Abstract
Purpose
The objective of this study was to investigate and present the early results on the efficacy, safety, and quality of life of 225Ac-DOTATATE targeted alpha therapy (TAT) in patients with advanced, progressive, 177Lu-DOTATATE refractory, and somatostatin receptor (SSTR) expressing metastatic GEP-NETs.
Methods
In this prospective study, we recruited patients with metastatic GEP-NETs who were stable or progressive disease on 177Lu-DOTATATE therapy. Systemic TAT using 225Ac-DOTATATE was performed in all the patients with 225Ac-DOTATATE (100 kBq/kg body weight) at an interval of 8 weeks. The primary end point was to assess the objective response (measured by RECIST 1.1 and functional M.D. Anderson criteria). The secondary end points included biochemical response assessment as per the Italian Trials in Medical Oncology (ITMO), adverse event profile as per CTCAE v5.0, and clinical response assessment by the quality of life (assessed with EORTC QLQ-GI.NET21 patient-based questionnaire).
Results
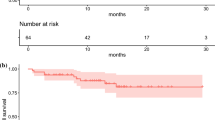
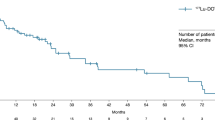
Between April 2018 and March 2019, 32 patients (17 females, 15 males, mean age 52 ± 9.2 years, 35–72 years) with either stable disease after completing 177Lu-DOTATATE therapy (14, 44%) or progressive disease on 177Lu-DOTATATE therapy (18, 56%) were included in the study. The morphological response was assessed in 24/32 patients that revealed partial remission in 15 and stable disease in 9. There was no documented disease progression or deaths in the median follow-up of 8 months (range 2–13 months). There was a significant decrease in the plasma chromogranin level post-225Ac-DOTATATE therapy (P < 0.0001).
Conclusion
Our short-term clinical results indicate 225Ac-DOTATATE TAT as a promising treatment option which adds a new dimension in patients who are refractory to 177Lu-DOTATATE therapy or have reached the maximum prescribed cycles of 177Lu-DOTATATE therapy.





Similar content being viewed by others
References
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. PROMID Study Group. A placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumour growth in patients with metastatic neuroendocrine midgut tumours: a report from the PROMID Study Group. J Clin Oncol 2009;7:4656–4663.
Delavault P, Caplin ME, Liyanage N, Blumberg J. The CLARINET study: assessing the effect of lanreotide autogel on tumour progression-free survival in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumours. J Clin Oncol. 2017;30(15_suppl). https://doi.org/10.1200/jco.2012.30.15_suppl.tps4153 Published online January 31, 2017.
Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–77.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumours. N Engl J Med. 2011;364:501–13.
Yao JC. Neuroendocrine tumours. molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2017;21:163–72.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumours. N Engl J Med. 2017;376:125–35.
Kim SJ, Pak K, Koo PJ, Kwak JJ, Chang. The efficacy of 177Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:1964–70.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35.
Ballal S, Yadav MP, Damle NA, Sahoo RK, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine tumours: a long-term-outcome, toxicity, survival, and quality-of-life study. Clin Nucl Med. 2017;42:e457–e66. https://doi.org/10.1097/RLU.0000000000001816.
Navalkissoor S, Grossman A. Targeted alpha particle therapy for neuroendocrine tumours: the next generation of peptide receptor radionuclide therapy. Neuroendocrinology. 2019;108:256–64.
Nayak TK, Norenberg JP, Anderson TL, Prossnitz ER, Stabin MG, Atcher RW. Somatostatin- receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(−)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl Med Biol. 2007;34:185–93.
Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R, et al. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumours. Clin Cancer Res. 2008;14:3555–61.
Norenberg JP, Krenning BJ, Konings IR, Kusewitt DF, Nayak TK, Anderson TL, et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumours in a preclinical animal model. Clin Cancer Res. 2006;12:897–903.
Chan HS, Konijnenberg MW, de Blois E, Koelewijn S, Baum RP, Morgenstern A, et al. Influence of tumour size on the efficacy of targeted alpha therapy with (213)Bi- [DOTA(0), Tyr(3)]-octreotate. EJNMMI Res. 2016;6:6.
Chan HS, Konijnenberg MW, Daniels T, Nysus M, Makvandi M, de Blois E, et al. Improved safety and efficacy of 213Bi-DOTATATE- targeted alpha therapy of somatostatin receptor expressing neuroendocrine tumours in mice pre-treated with L-lysine. EJNMMI Res. 2016;6:83.
Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–19.
Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, et al. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69:8941–8.
Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumours. J Nucl Med. 2009;50(Suppl 1):122S–50S. https://doi.org/10.2967/jnumed.108.057307.
Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumours in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Fayers PM, Aaronson NK, Bjordal K. On behalf of the EORTC Quality of Life Group et al. The EORTC QLQ-C30 scoring manual (3rd edition) Brussels: European Organisation for Research and Treatment of Cancer; 2001.
King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–67.
Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17:1008–19.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical clearance
Ethical clearance received Ref. No. IEC-517.
Informed consent
Informed consent obtained from all patients.
Disclaimer
This work has not been submitted elsewhere as a full article or has not under consideration to any other journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
The work has been presented as an oral presentation at the SNMMI 2019 and was nominated for the Oncology: Clinical Therapy and Diagnosis - Center for Therapy Excellence YIA Symposium. It was judged and received the first place for the Young Investigator Award Center for Therapy Excellence.
Sanjana Ballal, Madhav Yadav, and Chandrasekhar Bal. Early results of 225Ac-DOTATATE Targeted Alpha Therapy in Metastatic Gastroenteropancreatic Neuroendocrine Tumours: First Clinical Experience on Safety and Efficacy. J Nucl Med 2019 60:74
Rights and permissions
About this article
Cite this article
Ballal, S., Yadav, M.P., Bal, C. et al. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging 47, 934–946 (2020). https://doi.org/10.1007/s00259-019-04567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04567-2