Abstract
Immunotherapy is a relatively new treatment in the armamentarium of cancer treatment and has proven effective even in advanced stages. Immunotherapy utilizes the body’s own immune system to elicit a response against tumor cells. Response to immunotherapy can be different on imaging when compared with cytotoxic chemotherapy, since an effective immune response against a tumor can lead to an initial increase in size, termed pseudoprogression. Additionally, immune-response adverse events can occur which include colitis, enteritis, hepatitis, pneumonitis, pancreatitis, hypophysitis, and other inflammatory responses. The increasing use of immunotherapy requires the radiologist to be cognizant of this while assessing treatment response and possible immune-response adverse events.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Immunotherapy
- Response assessment
- iRECIST
- Toxicity
- Immune-related adverse events
- Colitis
- Pneumonitis
- Sarcoid-like reaction
-
Understand the mechanisms of action of immunotherapy agents
-
Recognize typical and atypical patterns of response to immunotherapy agents
-
Recognize the complications of immunotherapy agents
12.1 Introduction
Immunotherapy has emerged as a major advance in the treatment of cancer. Immunotherapy utilizes the body’s own immune system to target cancer cells and has proven effective against a variety of cancer subtypes, including melanoma, renal cell carcinoma, lung cancer, breast cancer, and lymphomas, to name a few. In this chapter, we will discuss the mechanism of action of common immunotherapy drugs, their response assessment, and adverse events.
12.2 Mechanism of Action
12.2.1 Cancer Immunity Cycle
Genetic alterations in cancer cells should, in theory, make them susceptible to attack by immune cells; however, cancer cells evade immune attack by producing certain surface proteins (such as PD-1) that inhibit T cells [1]. The creation of immune response against cancer cells requires a series of events, called the cancer immunity cycle. The major steps involved in this process include the release of antigens from dying cancer cells and their capture by the dendritic cells (antigen-presenting cell), presentation of the cancer antigens by the dendritic cells to T cells resulting in activation of effector T cells against cancer, infiltration of the tumor by activated effector T cells, recognition of the cancer antigen by the T cells and T cell binding, and finally killing the target cancer cell. Dying cancer cells release additional tumor-associated antigens leading to wider immune activation against cancer. However, the cancer immunity cycle may not be effective due to failure of any of the above steps, most importantly because tumor microenvironment may suppress effector T cells.
12.2.2 Goal of Immunotherapy
The goal of cancer immunotherapy is to create a robust and self-sustaining cancer immunity cycle without creating an unchecked autoimmune inflammatory response against the host cells. This is achieved by selectively targeting certain steps of the cycle without amplifying the entire cycle.
12.2.3 Anti-CTLA-4 Antibodies
Anti-CTLA-4 (Cytotoxic T-Lymphocyte Antigen-4) antibodies, such as Ipilimumab, target the T cell activation step within lymph nodes. CTLA-4 on the T cell surface is a major negative regulator of T cells. If unchecked, it binds with B7 on the antigen-presenting cell and leads to inhibition of T cell response. Anti-CTLA-4 antibodies block this interaction of CTLA-4 and lead to T cell activation (Fig. 12.1). This also helps explain the higher incidence of immune-related toxicities with anti-CTLA-4 antibodies. The activation of T cells is not necessarily limited against tumor-specific antigens. Lack of selectivity in T cell activation, combined with the fundamental importance of CTLA-4 as an immune checkpoint, leads to significant immune-related toxicities with agents such as ipilimumab [2].
Schematic showing the interaction between B7 on the antigen-presenting cell and CD28 on T cell leading to T cell activation (left), CTLA-4 on the T cell inhibiting this interaction leading to T cell inhibition (middle), and anti-CTLA-4 antibody (such as ipilimumab) blocking CTLA-4 leading to T cell activation (right)
12.2.4 PD-L1 and PD-1 Inhibitors
PD-L1 (Programmed death-ligand 1) is an immune modulator expressed in 20%–50% of human cancers, which binds to PD-1(Programmed cell death protein 1) on effector T cells, leading to blockade of cytotoxic mediators needed to kill the cancer cells. This is one of the most important mechanisms the cancer cells use to evade immune response. Agents that block PD-L1 on cancer cells (such as Atezolizumab, Avelumab, and Durvalumab) or PD-1 on T cells (such as Nivolumab and Pembrolizumab) restore antitumor immune response of effector T cells and result in excellent immune response (Fig. 12.2). Furthermore, acting within the tumor microenvironment, the PD-L1 and PD-1 inhibitors are more specific to cancer cells, and are thus associated with fewer and milder immune-related toxicities.
Key Point
Differences in the mechanism of action of anti-CTLA-4 antibodies and PD-L1/PD-1 inhibitors explain their different toxicity profiles. Anti-CTLA-4 antibodies lead to a more global activation of the immune system, leading to more frequent and severe toxicities, whereas PD-L1/PD-1 inhibitors are more specific to the tumor and therefore have fewer and milder toxicities.
12.2.5 Combination Immunotherapy
The approaches mentioned above, namely anti-CTLA-4 antibodies and PD-L1/PD-1 inhibitors are just two of many possible strategies to create immune response. Combining different agents acting at different steps of the cancer immunity cycle can create a more robust anticancer immune response, leading to potentially higher efficacy and frequency of response; however, such combination therapies may also be associated with a higher rate of toxicities.
Key Point
Once the immune system is activated, the response of immunotherapy agents is often durable, lasting even after the treatment is discontinued.
12.2.6 CAR T-Cell Therapy
Chimeric antigen receptor–engineered (CAR) T cell therapy is a novel form of immunotherapy predominantly used for hematologic malignancies. To date, there are four FDA-approved CAR T cell therapies. CAR T cell therapy involves harvesting of patient’s own T cells. These T cells are modified using viral vectors to express artificial chimeric antigen receptors that can recognize tumor-associated antigens. Patient is given chemotherapy to temporarily deplete the patient’s native lymphocytes, followed by infusion of CAR T cells. These cells then bind to the tumor-associated antigens thus activating an immune response [3].
12.3 Tumor Response to Immunotherapy
12.3.1 Development of Standards for Assessing Cancer Treatment Response
Imaging is important in assessing tumor response to treatment, of which contrast-enhanced CT plays a dominant role. The change in tumor burden with treatment, when compared to a baseline scan, is used as a surrogate for survival and quality of life [4]. Efforts in the 1980s by the World Health Organization (WHO) sought to standardize the process for assessing treatment response with bidimensional measurement of target lesions [5]. This was an important step since it allowed for data from multiple institutions to be compared in a reproducible and uniform fashion.
Around 2000, an international group called the Response Evaluation Criteria in Solid Tumors (RECIST), looking at 4500 patients from 14 clinical trials, simplified the process of standardized measurements and advocated for the use of unidimensional measurements of target lesions [6]. In 2009, RECIST 1.1 was developed which relies on the sum of unidimensional measurements of up to five target lesions (with a maximum of two per organ) [7]. Baseline CTs are performed less than 4 weeks prior to treatment. Target lesions are measured in longest dimension and have to be ≥10 mm. However, for lymph nodes as target lesions these need to be ≥15 mm in short-axis dimension. The sum of diameters of the target lesions is compared to a subsequent exam that is generally not less than 6–8 weeks after treatment. If all target lesions have disappeared and all measured lymph nodes are <10 mm in short-axis dimension, the treatment is designated as “complete response.” If there is at least a 30% reduction in the sum of diameters, the therapy elicited a “partial response.” “Progressive disease” occurs when there is at least a 20% increase in the size of the sum of diameters or the appearance of one or more lesions. Finally, if there is neither sufficient shrinkage or enlargement to meet partial response or progressive disease criteria, it is designated as a “stable disease.”
12.3.2 Limitations of RECIST 1.1 in Assessing Immunotherapy Response
The WHO, RECIST 1.0, and RECIST 1.1 were all developed to assess the response to cytotoxic therapy, in which tumor shrinkage correlates with increased survival. Immunotherapy poses a challenge to current tumor assessment criteria since its mechanism of action results in different imaging characteristics. Tumors treated with immunotherapy can show an initial increase in size and can take longer to shrink compared with cytotoxic drugs.
Key Point
Additionally, infiltration by immune cells with robust response to immunotherapy can lead to increased size of the lesions (termed pseudoprogression) despite a robust response.
Cases that were classified by RECIST 1.1 as “progressive disease” have been shown to be “stable disease,” “partial response,” or “complete response” when carried out more longitudinally [8]. Designating response as “progressive disease” when it is actually responding can lead to inappropriate, premature cessation of treatment.
12.3.3 Development of Immunotherapy-Specific Response Standards
In 2009, in response to these concerns, a revised version of the WHO criteria (using bi-dimensional measurements) was proposed, termed the immune-related response criteria (irRC). This system allowed for new lesions to be included in the sum of diameters calculation as well as the need for a confirmatory scan for patients with “progressive disease” to performed ≥4 weeks later. In 2013, subsequent recommendations incorporated elements of RECIST 1.1 into immune therapy (number of target lesions and use of unidimensional measurements) and termed irRECIST. However, these were inconsistently applied, and the lack of standardization led the RECIST working group to create iRECIST in 2017, which is a modified version of RECIST 1.1 [9].
Key Point
iRECIST follows RECIST 1.1 with a new category termed “unconfirmed progressive disease” (iUPD) in cases where the subsequent examination seems to meet RECIST 1.1 criteria for “progressive disease.” In such cases, a repeat scan is performed at 4–8 weeks, and if the sum of the dimensions continues to be 20% or greater from baseline or if there are new metastases, “confirmed progressive disease” (iCPD) is assessed [9].
12.4 Immune-Related Adverse Events (irAEs)
12.4.1 Overview
Immune-related adverse events (irAEs) are immunologic “flare” phenomenon. Clinically, irAEs have been reported in up to 72% of patients with high-grade toxicities in 24% of patients. On imaging, irAEs may be seen in up to 31% of patients with ipilimumab (anti-CTLA-4 antibody) and 14% of patients with nivolumab (anti-PD-1 agent); however, the actual frequency may vary based on the cancer population and the exact drugs used [10, 11]. The most common toxicities seen on imaging are colitis, pneumonitis, and sarcoid-like reaction (Table 12.1). irAEs are often mild and treatment can be continued if the patient tolerates it; however, when severe, they are treated with steroids and may necessitate treatment discontinuation.
12.4.2 Colitis
Immune-mediated colitis is the most common irAE, often seen within 2–3 months of starting treatment. It is often subtle on imaging, seen as fluid-filled bowel, mild bowel wall thickening, with or without surrounding fat stranding. Marked wall thickening, bowel perforation, and ascites are uncommon. Two distinct patterns of colitis are seen, namely diffuse and segmental [12]. Diffuse colitis involves the entire colon or a long segment and seen as fluid-filled colon and surrounding vascular engorgement with or without mild colonic wall thickening (Fig. 12.3). Segmental colitis often involves segments of preexisting diverticulosis, presumably secondary to inflammatory immune response, and seen as moderate degree of wall thickening and surrounding stranding (Fig. 12.4).
Key Point
It is important to communicate the pattern of colitis in the radiology report, because if treatment is needed, diffuse colitis is treated with steroids while segmental colitis may require treatment with steroids and antibiotics.
12.4.3 Pneumonitis
Immune-mediated pneumonitis is reported in approximately 5% of patients; however, subtle imaging findings of inflammation may be seen more frequently. It usually presents within 2–6 months of starting therapy and is more common with combination immunotherapy, and more common with anti-PD-1 agents than anti-PD-L1 drugs [13]. Most commonly imaged with CT, pneumonitis usually presents as areas of groundglass or consolidative opacities with lower lobe predominance and often peripheral (Fig. 12.5). Reticular changes may also be seen, especially when in the subacute phase. The findings are more commonly bilateral; however, may be unilateral. When presenting as a consolidation confined to a single lobe, it may mimic lobar pneumonia and may require treatment with both steroids and antibiotics if there is persistent confusion about the diagnosis. Some patients eventually develop a cryptogenic organizing pneumonia-like picture.
Key Point
Knowledge of immune-related pneumonitis is important even for abdominal radiologists as it is often seen in the lung bases on abdominal CT. Prompt communication of the toxicity is important as pneumonitis can quickly worsen and can be fatal.
12.4.4 Sarcoid-Like Reaction
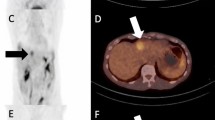
Sarcoid-like reaction is best known with ipilimumab, often presenting with mediastinal lymphadenopathy, pulmonary nodules, and splenic involvement (Fig. 12.6) [14]. It may also involve other nodal stations.
Sarcoid-like reaction on ipilimumab. FDG-PET/CT performed 3 months after the first dose of ipilimumab showed new mediastinal lymphadenopathy (short thin arrow), lung nodules (short thick arrow), and splenic uptake (long thin arrow). The report raised a possibility of metastatic disease; however, the findings resolved on the follow-up FDG-PET/CT at 6 months
Key Point
Sarcoid-like reactions may mimic disease progression. Mild increase or fluctuations in the size of previously uninvolved nodes upon starting immunotherapy should not be assumed to be metastatic disease. Progressive increase in nodal size on two or more scans is worrisome for metastatic involvement.
12.4.5 Hepatitis and Cholangitis
Immune-mediated hepatitis is an uncommon toxicity with often subtle and non-specific imaging findings. Imaging may be normal in mild cases. When severe, it may present with hepatomegaly, periportal edema, diffuse low attenuation or heterogeneous appearance of the liver and periportal lymphadenopathy (Fig. 12.7) [15]. On ultrasound, there is often prominent periportal echogenicity and gallbladder wall edema.
Immune-mediated cholangitis is uncommon and often difficult to diagnose. The imaging features are non-specific, appearing as biliary wall thickening and narrowing, mild biliary dilation, ill-defined peribiliary enhancement, and patchy diffusion restriction on MRI (Fig. 12.8). It is important to rule out other autoimmune disorders including PSC or IgG4-related disease.
12.4.6 Pancreatitis
Immune-mediated pancreatitis is an uncommon but important toxicity of immunotherapy, often requiring treatment discontinuation. It is often focal but may be diffuse and presents with edematous appearance of the pancreas with mild surrounding stranding (Fig. 12.9). Occasionally the appearance may resemble autoimmune pancreatitis with a sausage-shaped pancreas. Severe pancreatitis with necrotic changes and peripancreatic collections is almost never seen.
Key Point
Imaging findings of immune-mediated pancreatitis are often mild and inconclusive. Correlation with serum lipase and/or amylase levels may be needed.
12.4.7 Endocrine Adverse Events
Endocrine adverse events are more commonly seen with combination immunotherapy and can be seen in the form of adrenalitis, hypophysitis, or thyroiditis. With adrenalitis, depending on the timing of imaging, the adrenals may be mildly thickened with surrounding stranding or may be atrophic.
Key Point
Immune-related toxicity can be a biomarker of response. Patients with severe adverse events are also more likely to have a robust response.
12.4.8 Toxicities of CAR T-Cell Therapy
The major toxicities of CAR T cell therapy include cytokine release syndrome (CRS) and neurotoxicity. While not specific to CAR T cell therapy, CRS occurs in 58–93% of patients receiving this treatment, typically 2–3 days after the infusion. Mild CRS presents as fatigue, fever, and malaise while severe cases present with hemodynamic instability, altered liver function tests, respiratory failure, consumptive coagulopathy, and can lead to death [3]. Imaging findings are nonspecific and may demonstrate pulmonary edema and pleural effusions.
12.5 Conclusion
Immunotherapy has been shown to be effective in treating many common cancers and is increasingly used in clinical practice. However, the imaging features of immunotherapy in determining response are different than those seen after chemotherapy and radiation therapy. Additionally, there is a range of adverse effects of immunotherapy that can be seen on imaging. Therefore, the radiologist should be aware of the history of immunotherapy use in determining response to treatment as well as being aware of immune-response adverse events.
Take Home Messages
-
Immunotherapy activates the body’s own immune system to elicit a response against tumor cells.
-
Inflammation from a robust immune response can lead to pseudoprogression of the target lesions.
-
iRECIST category “unconfirmed progressive disease” (iUPD) may indicate pseudoprogression or true progression, requiring a repeat scan to be performed at 4–8 weeks.
-
Toxicities related to immunotherapy can lead to colitis, hepatitis, cholangitis, pancreatitis, sarcomatoid-like reactions, pancreatitis pneumonitis, hypophysitis, thyroiditis, and adrenalitis.
References
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Yoon JG, Smith DA, Tirumani SH, Caimi PF, Ramaiya NH. CAR T-cell therapy: an update for radiologists. AJR Am J Roentgenol. 2021;217(6):1461–74.
Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992–4.
Miller AB, Hoogstraten BF, Staquet MF, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–16.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20.
Tang YZ, Szabados B, Leung C, Sahdev A. Adverse effects and radiological manifestations of new immunotherapy agents. Br J Radiol. 2018;92:20180164.
Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with Ipilimumab. Cancer Immunol Res. 2015;3(10):1185–92.
Alessandrino F, Sahu S, Nishino M, Adeni AE, Tirumani SH, Shinagare AB, et al. Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor. Abdom Radiol (NY). 2019;44(5):1917–27.
Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, et al. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200(5):W468–74.
Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–81.
Vogel WV, Guislain A, Kvistborg P, Schumacher TNM, Haanen JBAG, Blank CU. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(2):e7–10.
Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Investig New Drugs. 2013;31(4):1071–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Shinagare, A.B., Fananapazir, G. (2023). Imaging Features of Immunotherapy. In: Hodler, J., Kubik-Huch, R.A., Roos, J.E., von Schulthess, G.K. (eds) Diseases of the Abdomen and Pelvis 2023-2026. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-031-27355-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-27355-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27354-4
Online ISBN: 978-3-031-27355-1
eBook Packages: MedicineMedicine (R0)