 (2014)
Yu, H.J., Wang, L., Shi, Q., Jiang, G.H., Zhao, Z.R. and Dong, X.C.: Dong study on nano-CaCO3 modified epoxy powder coatings. Prog. Organic Coating 55, 296–3000 (2006)
Xia, S.Q., Wang, L.G., Wang, E.Z., Yue, X., et al.: Study on the wear resistance of Al2O3 particles reinforced epoxy resin composite coating. In: Advanced Materials Research, vol. 821–822, pp. 1148–1151 (2013)
Ge, T., et al.: Design alternate epoxy-reduced graphene oxide/epoxy-zinc multilayer coatings for achieving long-term corrosion resistance for Cu. Mater. Des. 186, 108299 (2020)
Shi, X., Nguyen, T.A., Suo, Z., Liu, Y., Avci, R.: Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 204, 237–245 (2009)
Pham, G.V., Trinh, A.T., To, T.X.H., Nguyen, T.D., Nguyen, T.T., Nguyen, X.H., et al.: Incorporation of Fe3O4/CNTs nanocomposite in an epoxy coating for corrosion protection of carbon steel. Adv. Nat. Sci.: Nanosci. Nanotechnol. 5, 035016 (2014)
Bakhshandeha, E., Jannesaria, A., Ranjbarb, Z., Sobhania, S.: Mohammad Reza Saebaa, Anti-corrosion hybrid coatings based on epoxy–silica nano-composites: toward relationship between the morphology and EIS data. Prog. Org. Coat. 77, 1169–1183 (2014)
Borsoia, C., Scienzab, L.C., Zatterac, A.J.: carlos arthur ferreira effect of the incorporation of micro and nanocellulose particles on the anticorrosive properties of epoxy coatings applied on carbon steel. Mater. Res. 21(6), e20170269 (2018)
Valença, D.P., Alves, K.G.B., Melo, C.P.D., Bouchonneau, N.: Study of the efficiency of Polypyrrole/ZnO nanocomposites as additives in anticorrosion coatings. Mater. Res. 18 (supl.2), 273–278 (2015)
Aigbodion, V.S.: Explicit simultaneous enhancement of adhesion strength and wear resistance of functional value-added epoxy-functionalized rice husk ash nanoparticle composite coating. Int. J. Adv. Manuf. Technol. 109(7–8), 2205–2214 (2020). https://doi.org/10.1007/s00170-020-05758-0
Jha, A.K., Kumar, V., Prasad, K.: Biosynthesis of metal and oxide nanoparticles using orange juice. J. Bionanosci. 5, 162–166 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Aigbodion, V.S., Ozor, P.A. (2023). New Insights into the Polymer Coating of Mild Steel Using Activated Orange Juice Functionalized Rice Husk Nanoparticles. In: Kohl, H., Seliger, G., Dietrich, F. (eds) Manufacturing Driving Circular Economy. GCSM 2022. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-28839-5_115
Download citation
DOI: https://doi.org/10.1007/978-3-031-28839-5_115
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28838-8
Online ISBN: 978-3-031-28839-5
eBook Packages: EngineeringEngineering (R0)