Abstract
The theranostic concept to use diagnostic and therapeutic nuclides to image and treat cancer was established many years ago for thyroid disease. With the success of [177Lu]-labeled therapeutic agents targeting neuroendocrine tumors or prostate cancer, the interest for this concept has been significantly rising. The possibility to localize and quantify a therapeutic target within the patient opens up novel possibilities, but also challenges for patient selection, dosimetry, and response assessment. Especially for response assessment we however, still rely on the morphologic changes. With the increasing use of immunotherapy and other biological approaches, image interpretation needs a profound understanding of the therapeutic mechanism and impact on imaging parameters for accurate assessment of tumor response and guidance for appropriate therapy selection.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
To understand the theranostic concept and the potential differences between alpha and beta therapy.
-
To know about the advantages and limitations of receptor PET-based patient selection.
-
Review the developing role of PET-based response assessment.
-
To learn about the evidence, pearls and pitfalls of morphology-based response assessment.
-
To introduce the role of combining DWI with receptor targeted PET in patient evaluation.
3.1 Introduction
With the introduction of the combination of anatomical imaging with CT and molecular imaging based on positron emission tomography (PET) in the early 2000, there was a steady increase in clinical applications in oncology. The most commonly used tracer is fluorodeoxyglucose [18F]-FDG, taken up by cells with high expression of insulin-independent glucose transporter (GLUT1, 2, or 3), if the patient was fasting for at least 4 h. GLUT expression, however, is also increased in a variety of inflammatory lesions, reducing the specificity. Despite intensive research for a more specific tracer for malignant tissue compared to FDG [1, 2] the high sensitivity was still not reached by any other approach for lymphomas and most solid tumors. Only for tumors with a usually low metabolic activity (neuroendocrine tumors (NET) and prostate cancer (PCa)), alternative approaches based on receptor-based imaging have been established: targeting the prostate-specific membrane antigen (PSMA) for PCa and somatostatin receptor 2 (SSTR-2) imaging for NET. These targets are currently also in the focus of a theranostic approach; we would like to focus in the first part of this chapter. The second part will be focused on response assessment with both morphologic and molecular imaging in general and for specific therapeutic approaches.
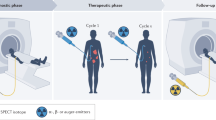
3.2 Introduction to Theranostics
Theranostics refers to the use of molecules for both imaging and therapy. Although this was originally developed over 75 years ago with the introduction of iodine therapy (I-123 for imaging and I-131 for treatment), it was the development of SSTR-2 targeted imaging agents and subsequent therapy, which led to the renewed focus on radioligand therapies (RLTs). In general, most theranostic agents have a ligand that targets a protein overexpressed on the surface of a tumor. These ligands are then labeled with either a radionuclide used for imaging or a radionuclide used for therapy. The most common imaging radionuclides used clinically for theranostics are positron emitting (β+) used in PET and include fluorine-18, gallium-68, and copper-64. Although gamma emitters (γ) can also be used, including technetium-99m and indium-111, the field has focused on PET radiopharmaceuticals. Radionuclides for therapy are broken down into alpha (α) and beta (β−) emitters. Beta emitters, which emit an electron, include yttrium-90, lutetium-177, and iodine-131. Alpha emitters, which emit a helium atom, include actinium-225, lead-212, and radium-223.
Theranostics is considered a personalized therapy, as patients are selected using the imaging study to demonstrate the presence of the target, before the treatment is administered. This allows one to have some understanding of how patients will respond to treatment. For example, in patients with prostate cancer, higher uptake on the pre-treatment PSMA PET have a better response to PSMA-targeted RLT [3].
3.2.1 Therapeutic Radionuclides
There are many factors that influence which radionuclide groups use for RLT, including half-life, chemistry, and the distance the emitted particle travels. Having a half-life that enables central synthesis and distribution is critical for commercialization and is why radionuclides such as astatine-211, with its 7 h half-life, is not frequently utilized. Lutetium-177 has a 6.6-day half-life, allowing for central synthesis. The distance the emitted particle is also important and impacts treatment efficacy. Yttrium-90 emits a beta particle that travels over 1 cm, while the lutetium-177 beta particle travels between 1 and 2 mm. This means that yttrium-90 would be better at treating larger lesions than lutetium-177 and may explain the higher rate of renal toxicity with yttrium-90 that has been reported [4]. Alpha particles have a very high energy but deposit their doses over a much shorter distance, typically 50–70 micrometers. This shorter range has led some to believe that alpha particles might be able to kill single cells, but it must be remembered that the majority of the energy is deposited at the Bragg peak, at the end of the path, so the deposited energy is mostly 1 to 2 cells away from the cell of origin. Nonetheless, there is considerable interest in alpha particles given their potential for higher efficacy due to the high energy deposition [5,6,7]. There is currently one Phase III study evaluating [225Ac]-DOTATATE in patients with gastroenteropancreatic (GEP) neuroendocrine tumors (NETs) (NCT05477576).
Key Points
The currently most commonly used radionuclide for RLT is Lutetium-177—a beta emitting nuclide with a half-life of 6.6 days. Yttrium-90, also a beta emitter, has a higher energy and could be used for larger lesions, or targets with more distance (spheres). And Actinium-225 is an alpha emitting nuclide, with the potential of higher efficacy but also more potential side effects.
3.2.2 Unique Role of Dosimetry
One unique aspect of many RLTs is that they can be directly imaged after treatment in order to measure the actual dose deposited in the tumors. Although this is also true for I-131 in thyroid cancer, it was performed infrequently with thyroid patients. Of particular interest is lutetium-177, which has a 12% gamma emission, allowing for high quality post-treatment images. As software is developed that makes quantifying dosimetry more streamlined, it is hoped that this approach will be incorporated into clinical practice. Currently, post-treatment imaging is used mostly qualitatively to determine if there has been evidence of progression or response, rather than to modulate administered activity based on measured dosimetry from the previously administered cycle. It should be noted that alpha emitters such as actinium-225 are difficult to image using SPECT/CT due to their low gamma emissions and much lower administered activities. There is significant work underway to try to improve our ability to image alpha emitters.
3.2.3 Current Theranostic Agents and Agents in Development
There are two major applications for theranostics in clinic today: [177Lu]-DOTATATE (Lutathera, Novartis) and [177Lu]-PSMA-617 (Pluvicto, Novartis) RLT. [177Lu]-DOTATATE targets the SSTR in patients with NETs and is used in patients who progress after somatostatin analog therapy. [177Lu]-DOTATOC is a nearly identical agent, which is currently being evaluated in two Phase III trials (NCT03049189 and NCT04919226). Yttrium-90 labeled compounds have been evaluated but have been largely replaced with lutetium-177 labeled compounds. [177Lu]-PSMA-617 targets PSMA in patients with metastatic castration-resistant prostate cancer (mCRPC) and was shown to prolong life in patients after chemotherapy and androgen receptor targeted therapies in the VISION trial [8]. [177Lu]-PSMA-I&T is a similar compound currently being evaluated in two Phase III studies in the pre-chemotherapy mCRPC setting (NCT05204927 and NCT04647526). [177Lu]-PSMA-617 is also being evaluated in the metastatic castration-sensitive setting (NCT04720157).
There are a number of exciting targets outside of PSMA which are currently being evaluated. Radioligands targeting the fibroblast activation protein (FAP) are of interest, as the target is expressed across multiple cancer types rather than being specific to a cancer like SSTR and PSMA. There is limited evidence of efficacy to date with small retrospective series published to date [9, 10]. [177Lu]-FAP-2286 is being evaluated in a dose expansion Phase I trial (NCT04939610). Other targets in evaluation includes the gastrin-releasing peptide receptor [11], C-X-C motif chemokine receptor 4 (CXCR4) [12, 13] and urokinase-type plasminogen activator receptor (uPAR) [14]. It is unclear if any of these compounds will have the clinical impact of SSTR and PSMA-targeted RLTs, but there is considerable investment being made in the field.
3.2.4 Patient Selection for Internal Radiotherapy
The use of the targeted imaging to select patients for RLT has been adopted very early on. The NETTER trial that lead to the approval for [177Lu]-DOTATATE for midgut NET selected patients with well-differentiated tumors Grade 1-2 and high uptake based on SSTR-scintigraphy [15]. The concensus statement by the North American Neuroendocrine Tumor Society (NANETS) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) agreed that positivity on SSTR imaging is defined as an intensity in uptake in sites of disease that exceeds the normal liver for both imaging modalities [111In]-pentetreotide single-photon emission computer tomography (SPECT) or [68Ga]-DOTATATE PET [16]. However, already for neuroendocrine disease several authors suggested that imaging the target is fundamental, but not enough [17]. Tumor heterogeneity and dedifferentiation in the course of a malignant disease lead to tumor parts that lost the expression of the target, and therefore cannot be detected by the receptor PET. Therefore, a combination with FDG PET/CT is suggested, in patients with only faint uptake or negative lesions on [68Ga]-DOTATATE [18].
Also the VISION trial leading to the approval for [177Lu]-PSMA-617 used the corresponding [68Ga]-PSMA-11 PET scan for patient selection [8]. Eligible patients had PSMA-positive metastatic lesions and no PSMA-negative lesions; PSMA-positive status was defined as uptake greater than that of liver parenchyma in one or more metastatic lesions of any size in any organ system. Given that based on these criteria only 12% of the patients were excluded due to PET criteria, the discussion was opened, if the costs of [68Ga]-PSMA-11 PET are justified and needed, if the patient exclusion is so low. On the contrary, given that bone metastasis are often not well seen on CT, the additional value of FDG PET/CT might even be higher for PCa than NET patients. Preliminary results for studies selecting patients based on [68Ga]-PSMA-11 and FDG PET showed indeed a slightly higher PSA response rate compared to the VISION trial (TheraP: 65%, compared to VISION: 46%) [19]. Follow-up data on the TheraP trial now published further support that patients with an SUVmean ≥10 on PSMA PET had an excellent response to [177Lu]-PSMA-617, while patients with FDG active disease larger than ≥200 mL did worse in both arms (Cabazitaxel and [177Lu]-PSMA-617), indicating that the combination of metabolic and receptor PET information might be a good way to tailor therapy intensity in the future [3].
3.3 Monitoring Disease
The World Health Organization (WHO) recognized the need for standardized criteria across clinical trials very early, publishing the initial “WHO handbook for reporting results of cancer treatment” in 1979 [20]. Since then, a number of guidelines and updates have been published.
3.3.1 Response Based on Morphology
The most commonly applied response criteria for systemic disease are Response Evaluation Criteria In Solid Tumors (RECIST) that have been updated to RECIST 1.1 [21]. Based on the changes of target and non-target lesions patients are categorized in four groups: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (Table 3.1). To simplify readouts only five target lesions (TL), maximum two in the same organ, are assessed by the maximum diameter. Lymph nodes are measured by the short axis and need to be larger than 1.5 cm to be considered as target lesions. Sclerotic bone lesions are considered non-target lesions (NTL), as well as cystic lesions, if they do not have large solid components. The sum of all diameters from TLs need to decrease more than 30% to be considered as a partial response or increase by 20% (more than 5 mm) to indicate progressive disease.
3.3.2 Response Based on Morphology for Immunotherapy
Chemotherapies directly kill tumor cells leading to shrinkage of tumor volume depending on the efficacy of the therapy. Immunotherapies activate the immune system of the patient with different response patterns depending on the mechanism of drug action [22]. Morphologic response after immunotherapy will often need more time compared to conventional therapies due to an initial increase in volume due to infiltration of inflammatory cells into the tumor (Fig. 3.1).
For accurate interpretation of the response pattern, understanding of the drug mechanism is crucial. Therapeutics leading to an increasing migration of T-cells into the tumor (CTLA-4 blocking, e.g., Ipilimumab) will be more likely to have an initial increase in tumor size [23]. iRECIST is based on RECIST 1.1 to select TL and NTL, if however PD is seen on first follow-up, this is interpreted as iUPD (Unconfirmed Progressive Disease), only if tumor increase of more than 30% is confirmed on the second follow-up (4–8 weeks later) a confirmed progression iCPD will be noted (Fig. 3.2). If the tumor remains stable, iUPD will remain the interpretation and only if criteria for partial or complete response are met, iPR or iCR will be given [22].
Maximum intensity projection (MIP) images in a patient with melanoma treated with nivolumab. Baseline imaging demonstrates a peritoneal nodal that resolves at the first time point (black arrow, a and b). A new lesion is seen at the 3-month PET (b, red arrow) which is consistent with unconfirmed progressive disease (iUPD). All diseases resolve on subsequent imaging (c, d). Note that the patient developed immune-related colitis (b, white arrowhead)
Key Points
Initial progression after start of immunotherapy has to be confirmed after 4–8 weeks to distinguish pseudoprogression from confirmed progression.
Besides accurate interpretation of early pseudoprogression, the recognition of immune-related response (irR) is crucial as well. Inflammatory changes can result in activation of sarcoidosis with enlarged lymph nodes (Fig. 3.3) or adrenalitis with enlarged suprarenal glands. This is not only challenging for morphologic imaging but also for PET/CT since irR can show intensive 18F-fluorodeoxyglucose (FDG) uptake [23]. Immunotherapies are already a clinically established option for patients with melanoma or lung cancer. However, more indications also for abdominal diseases will follow soon, such as prostate cancer (Sipuleucel-T), kidney cancer (nivolumab), or bladder cancer (pembrolizumab).
Maximum intensity projection (MIP) images and axial fused images through the mid abdomen in a patient with Mantle Cell Lymphoma treated with pembrolizumab. Baseline imaging demonstrates splenomegaly and a large abdominal mass. Three months after treatment initiation, the has been resolution of the abdominal mass and the splenomegaly, and development of numerous mediastinal hypermetabolic nodes consistent with a sarcoid-like reaction
3.3.3 Response Based on FDG PET
Numerous publications showed a good correlation between the decrease in [18F]-FDG accumulation in tumor lesions and therapy response [24]. Therefore, PET response evaluation was postulated, including EORTC PET response recommendations (1999) and the PET response criteria in solid tumors (PERCIST) pioneered by Wahl et al. in 2009 [25]. Both methods follow the model of RECIST with four adapted response categories: complete metabolic response (CMR), partial metabolic response (PMR), stable metabolic disease (SMD), and progressive metabolic disease (PMD). Numerous studies compared the original EORTC PET criteria with PERCIST showing similar results (Table 3.2). However, the use of PERCIST seems preferable for clinical trials due to a better standardization [26]. Both methods recommend the use of standardized uptake values (SUV) normalized to the lean body mass (SUL). However, if possible PERCIST favors the use of an SULpeak (average value in the hottest 1cm3 sphere within the tumor), instead of SULmax (only one maximum voxel) to reduce the intrinsic variability.
Despite all these efforts, one has to recognize that PERCIST assessment has not been used to evaluate response in clinical trials yet. As a primary or secondary endpoint for prospective studies, we still rely on morphologic imaging.
It is important to note that all these recommendations are focusing on [18F]-FDG PET for the evaluation of metabolic response of solid tumors. Tumor dedifferentiation does not correlate with receptor expression in the same way like [18F]-FDG. Therefore, PERCIST per se is not necessarily applicable for the increasing use of receptor imaging in oncologic diseases, for example, SSTR PET for NETs or PSMA for prostate cancer.
3.4 Monitoring Liver Disease After SIRT
Selective internal radiotherapy (SIRT) using yttrium-90 resins or glass microspheres is an increasingly used palliative therapy option for patients with non-resectable primary liver tumors or metastatic hepatic disease. Clinical evaluation of patients prior to SIRT includes contrast-enhanced CT of the chest and the abdomen to rule out extensive extrahepatic disease, liver MRI to assess tumor burden and selective angiography to evaluate vascular anatomy and hepatic shunt with technetium-99m labeled aggregated macroalbumin [99mTc]-MAA) [27, 28].
3.4.1 Monitoring SIRT with CT/MRI
Early response assessment after SIRT using anatomical imaging can be limited due to delayed reduction in tumor size and initial pseudoprogression due to edema and sharper demarcation of tumor boundaries on conventional imaging (Fig. 3.4). Early investigations with serial ceCT images showed a maximum decrease in tumor size at 3–21 months (median 12 months), concluding that blood tumor markers (e.g., CEA for colorectal metastasis) were superior in therapy response assessment compared to ceCT [28].
The calculation of arterial perfusion (AP) using dynamic contrast-enhanced CT prior to SIRT was the best predictor for good treatment response and overall survival in patients [29]. Furthermore, follow-up perfusion CT showed a significant decrease of AP in hepatic metastasis 4 weeks after SIRT in patients with long-term response, compared to non-responders [30]. Dual-energy CT is a new technology, allowing the creation of iodine maps as promising tools to evaluate and quantify tumor viability, utilizing iodine maps measuring the amount of iodine per lesion. Although this approach requires validation and standardization, it showed promising first results for hepatic radiofrequency ablation [31] and could also be a promising tool for SIRT therapy response assessment.
Functional MRI was suggested to be used to assess response to SIRT, as well. A post-therapeutic increase in ADCmin of more than 22%, 4 weeks after SIRT was significantly associated with a superior overall survival (18 vs. 5 months, p < 0.001), while tumor size did not show any significant decrease after 4 weeks [32].
3.4.2 Monitoring SIRT with PET
Early on FDG PET was used to assess the reduction of hepatic metastatic load after SIRT [33]. Such studies displayed superior early response assessment with FDG PET/CT compared to ceCT [34, 35]. Other groups investigated the PET-based volume-metrics such as the metabolic tumor volume (MTV) or the total lesion glycolysis rate (TLG) and came to the conclusion that those parameters correlate better with outcome compared to plain tumor size or SUVmax[36]. For accurate response assessment with FDG PET potential pitfalls have to be considered. To name the two most prominent limitations: False-negative results caused by partial volume effects in small lesions (<1 cm) or diabetes and false-positive results caused by abscess formation or inflammatory changes [37]. However, a combination of FDG PET/CT with ceCT can solve most of these limitations.
Despite the fact that earlier prediction of response to SIRT with FDG PET is possible, there is no established clinical role for FDG PET in assessing tumor response after SIRT. This might be attributable to a lack of additional treatment options. Therefore, early knowledge of a limited response to this palliative therapy will change treatment only in very few cases. Nevertheless, it is crucial to know the potential limitations and possibilities of the various imaging modalities to prevent false interpretation of early morphologic changes in the accurate judgment of therapeutic response.
3.5 Monitoring Neuroendocrine Tumors
NETs are a heterogeneous tumor type classified based on their grade and their site of origin. Grade is broken into three categories: Grade 1 referring to well-differentiated tumors with a Ki-67 less than 2, Grade 2 for well-differentiated tumors with a Ki-67 between 3 and 20, and Grade 3 for poorly differentiated tumors with a Ki-67 greater than 20. Recently, Grade 3 has been broken down into well differentiated and poorly differentiated tumors. The most common site of origin for NETs are the small bowel and the pancreas. Prior to the approval of [177Lu]-DOTATATE, somatostatin analogs (SSAs), and everolimus were used for small bowel NETs, and SSAs, everolimus, sunitinib, and capecitabine/temozolomide for pancreatic NETs. Everolimus is also indicated for the treatment of bronchial NETs. It should be noted that in all clinical trials for NETs, the primary endpoint has been progression-free survival using RECIST-based evaluation.
The NETTER-1 trial investigated the efficacy of peptide receptor radionuclide therapy (PRRT) with [177Lu]-DOTATATE compared to 60 mg of sandostatin. The primary endpoint was progression-free survival where was defined as primary endpoint documented with either CT or MRI. An increase of progression-free survival fraction from 10.8% in the sandostatin arm to 65.2% with [177Lu]-DOTATATE was observed at 20 months. One important issue with NETs is that the disease can be slow growing, particularly in Grade 1 and 2 patients, and therefore the detection of small changes in tumor volume can be clinically significant although hard to tell. Because of the slow growth in disease, some groups have started to suggest replacing RECIST with tumor growth rate as an endpoint [38]. Due to the slow rate of growth, using the most reproducible imaging modality available is important, and for patients with liver dominant disease hepatobiliary phase MRI is the optimal imaging modality [39].
Similar to SIRT and immunotherapies, the morphologic response can lag significantly behind physiologic changes. Additionally, pseudoprogression can confound interpretation due to edema and improved tumor delineation of liver lesions. This is crucial since progression under therapy is a potential reason to stop current treatment according to the European Neuroendocrine Tumor Society (ENETS) consensus guidelines. Therefore, a harmonization and combination of anatomical and molecular imaging as well as biomarkers was suggested for monitoring PRRT [40]. No standardized procedures have been established yet on how to interpret molecular imaging (e.g., 68Ga-DOTATATE PET/CT) results after PRRT. Only one publication summarizing results of 33 patients undergoing early therapy response assessment with 68Ga-DOTATATE after 1 cycle of PRRT has been published so far. They came to the conclusion that a decrease of the tumor to spleen ratio (SUVT/S) correlated well with progression-free survival (p = 0.002), while a decrease in SUVmax did not reach significance [41] (Fig. 3.5). Therefore at this time, evaluation of response is still base on cross-sectional imaging, and SSTR PET can be acquired 9–12 months after the completion of treatment as a new baseline [16, 42].
3.6 Monitoring Metastasized Prostate Cancer
3.6.1 Conventional Monitoring of Metastasized Prostate Cancer with CT and Bone Scans
Imaging response assessment for prostate cancer is notoriously difficult on anatomical imaging and therefore plays only secondary role for treatment evaluation in new drug trials that commonly focus on overall survival (OS) as primary endpoint instead and for early stages now metastasis-free survival (MFS) as a primary endpoint [43, 44]. For standardized response assessment, the Prostate Cancer Clinical Trials Working Group 3 (PCWG-3) published an updated version in 2016 [45]. The recommendations for extraskeletal disease are still in line with the RECIST 1.1 criteria, with the exception that up to 5 lesions per site (e.g., lung, lymph node, liver) should be measured to address the disease heterogeneity. Lymph nodes are considered measurable with a short axis of ≥1.5 cm and visceral lesions have to be ≥1.0 cm to be considered target lesions. Lymph nodes between 1 and 1.5 cm can be considered malignant but only as non-target lesions. Given that sclerotic lesions can increase under therapy and persist for a long time on CT only lytic lesions ≥1.0 cm can be considered target lesions based on CT. To assess response, bone scans are incorporated into the PCWG-3 assessment. Given that healing bone metastases will initially react with increasing mineralization (Fig. 3.6). This will therefore also increase the activity on 99mTc-bone scans or 18F-NaF PET/CT, the so called tumor flare, typically lasting for 3 months after therapy but can be seen as late as 6 months after treatment [46]. An initial increase in lesions after 8 weeks on a new therapy is therefore interpreted as a bone flare and progression on bone scans defined only after confirmation of at least 2 new lesions twice (2+2 rule).
Key Points
Sclerotic bone lesions on CT should not be considered as target lesions for response assessment. New sclerotic lesions after therapy change are not considered progressive disease. A confirmation is needed due to the flair phenomenon.
Based on analysis of the data from the PREVAIL trial investigating the effect of enzalutamide in chemotherapy naïve man, Rathkopf et al. not only demonstrate the robustness of the PCWG criteria but also a high positive correlation between rPFS and OS 0.89 by Spearman ρ [47]. Based on this data, several trials now use rPFS based on the PCWG-3 criteria as their primary endpoints including studies for Lu-PSMA-617 [8].
3.6.2 Monitoring Metastasized Prostate Cancer with PET/CT
The use of FDG PET/CT is very limited in prostate cancer patients since only a small subgroup of highly dedifferentiated tumors will have increased glucose uptake. Assessment of nodal, visceral, and osseous metastasis with one exam is possible with 18F or 11C-Choline or 68Ga-prostate-specific membrane antigen (PSMA) PET/CT. A good correlation between apoptosis and decrease in SUV on 11C-Choline PET/CT scans was shown after neoadjuvant docetaxel chemotherapy and complete androgen blockade in locally advanced prostate cancer patients [48]. The prospective use of Choline PET/CT for response assessment of standardized docetaxel first-line chemotherapy on the other hand showed no correlation between changes in Choline uptake and clinical assessments of progression based on RECIST 1.1 and PSA values [49].
The clinical utility of 68Ga-PSMA PET for treatment response is not established, yet (Fig. 3.7). First investigations showed promising results using 68Ga-PSMA PET to evaluate 223Ra therapy response [50]. The use of 68Ga-PSMA PET for response assessment to androgen-deprivation therapy (ADT) will need careful prospective evaluation since preliminary invitro results showed that ADT is increasing the cellular expression of PSMA; therefore, a novel “tumor flare” might be observed in these patients [51]. With RECIP 1.0 based on the appearance of new lesions and the change in PSMA-positive tumor volume (+20% for DP and -30% for PR), a simple algorithm was proposed incorporating PSA and PSMA PET information to improve response assessment [52].
3.7 The Role of MRI and PET/MRI for Response Evaluation
There has been historically limited interest in the combination of diffusion-weighted imaging (DWI) and FDG PET in the setting of PET/MRI as restricted diffusion often mirrors elevated metabolism as measured on PET. Therefore when performing an FDG PET/MRI, there is little value in performing DWI. In the setting of theranostics, patients are imaged with targeted radiopharmaceuticals as described above, for example, PSMA- and SSTR-targeted agents. In this setting, there can be additional information from DWI that is not captured with the PET imaging. Additionally, NETs and mCRPC is heterogeneous in its hypermetabolism and diffusion restriction; therefore, MRI can detect disease that is not visualized on PET. For example, in Fig. 3.8, this patient with well-differentiated Grade 3 NET has extensive liver disease that is not FDG positive.
The same is true in prostate cancer. Figure 3.9 shows a patient with innumerable liver metastases imaged using PET/MRI. The lesions have uptake less than the background liver, but are clearly seen on DWI, and so the patient is not a good candidate for PSMA RLT. This is an important approach for patient selection, as patients with PSMA-negative disease can be missed if only PSMA PET is used for screening. The TheraP trial used the combination of FDG and PSMA PET and excluded patients who had FDG-positive/PSMA-negative disease [19]: TheraP a randomized, open-label, Phase II trial. However, it is not always possible to image patients with both PSMA and FDG PET in order to select patients, and therefore an alternative that allows one to visualize PSMA-negative disease is needed. DWI imaging may be able to help replace FDG PET in order to find PSMA-negative disease.
Although this case demonstrates that DWI can detect PSMA-negative disease, the liver is not the main issue, as cross-sectional imaging can easily evaluate for liver metastases. Much more clinically relevant is bone disease. In PSMA PET, it is difficult to determine if a bone lesion is expressing low levels of PSMA (i.e., PSMA negative) or if the bone lesion has been treated and has a low cellularity. The prior would portend a poor outcome, while the latter would not. In Fig. 3.10, you can see how DWI can detect PSMA-negative disease in the bones that would otherwise not be able to be detected.
PSMA PET/MRI in a 59-year-old man being evaluated for PSMA RLT. The PSMA PET demonstrates PSMA avid lung disease, but the whole-body DWI demonstrates numerous additional sites of metastatic disease that are not PSMA avid. There is PSMA-negative disease immediate anterior to the liver that markedly restricts diffusion (solid red circle). In particular, note how DWI can detect osseous metastases that is only minimally positive on PSMA PET (dotted red circle)
Key Points
DWI can be a very helpful tool to determine PSMA-negative disease. Especially for bone lesions, this could be of significant added value since the vitality of these lesions can’t be evaluated on CT.
What may end up being most valuable in terms of using diffusion-weighted imaging for imaging of patients with theranostics is the ability to follow patients over time. In many centers, it is not feasible to image patients using PSMA- or SSTR-PET at multiple timepoints, and moreover, this approach would miss PSMA-negative disease that might develop. In this setting, following patients using whole-body MRI may be preferred. The METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) has already been developed to use whole-body DWI as response criteria [53]. In addition to assessing anatomic changes for nodal and visceral lesions similar to RECIST, MET-RADS-P incorporates WB DWI as a key imaging technique for evaluating bone response, which has been limited using conventional imaging (e.g., CT and bone scan) [54]. The combined use of targeted PET and whole-body DWI holds significant promise for both patient selection and response assessment in patients undergoing RLT.
3.8 Concluding Remarks
With the introduction of hybrid imaging, the use of different PET tracers to assess metabolism or receptor expression has expanded a lot. The potential to use the same molecules in a second step not only for diagnosis but also therapy is currently generating a novel momentum. The optimal combination of morphologic and molecular information for patient selection for therapy as well as monitoring disease has still to be determined. A profound understanding of the tumor biology as well as imaging possibilities is key for optimal patient care.
Take-Home Messages
-
Systemic therapy response assessment with imaging is generally based on morphology. With the introduction of non-cytotoxic therapies, response patterns became very heterogeneous.
-
Especially immunotherapy or local therapy of liver metastasis such as SIRT can lead to an initial pseudoprogression as a part of the response pattern with delayed morphologic response.
-
In analogy to RECIST, response criteria have been proposed for FDG PET/CT (PERCIST) and shown to correlate with outcome. PERCIST might be a good surrogate marker for response, however until now no clinical prospective trials are based on FDG PET/CT for solid tumors.
-
Morphologic assessment of bone metastasis is only possible for lytic lesions with a soft-tissue component. New sclerotic lesions on CT are not necessarily a sign of tumor progression but might be a sign of response of previously occult lesions.
References
Hicks RJ. Beyond FDG: novel PET tracers for cancer imaging. Cancer Imaging. 2003;4:22–4. https://doi.org/10.1102/1470-7330.2003.0032.
Burger IA, Zitzmann-Kolbe S, Pruim J, Friebe M, Graham K, Stephens A, et al. First clinical results of (D)-18F-Fluoromethyltyrosine (BAY 86-9596) PET/CT in patients with non-small cell lung cancer and head and neck squamous cell carcinoma. J Nucl Med. 2014;55:1778–85. https://doi.org/10.2967/jnumed.114.140699.
Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022; https://doi.org/10.1016/S1470-2045(22)00605-2.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. https://doi.org/10.1007/s00259-014-2893-5.
Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with (225)Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934–46. https://doi.org/10.1007/s00259-019-04567-2.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. https://doi.org/10.2967/jnumed.117.203539.
Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving (225)Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–9. https://doi.org/10.2967/jnumed.119.229229.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–103. https://doi.org/10.1056/NEJMoa2107322.
Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using (177)Lu-FAP-2286: first-in-humans results. J Nucl Med. 2022;63:415–23. https://doi.org/10.2967/jnumed.120.259192.
Fendler WP, Pabst KM, Kessler L, Fragoso Costa P, Ferdinandus J, Weber M, et al. Safety and efficacy of 90Y-FAPI-46 radioligand therapy in patients with advanced sarcoma and other cancer entities. Clin Cancer Res. 2022;28:4346–53. https://doi.org/10.1158/1078-0432.CCR-22-1432.
Dalm SU, Bakker IL, de Blois E, Doeswijk GN, Konijnenberg MW, Orlandi F, et al. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J Nucl Med. 2017;58:293–9. https://doi.org/10.2967/jnumed.116.176636.
Buck AK, Grigoleit GU, Kraus SK, Schirbel A, Heinsch M, Dreher N, et al. C-X-C motif chemokine receptor 4-targeted radioligand therapy in patients with advanced T-cell lymphoma. J Nucl Med. 2022; https://doi.org/10.2967/jnumed.122.264207.
Merkx RIJ, Rijpkema M, Franssen GM, Kip A, Smeets B, Morgenstern A, et al. Carbonic anhydrase IX-targeted alpha-radionuclide therapy with 225Ac inhibits tumor growth in a renal cell carcinoma model. Pharmaceuticals (Basel). 2022:15. https://doi.org/10.3390/ph15050570.
Persson M, Juhl K, Rasmussen P, Brandt-Larsen M, Madsen J, Ploug M, et al. uPAR targeted radionuclide therapy with (177)Lu-DOTA-AE105 inhibits dissemination of metastatic prostate cancer. Mol Pharm. 2014;11:2796–806. https://doi.org/10.1021/mp500177c.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. https://doi.org/10.1056/NEJMoa1607427.
Hope TA, Bodei L, Chan JA, El-Haddad G, Fidelman N, Kunz PL, et al. NANETS/SNMMI consensus statement on patient selection and appropriate use of (177)Lu-DOTATATE peptide receptor radionuclide therapy. J Nucl Med. 2020;61:222–7. https://doi.org/10.2967/jnumed.119.240911.
Zhang J, Liu Q, Singh A, Schuchardt C, Kulkarni HR, Baum RP. Prognostic value of (18)F-FDG PET/CT in a large cohort of patients with advanced metastatic neuroendocrine neoplasms treated with peptide receptor radionuclide therapy. J Nucl Med. 2020;61:1560–9. https://doi.org/10.2967/jnumed.119.241414.
Kaewput C, Vinjamuri S. Role of combined (68)Ga DOTA-peptides and (18)F FDG PET/CT in the evaluation of gastroenteropancreatic neuroendocrine neoplasms. Diagnostics (Basel). 2022:12. https://doi.org/10.3390/diagnostics12020280.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
WHO handbook for reporting results of cancer treatment; 1979.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e52. https://doi.org/10.1016/S1470-2045(17)30074-8.
Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44:67–77. https://doi.org/10.1007/s00259-017-3691-7.
Rymer B, Curtis NJ, Siddiqui MR, Chand M. FDG PET/CT can assess the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy: evidence from meta-analysis and systematic review. Clin Nucl Med. 2016;41:371–5. https://doi.org/10.1097/RLU.0000000000001166.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. https://doi.org/10.2967/jnumed.108.057307.
Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44:55–66. https://doi.org/10.1007/s00259-017-3687-3.
Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg. 2001;5:294–302.
Boppudi S, Wickremesekera SK, Nowitz M, Stubbs R. Evaluation of the role of CT in the assessment of response to selective internal radiation therapy in patients with colorectal liver metastases. Australas Radiol. 2006;50:570–7. https://doi.org/10.1111/j.1440-1673.2006.01630.x.
Morsbach F, Pfammatter T, Reiner CS, Fischer MA, Sah BR, Winklhofer S, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol. 2013;48:787–94. https://doi.org/10.1097/RLI.0b013e31829810f7.
Reiner CS, Morsbach F, Sah BR, Puippe G, Schaefer N, Pfammatter T, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014;25:747–59. https://doi.org/10.1016/j.jvir.2014.01.025.
Lee SH, Lee JM, Kim KW, Klotz E, Kim SH, Lee JY, et al. Dual-energy computed tomography to assess tumor response to hepatic radiofrequency ablation: potential diagnostic value of virtual noncontrast images and iodine maps. Invest Radiol. 2011;46:77–84. https://doi.org/10.1097/RLI.0b013e3181f23fcd.
Schmeel FC, Simon B, Sabet A, Luetkens JA, Traber F, Schmeel LC, et al. Diffusion-weighted magnetic resonance imaging predicts survival in patients with liver-predominant metastatic colorectal cancer shortly after selective internal radiation therapy. Eur Radiol. 2017;27:966–75. https://doi.org/10.1007/s00330-016-4430-3.
Wong CY, Qing F, Savin M, Campbell J, Gates VL, Sherpa KM, et al. Reduction of metastatic load to liver after intraarterial hepatic yttrium-90 radioembolization as evaluated by [18F]fluorodeoxyglucose positron emission tomographic imaging. J Vasc Interv Radiol. 2005;16:1101–6. https://doi.org/10.1097/01.RVI.0000168104.32849.07.
Annunziata S, Treglia G, Caldarella C, Galiandro F. The role of 18F-FDG-PET and PET/CT in patients with colorectal liver metastases undergoing selective internal radiation therapy with yttrium-90: a first evidence-based review. ScientificWorldJournal. 2014;2014:879469. https://doi.org/10.1155/2014/879469.
Szyszko T, Al-Nahhas A, Canelo R, Habib N, Jiao L, Wasan H, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun. 2007;28:15–20. https://doi.org/10.1097/MNM.0b013e328011453b.
Fendler WP, Philippe Tiega DB, Ilhan H, Paprottka PM, Heinemann V, Jakobs TF, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med. 2013;54:1202–8. https://doi.org/10.2967/jnumed.112.116426.
Dierckx R, Maes A, Peeters M, Van De Wiele C. FDG PET for monitoring response to local and locoregional therapy in HCC and liver metastases. Q J Nucl Med Mol Imaging. 2009;53:336–42.
Dromain C, Pavel ME, Ruszniewski P, Langley A, Massien C, Baudin E, et al. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer. 2019;19:66. https://doi.org/10.1186/s12885-018-5257-x.
Morse B, Jeong D, Thomas K, Diallo D, Strosberg JR. Magnetic resonance imaging of neuroendocrine tumor hepatic metastases: does hepatobiliary phase imaging improve lesion conspicuity and interobserver agreement of lesion measurements? Pancreas. 2017;46:1219–24. https://doi.org/10.1097/MPA.0000000000000920.
Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology. 2017; https://doi.org/10.1159/000475526.
Haug AR, Auernhammer CJ, Wangler B, Schmidt GP, Uebleis C, Goke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–56. https://doi.org/10.2967/jnumed.110.075002.
Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74. https://doi.org/10.2967/jnumed.117.202275.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. https://doi.org/10.1056/NEJMoa1014618.
Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. https://doi.org/10.1056/NEJMoa1213755.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92.
Rathkopf DE, Beer TM, Loriot Y, Higano CS, Armstrong AJ, Sternberg CN, et al. Radiographic progression-free survival as a clinically meaningful end point in metastatic castration-resistant prostate cancer: the PREVAIL randomized clinical trial. JAMA Oncol. 2018;4:694–701. https://doi.org/10.1001/jamaoncol.2017.5808.
Schwarzenbock SM, Knieling A, Souvatzoglou M, Kurth J, Steiger K, Eiber M, et al. [11C]Choline PET/CT in therapy response assessment of a neoadjuvant therapy in locally advanced and high risk prostate cancer before radical prostatectomy. Oncotarget. 2016;7:63747–57. https://doi.org/10.18632/oncotarget.11653.
Schwarzenbock SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, et al. Prospective evaluation of [11C]Choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2105–13. https://doi.org/10.1007/s00259-016-3439-9.
Bieth M, Kronke M, Tauber R, Dahlbender M, Retz M, Nekolla SG, et al. Exploring new multimodal quantitative imaging indices for the assessment of osseous tumour burden in prostate cancer using 68Ga-PSMA-PET/CT. J Nucl Med. 2017; https://doi.org/10.2967/jnumed.116.189050.
Meller B, Bremmer F, Sahlmann CO, Hijazi S, Bouter C, Trojan L, et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015;5:66. https://doi.org/10.1186/s13550-015-0145-8.
Gafita A, Rauscher I, Weber M, Hadaschik B, Wang H, Armstrong WR, et al. Novel framework for treatment response evaluation using PSMA-PET/CT in patients with metastatic castration-resistant prostate cancer (RECIP 1.0): an international multicenter study. J Nucl Med. 2022; https://doi.org/10.2967/jnumed.121.263072.
Padhani AR, Lecouvet FE, Tunariu N, Koh DM, De Keyzer F, Collins DJ, et al. METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol. 2017;71:81–92. https://doi.org/10.1016/j.eururo.2016.05.033.
Yoshida S, Takahara T, Ishii C, Arita Y, Waseda Y, Kijima T, et al. METastasis reporting and data system for prostate cancer as a prognostic imaging marker in castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18:e391–e6. https://doi.org/10.1016/j.clgc.2019.12.010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Burger, I.A., Hope, T.A. (2023). Advances in Molecular Imaging and Therapy and Its Impact in Oncologic Imaging. In: Hodler, J., Kubik-Huch, R.A., Roos, J.E., von Schulthess, G.K. (eds) Diseases of the Abdomen and Pelvis 2023-2026. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-031-27355-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-27355-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27354-4
Online ISBN: 978-3-031-27355-1
eBook Packages: MedicineMedicine (R0)