Abstract
Cartilages are unique in the family of connective tissues in that they contain a high concentration of the glycosaminoglycans, chondroitin sulfate and keratan sulfate attached to the core protein of the proteoglycan, aggrecan. Multiple aggrecan molecules are organized in the extracellular matrix via a domain-specific molecular interaction with hyaluronan and a link protein, and these high molecular weight aggregates are immobilized within the collagen and glycoprotein network. The high negative charge density of glycosaminoglycans provides hydrophilicity, high osmotic swelling pressure and conformational flexibility, which together function to absorb fluctuations in biomechanical stresses on cartilage during movement of an articular joint. We have summarized information on the history and current knowledge obtained by biochemical and genetic approaches, on cell-mediated regulation of aggrecan metabolism and its role in skeletal development, growth as well as during the development of joint disease. In addition, we describe the pathways for hyaluronan metabolism, with particular focus on the role as a “metabolic rheostat” during chondrocyte responses in cartilage remodeling in growth and disease.
Future advances in effective therapeutic targeting of cartilage loss during osteoarthritic diseases of the joint as an organ as well as in cartilage tissue engineering would benefit from ‘big data’ approaches and bioinformatics, to uncover novel feed-forward and feed-back mechanisms for regulating transcription and translation of genes and their integration into cell-specific pathways.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
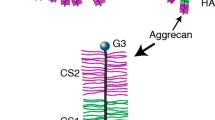
Cartilages are unique in the family of connective tissues in that they contain a high concentration of the glycosaminoglycans (GAG), chondroitin sulfate (CS) and keratan sulfate (KS) that are attached to the core protein of the proteoglycan, aggrecan. Aggrecan is organized in the extracellular matrix via a domain-specific molecular interaction with hyaluronan (HA) and with a link protein, and it is present throughout the collagen and glycoprotein network.
The high concentration of these organized GAGs have a well-documented essential role for articular cartilages to absorb alterations in biomechanical stresses during movement of an articular joint. At the structural level this is due to their biophysical characteristics at physiological pH, which include hydrophilicity and high osmotic swelling pressure due to the negative charges on their carbohydrate subunits (carboxyl and sulfate groups) and on their conformational flexibility and efficiency at filling space due to their sizes.
In this chapter we review the history and current knowledge of the cell-mediated regulation of aggrecan metabolism (Fig. 1.1) including: (a) the posttranslational modification of the core protein with CS and KS and its extracellular organization into ‘aggregates’ with HA and link proteins; (b) the proteolytic processing of the core protein by a specific set of extracellular proteases (ADAMTSs and MMPs); and (c) the function of hyaluronan (HA) metabolism in the context of serving as a “metabolic rheostat” during chondrocyte responses in cartilage remodeling during growth and disease.
Throughout the Chapter, components of the metabolic pathways that have been shown to be affected by biomechanical perturbation of tissues will be highlighted. In this research area, the Grodzinsky lab, together with an extensive network of collaborators, spearheaded in vitro bioreactor experiments using cartilage explants or chondrocytic cell constructs, to delineate the effects of static and dynamic compression, and of sheer stress, on the illustrated pathways in aggrecan post-translational processing. This set in motion a research approach used by multiple laboratories to extend our understanding of mechanotransduction pathways in chondrocytes and progenitor cells for cartilage engineering purposes ([77, 106, 144, 228, 239, 279] and references therein). In addition, throughout the comprehensive list of key references in the covered research areas the publications from the Grodzinsky lab and its past members are annotated in the Bibliography.
2 Chondroitin Sulfate and Keratan Sulfate Fine Structure on Aggrecan
Core protein linkage regions for synthesis and polymerization of CS and KS on the aggrecan core protein domains are illustrated in Fig. 1.2. CS is O-glycosidically linked to the serine residues along the CS rich regions 1 and 2 of the core protein via a linkage region oligosaccharide (-Xyl-Gal-Gal-GlcA) followed by unbranched chains consisting of disaccharides, (→4)β-GlcA (1→3)βGalNAc(1→), in which the amino sugar can be substituted on the C4 and/or C6 by a sulfate ester.
KS on aggrecan, also known as ‘skeletal’ KS, [180, 214] is O-linked to a serine or threonine in the KS domain, via a mucin core-2 linkage structure, (-GalNAc β(1–6)GlcNAc(1→). The GAG polymer is based on a polylactosamine backbone, with repeated disaccharides of (→4) βGal β (1–3) GlcNAc (1→). Both sugars in the disaccharide repeat can be sulfated on their C6 carbon, and an additional fucose can be substituted on the GlcNAc-6S. Many of these chains also capped with a sialic acid at the non-reducing terminal.
2.1 Aggrecan CS Chain Length and Sulfation Are Different in Skeletal Growth and Mature Cartilages
It is well established that chain length of CS and the type of sulfation on the C-4 or C-6 position of GalNAc residues in CS can vary with cartilage source depending on species and anatomical location. Detailed analyses of aggrecan CS fine structures in cartilage growth and maturation have provided more insights into conserved adaptations of CS biosynthesis to altered biophysical and biomechanical demands of a particular cartilage type.
Thus, examination of the GAG fine structure on growth and mature cartilage aggrecan core protein GAG domains using HPLC [163, 199] and FACE analyses [33, 201] established both location and age-related changes. For example, CS fine structure analyses of fetal growth plate cartilage aggrecan revealed a gradient in CS composition from the reserve zone to the hypertrophic zone, characterized by a marked increase in chain length accompanied by increased 6-sulfation and a concomitant decrease in 4-sulfation [55]. Furthermore, major changes in both CS chain length and sulfation pattern during postnatal maturation of human knee cartilage from the epiphyseal growth to a mature articular phenotype [200, 214, 285] were also detected. Upon skeletal maturation, chain length decreased by as much as 50%, and transitioned from an equal abundance of 4- and 6-sulfated GalNAc residues in growth cartilage to a predominance of 6-sulfated GalNAc residues. In addition CS chains in the CS2 region were shorter than those in the CS1 domain and carried a non-reducing terminal 4, 6-disulfated GalNAc residue instead of a 4S-GalNAc residue. A similar pattern in decreased chain length and increased 6-sulfation of both internal and terminal GalNAc residues was also observed by analyses of equine carpal articular cartilage CS [27].
2.2 GAG Biosynthesis Is a Multienzyme Process That Takes Place During Core Protein Trafficking Through the ER and Golgi
Studies to-date have shown that the conserved heterogeneity in GAG fine structures, unlike protein synthesis, do not follow a template, and it is regulated by individual cell phenotypes as well as by the structure of the proteoglycan core proteins that provide the acceptors. It is now recognized that conserved GAG structures are generated by transcriptional [124, 164, 288] and topographical [127, 238, 248, 249] control of the numerous enzymes responsible for linkage region synthesis and by GAG polymerization and sulfation (Table 1.1).
2.3 Skeletal Disorders Caused by Defective Genes Encoding Biosynthetic Enzymes for Sulfated Glycosaminoglycans
The generation of knock out mouse strains deficient in these enzymes revealed that many had an embryonic lethal phenotype due to defective cell proliferation and organ development, or altered neuronal function. However, they did not reveal a specific function for their role in cartilage growth and maturation (Table 1.2). On the other hand, human genetic studies revealed that defects in GAG-biosynthetic glycosyltransferases, epimerases or sulfotransferases cause distinct phenotypes of congenital disorders in cartilage growth, such as skeletal dysplasia, chondrodysplasia, multiple exostoses, and Ehlers-Danlos syndrome. This has furthered our understanding of the functional importance in the CS substitution on the aggrecan core protein (Table 1.3). In addition to the studies listed, individuals with either Kashin–Beck disease (KBD) [84], who show a dysfunction of CS sulfation enzymes, or a rare polymorphism in the aggrecan core protein [61, 122] are pre-disposed to the development of multi-joint or hand osteoarthritis, respectively.
2.4 Intracellular Localization and Topographical Organization of Enzymes for Aggrecan GAG Synthesis
The initiation of the linkage region by xylosyltransferases (I or II) [80, 203, 247] using UDP-xylose for addition of xylose to CS-region serine residues on aggrecan has been shown to occur in a pre-Golgi compartment, either at endoplasmic reticulum (ER) exit sites or in the ER-Golgi intermediate compartment [115, 174, 267]. However, the locations of these enzymes are also proteoglycan core protein and/or cell type specific since xylosyltransferases (I and II) were identified in the cis-Golgi region in rat liver cells and chondrosarcoma cells [149, 181]. Glycosyl- and sulfotransferases for extension and sulfation of the CS chains in the C4 or C6 positon of the GalNAc residues takes place in the Golgi stacks and extends into the trans-Golgi network (TGN) [249, 264].
Much less is known about the topographical location of the O-linked KS synthesis enzymes, largely impeded by the fact that their activity rapidly declines when tissues or cells are maintained ex vivo [75, 179]. For example, it has not been determined whether CS and KS synthesis occur simultaneously or whether GAG-specific enzymes are segregated in Golgi sub-compartments, or whether there is a regulated temporal recruitment as the core protein is trafficked through the secretory pathway enzymes in the same compartment.
2.5 ER/Golgi Topography and Organelle Microenvironment of GAG Synthesizing Enzymes
The ER/Golgi membrane localization of the GAG synthesis enzymes has been confirmed from their protein sequences, but details of their arrangement in these compartments are still debated [66]. For example, it has been proposed that the enzymes are at different membrane locations throughout the Golgi, and in that configuration, they would randomly synthesize chains depending on overall luminal availability of UDP-sugars and PAPS substrates. More recently, studies with chemically modified xylosides that serve as “substitute” acceptors for CS synthesis in the Golgi [43, 269] suggest that distinct functional macromolecular assemblies of elongation and sulfation enzymes, termed “GAGOSOMES”, are present. These complexes would concurrently catalyze the UDP-sugar addition and sulfate transfer to generate diverse GAG chain structures. This type of mechanism could indeed account for the differences in CS chain structures present on the CS1 and CS2 domains of aggrecan. The need for a specialized configuration of the Golgi compartment to achieve coordinated glycosylation reactions has also been suggested from genetic mutations in proteins such as COG4, CORAB and GOG8 associated with Golgi subdomains. These proteins have been shown to cause congenital disorders of glycosylation, including GAG biosynthesis, due to mis-localization of the transferase enzymes [2, 99, 167]. Topographical organization of the GAG biosynthetic enzymes is also a necessary prerequisite for targeted transport of nucleotide sugar precursors [242] for glycosylation and PAPS for sulfation [18, 57] from their production sites in the cytosol into the ER/Golgi lumen. In this regard, genetic deletion of the nucleotide sugar transporter Slc35d1 caused a skeletal defect in the knockout mice, and this was due to a sparse substitution of significantly shortened CS chains on aggrecan [98]. Other factors that could influence a functional Golgi membrane structure and luminal environment, and thereby regulate core protein glycosylation, include pH [213], ionic strength, [137] and cellular stress responses [225].
2.6 Alterations in CS Fine Structure by Biomechanical Stimuli – What Parts of the Post-translational Pathway Are They Targeting?
While there have been studies on the effects of growth factors (e.g. TGFβ1, IGF1) and cytokines on cartilage GAG synthesis, [161] and on CS synthesis [22, 171, 188], there have been relatively few studies to determine the effects of biomechanical stimuli on modulation of CS and KS synthesis enzymes. Cyclic compression of bovine cartilage explants in vitro resulted in the synthesis of CS chains with increased GalNAc6-sulfation and a concomitant decrease in GalNAc4-sulfation, and with fewer chains terminating with disulfated GalNAc4,6S [28, 227]. In vivo treadmill exercise in horses [28] increased CS chain size, which was accompanied by a greater proportion of un-sulfated regions in the chains, suggesting a differential effect on the supply of UDP-precursors and PAPS to the CS-synthesizing enzymes, or a selective decrease in activity of the sulfotransferases.
However, a considerable number of studies have reported structural changes in the cytoskeleton and intracellular organelles, such as mitochondria, ER/Golgi [145] and the nucleus, and in structures in response to biomechanical stimuli, including compression, hydrostatic and osmotic pressure [29, 32, 53, 56, 64, 82, 95, 123, 125, 128, 137,138,139, 145, 168, 169, 253]. Likewise such mechanical perturbations of the tissues or the cells is expected to modify ion channel activity, Ca2+ signaling [53, 101, 196, 299, 300] and glucose transport and utilization [138, 160, 241, 276, 286] that can affect steps in glycosylation pathways.
In summary, mechano-signal transduction, [77] which targets the aggrecan GAG substitution pathways, is likely to induce changes in the GAG precursor synthesis and/or topographical organization of the GAG synthesis enzymes, rather than in transcriptional regulation of the GAG biosynthetic enzymes (Fig. 1.1).
3 Aggrecan Metabolic Turnover in the ECM of Healthy and Osteoarthritic Cartilages
The cartilage ECM composition changes in order to adapt to various postnatal stages of growth and maturation, and is also affected by arthritic diseases. The mechanisms that such metabolic turnover events have on aggrecan have been well studied. For example, Maroudas and coworkers [156, 268] measured the D/LAsp ratio and the advanced glycation end product, pentosidine, in aggrecan purified from adult human cartilages and reported a half-life of ~3 years in vivo. A different approach [91] utilized an in vitro cartilage explant culture method with medium supplemented with 35S radiolabel to tag the CS-bearing region of newly synthesized aggrecan. By quantitating both the matrix retention and release into the culture medium of newly synthesized and resident CS-core protein fragments, turnover constants and half-lives for both pools of aggrecan in vitro were determined to be between 6-20 days. This method was subsequently used by others [35] to show that the half-life of aggrecan in the ECM can be prolonged by the inclusion of serum or anabolic growth factors [35, 172] or was shortened by proinflammatory stimulators [88] in the culture medium. It is also influenced by the type of cartilage [197] or the disease state [37, 219], and can be modulated by biomechanical perturbations [58, 133, 191, 205,206,207, 217].
3.1 Enzymatic Mechanism of Aggrecanolysis
Explant culture experiments demonstrated that a cell-dependent process generates aggrecan species that can no longer bind to HA and therefore diffuse from the tissue. This in turn motivated a research area to determine the molecular mechanism for the “aggrecanolysis”.
Our understanding of “aggrecanolysis” in the human joint was clarified by detailed analysis of aggrecan intermediates in chondrocyte and cartilage culture medium [103, 222], and this was shown to occur naturally in human cartilage and synovial fluids [220] (Fig. 1.3). The most studied aspect has been the proteolysis of the interglobular domain (IGD) of aggrecan with the release of the glycosaminoglycan (GAG)-attachment regions which is destructive to the tissue biomechanical function [20, 21] as it causes loss of the CS from the cartilage ECM.
Proteolysis sensitive sites in the human aggrecan core protein: The amino acid sequences in the sciscle bonds were either identified by protein sequencing of fragments isolated from human cartilages or synovial fluids (for MMPs, N-F and for ADAMTS, E-A, E-G and E-L) [220] or predicted from the published aggrecan core protein sequences [60]
Although there had been much debate around data suggesting a role for MMP3 (Stromelysin) in aggrecanolysis, a team of scientists at the pharmaceutical company DuPont [258] purified the aggrecan degrading proteolytic enzymes from the medium of catabolically stimulated bovine cartilage explant cultures. They belonged to the “A Disintegrin and Metalloproteinase with the ThromboSpondin motifs” (ADAMTS) family of metalloproteinases. They were termed aggrecanase-1 (ADAMTS-4) and aggrecanase-2 (ADAMTS-5).
3.2 Targeted Inhibition of Aggrecanolysis – A Potential Treatment for Human Osteoarthritis?
Given that aggrecan depletion of the articular cartilage is a hallmark of chronic OA and that ADAMTS5 has been proposed as the primary aggrecanase responsible for the destructive cleavages [73, 78, 246], it appeared likely that inhibitors of this enzyme would have therapeutic value as a Disease Modifying OA Drug (DMOAD).
A number of preclinical studies with in vitro explant cultures and/or animal models of OA using small molecular weight inhibitors of ADAMTS5 [25, 41, 45, 46] and catalytic-site directed neutralizing antibodies [192] showed promising results, and several of these potential therapeutics were tested in clinical trials (Table 1.4). However to-date, although showing promising DMOAD activity in pre-clinical models of OA [40, 134, 166], none were effective in the human disease, or showed detrimental side effects and thus not approved for clinical use. For example, in human OA explants, a humanized ADAMTS-5-selective monoclonal antibody (GSK2394002) was able to decrease the levels of aggrecan fragments released. However, toxicity studies of this antibody in a primate model of OA showed impairment of cardiovascular function as a side effect, and clinical trial studies were not developed. A novel type of therapeutic anti-ADAMTS-5 antibody, the Nano-body (M6495, Ablynx) blocked OA progression in mice following destabilization of the medial meniscus (DMM) surgery and reduced circulating levels of aggrecanase-generated aggrecan fragments when administered in a primate model [26]. A different set of antibodies that inhibited either the ability of ADAMTS5 for auto-activation or its interaction with an activating factor, such as LRP1, have also been shown to protect against aggrecanolysis in vitro [223, 224]. However, no information is available if they were investigated for their clinical therapeutic usefulness.
In summary, future plans for the generation of aggrecanase inhibitors as clinically sound therapeutics for targeted mitigation of aggrecan depletion from the cartilage ECM during OA pathogenesis may remain impeded by the findings that these enzymes have multi-tissue and organ distributions and functions. For example ADAMTS5 is essential for dermal wound healing [266], maintenance of tendon fibrillar structure/function [275], regulation of metabolic health by adipose tissue [17], and cardiovascular homeostasis [16]. An alternative future approach to restoring the aggrecan-dependent physiochemical and biomechanical properties of the cartilage matrix may require the cartilage-targeted delivery of engineered cleavage-resistant aggrecan-or GAG-mimetics, singly or in molecular complexes with other components. Such an approach could develop from technological advances made to-date in chemo-enzymatic synthesis of functional GAG structures and domains [175, 240].
4 Hyaluronan Metabolism and Its Relevance to Cartilage Structure and Function
Hyaluronan is a high-molecular weight polysaccharide composed of repeating disaccharide units, (→4) β-GlcA (1→3) βGlcNAc (1→) with a wide range of structural and metabolic functions in all tissues and body fluids [89]. These functions include lubrication, water homeostasis, macromolecular filtering, interactions with “hyaladherins” in matrix organization [49, 158, 274, 303] and regulation of cellular activities during development and in a range of pathologies [76, 92, 130, 194, 257]. This section provides a brief summary of the extensive research into the role of HA in cartilage structure/function and follow with highlights of recent advances in HA metabolism that could be incorporated into studying the cell biological responses of tissues under mechanical perturbations.
4.1 Hyaluronan in Cartilage Matrix Structure and Articular Joint Mechanics
The role of HA in cartilage has largely been considered in the light of its physical properties, namely for organizing aggrecan throughout the extracellular cartilage matrix A first report of a specific interaction of aggrecan with HA was reported by Hardingham and Muir [86, 87, 260], followed by more detailed analyses of the role of HA chemistry [90] and the role of the link glycoproteins in stabilization of the protein carbohydrate interactions [23, 67, 182, 252]. The biochemical analyses was later confirmed by electron microscopic methodology to visualize the structural arrangement of aggrecan monomers [96, 215] and link proteins [30, 31, 173] along the extended HA polymer backbone. In vitro cell biological studies with rat chondrosarcoma cells, and with pig and rabbit articular chondrocytes, confirmed that the ternary complex between aggrecan, link protein and hyaluronan was formed extracellularly, soon after secretion of the glycosylated proteins from the cell [120, 121, 198, 209, 216].
A different protein-HA modification, first discovered in the cumulus oophorus extracellular matrix [74] has also been identified in the extracellular matrix of OA cartilage [296]. These macromolecular HA complexes are formed in the extracellular matrix by covalent transfer of heavy chains (HCs) from inter-alpha-inhibitor (ITI) to HA. ITI is a modified CS proteoglycan with a core protein, bikunin that has 1, 2 or 3 HCs attached by an ester linkage between an aspartate in the HC and the 6-OH of a GalNAc in the CS chain [150]. The HC is transferred to the 6-OH on GlcNAc in HA [301] by tumor necrosis factor-induced protein-6 (TSG-6) [48, 176]. Subsequent investigations have identified the formation of such HC-HA matrices as part of a cellular response in tissue inflammations in a wide range of chronic diseases [136, 274], including asthma [250] Crohn’s disease [195], diabetic nephropathy [141], and degenerative suspensory ligament desmitis [202]. In both, OA and RA, HA-HC complexes are abundantly present in synovial fluid aspirates from patients [116, 229, 293, 296] and in animal models [68, 135] likely having been shed into the fluid after formation in inflamed synovium and/or degenerated cartilage.
In addition to the role of HA in organization of tissue and cell-specific extracellular matrices, it generates the viscoelastic properties of synovial fluid [185, 251], and in cooperation with the mucin-like molecule, PRG4 (aka Superficial Zone Protein or Lubricin), it provides boundary lubrication of the articular cartilage surfaces in diarthrodial joints [230]. Notably, in both OA and RA, decreased size and increased polydispersity of molecular the weight distribution of HA polymers in synovial fluid have been reported [12, 13] in keeping with the proposed impaired cartilage boundary lubrication in degenerative joint diseases [24]. Such observations led to the wide clinical use of intra-articular injections of high molecular weight HA as potential therapeutic ‘viscosupplementation’ for arthritic joints [4, 10, 11, 211].
4.2 Engagement of Hyaluronan Receptors Modulates Cell Responses
The studies of HA receptors, CD44, RHAMM, LYVE, Layilin and Stabilin2 and their downstream effects on cellular functions have been extensively investigated, particularly in the areas of development, cancer and respiratory diseases, as well as neuro- and vascular pathologies. A number of comprehensive recent reviews on this topic are available [76, 111, 131, 146, 162, 193, 263, 281]. Several of these receptors, in particular CD44, have also been shown to be active in cartilage matrix development and inflammatory pathologies, and those reports are summarized in Table 1.5. In the context of biomechanical effects on receptor HA interactions it is notable that ligand responses to tensile or flow stresses have been reported [14, 15, 184, 208], which would imply that application of physiological forces, such as tensile stress, sheer stress and fluid flow can affect receptor-HA interactions. This would provide an important function of these cell/matrix interactions as force sensing mechanisms [71, 148].
5 HA Metabolism Pathways Support Cell Survival
The biophysical, structural and cell biological roles of HA polymers reviewed above should be viewed in relation to their biosynthesis and degradation pathways. Over the past 5 decades many laboratories contributed research data that have built a comprehensive picture of these pathways (see Fig. 1.4).
Schematic illustration of coordination of HA synthesis, catabolism and HA-protein interactions: HA-Synthesis steps include HAS1, 2 or 3 protein transcription, modification and translocation to the plasma membrane, polymerization of HA chains using cytosolic UDP-GlcA and UDP-GlcNAc precursors and extrusion into the extracellular space. Cell signaling can be induced by HA/cell surface receptor interactions (CD44, RHAMM, Layilin). Interaction of HA with binding proteins (Acan, LP, TSG6, HCs) in the pericellular and interterritorial matrix generate specialized macromolecular complexes. HA-catabolism is mediated either by receptor mediated internalization (via LYVE-1 or Stabilin-2) of high molecular weight polymer or of low molecular weight fragments generated by cell surface hyaluronidases (TMEM2 or CEMIP) and completed in the lysosomal compartment by resident hyaluronidases (HYAL1, HYAL2 or HYAL3)
5.1 Enzymatic Pathways in HA Synthesis and Catabolism
The first insights into the mechanism of HA synthesis were reported in 1959, using Streptococcus membranes [154] that contained an enzyme activity (HA synthase (HAS)), which uses GlcA-UDP and GlcNAc-UDP as substrates to polymerize HA chains, and its gene was cloned in 1993 [52]. This was followed by identification of mammalian HAS genes (HAS1, HAS2 and HAS3) from a number of laboratories (reviewed in [282, 284]). They are transmembrane proteins, and have similar domain organizations that allows the direct translocation of the HA polymer into the extracellular space during HAS-catalyzed synthesis [153, 280]. Rates of polymer synthesis and size of the extruded HA chain are dependent on expression, translation and plasma membrane targeting of the enzyme proteins [255] as well as on the supply of the UDP-sugar precursors from the cytoplasm [92, 112]. A detailed study of HAS2 has revealed additional levels of post-translational control, including phosphorylation, [270], O-GlcNAcylation, [112], ubiquitination and dimerization [114]. Furthermore, the establishment of HAS knock out mouse strains provided important insights into the distinct roles of the three HAS proteins in development, growth and pathologies (summarized in Table 1.6).
In addition to the biosynthetic pathways, the degradative mechanisms for HA in tissues is also becoming more clearly defined. The existence of lysosomal hyaluronidases has long been established [110, 259], and their involvement following receptor mediated endocytosis via CD44 [47, 85], LYVE-1 [204] and HARE (Stabilin 1) [283]. However, extracellular hyaluronidase activities remained elusive until the identification of two extracellular hyaluronidase activities: (1) TMEM2, a type II transmembrane protein with hyaluronidase activity at neutral pH, [105, 256, 291] is expressed widely in adult mouse tissues, including vascular and lymphatic endothelial cells and liver, the major sites of HA clearance; and (2) KIAA1199 (CEMIP) [294, 295]. CEMIP was initially described as having a pivotal role in cancer cells, aiding their migration during tissue invasion and metasis [72, 262]. However, a number of recent reports have demonstrated its involvement in both cartilage pathologies [54, 59, 235, 299, 300] and osteoblast differentiation [39] making this an interesting candidate gene and protein to examine in relation to biomechanical stimulants imposed on cartilage and bone tissues (see Fig. 1.4).
5.2 Synergy Between Glucose Metabolism and HA Synthesis Adjusts the Cellular Energy Status
More recent studies on HA metabolism in cancer biology and diabetes have clearly demonstrated that biosynthesis of the HA is closely linked to intracellular glucose metabolism. This is through both aerobic and anaerobic glycolysis for energy production [265], and by the generation of the two sugar nucleotides, UDP-GlcNAc and UDP-GlcA. Together these sugar nucleotides regulate HA production by modification of both the biosynthetic activity [272, 304] and the half-lives of the membrane-associated HAS enzymes [271].
Biosynthesis of the two nucleotide precursors takes place in the cytoplasm (Fig. 1.5) and is driven by the availability of intracellular glucose taken up by the cell from the interstitial fluid by glucose transporters and its subsequent conversion to Glc6P [36]. UDP-GlcNAc is then synthesized via the hexosamine biosynthetic pathway [187], that also engages products from amino acid metabolism (glutamine) and lipid metabolism (Acetyl-CoA). UDP-GlcA biosynthesis on the other hand, depends on the activity of two enzymes, UDP-Glucose pyrophosphorylase (UPP), which uses glucose-1-phosphate (Glc1P) and UTP to generate UDP-Glc for conversion to UDP-GlcUA by UDP-Glucose dehydrogenase (UGDH) [244, 304]. Both enzymes show a wide tissue distribution, including cartilages [44, 152].
Schematic Illustration of Integration of Glucose Metabolism for Cytosolic Production of HA Biosynthesis Precursors UDP-GlcNAc and UDP-GlcUA: Extracellular glucose is transported into the cytoplasm by specific glucose transporters, where it is shunted for energy production via glycolysis and for production of the HA synthesis precursors UDP-GlcNAc and UDP-GlcUA via the hexosamine biosynthetic pathway or by UDP-glucose pyrophosphorylase/UDP-glucose dehydrogenase, respectively. Potential regulatory sites for mechanical stimuli of cells/tissues are indicated by bold black arrows. It should be noted that HA synthases have ‘direct’ access to cytosolic UDP-precursors, whereas UDP precursors for the chondroitin and keratan polymerases, or for other enzymes of glycol-conjugate synthesis, require an additional translocation/transport step into the ER/Golgi compartments
To date, the mechanistic linkage of glucose metabolism and HA synthesis has not been studied in detail in the context of cartilage during growth, maturation and pathologies, with only one recent review pointing to its importance in the developmental biology of the tissue [100]. An interest in the importance of the HBP in OA pathology was initiated by the observations that high concentrations of extracellular glucosamine or mannosamine could inhibit in vitro cytokine-induced aggrecan degradation by ADAMTS proteinases [190, 221] and inhibit disease progression in animal models of OA [183, 273]. Clinical use of oral dosages of glucosamine as a potential DMOAD [19, 70, 109, 165, 212] is still debated.
5.3 Are Biophysical Stressors Important in Regulation of HA Metabolism by Chondrocytes?
The subject of biomechanical effects on HA metabolism has been most broadly studied in endothelial cells and their response to sheer stresses generated by blood flow [81, 155, 277], as well as in epithelial cells in the alveolar lining [97]. Other mechanical perturbances, such as cyclic mechanical stretch or strain, shear stress, surface motion or mechanical injury [63, 119, 138, 142, 210, 254, 298] imposed on connective tissue cells, including fibrochondrocytes and articular chondrocytes, have also been shown to modulate HA production. The later studies have not provided any information on potential transduction pathways for stimulated HA production, but likely mechanisms could come from the newly emerging databases on cartilage “metabolomics’ [5, 50, 232, 236, 243]. Key regulatory points would include glucose transport [168, 169, 241], subsequent Glc6P shunting to aerobic [113] or anaerobic glycolysis [292, 302] for energy production, and/or synthesis of UDP-GlcNAc and UDP-GlcUA to regulate HAS activities. Given the critical structural and cell regulatory roles of HA reviewed above, a more detailed understanding of HA metabolism and its response under biomechanical perturbation of tissues and cells would provide novel opportunities to uncover treatment of cartilage pathologies [261], as well as optimization of procedures for the production of tissue engineered cartilages [160, 245].
6 Conclusion
Despite the extensive knowledge base in cartilage extracellular matrix structure and metabolism in health and diseases, there remain multiple opportunities to apply ‘big data’ generation and bioinformatics mining approaches to gain further insights to the feed-forward and feed-back mechanisms between genes, their products and cellular pathways. These goals could be achieved by applying such approaches to examine engineered tissues, animal models and clinical biorepositories.
References
Adhikara IM, Yagi K, Mayasari DS, Suzuki Y, Ikeda K, Ryanto GRT, Sasaki N, Rikitake Y, Nadanaka S, Kitagawa H, Miyata O, Igarashi M, Hirata KI, Emoto N (2021) Chondroitin Sulfate N-acetylgalactosaminyltransferase-2 impacts foam cell formation and atherosclerosis by altering macrophage glycosaminoglycan chain. Arterioscler Thromb Vasc Biol 41(3):1076–1091
Adusumalli R, Åsheim HC, Lupashin V, Blackburn JB, Prydz K (2021) Proteoglycan synthesis in conserved oligomeric Golgi subunit deficient HEK293T cells is affected differently, depending on the lacking subunit. Traffic 22(7):230–239
Aguiar DJ, Knudson W, Knudson CB (1999) Internalization of the hyaluronan receptor CD44 by chondrocytes. Exp Cell Res 252(2):292–302
Altman R, Hackel J, Niazi F, Shaw P, Nicholls M (2018) Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum 48(2):168–175
Anderson JR, Phelan MM, Foddy L, Clegg PD, Peffers MJ (2020) Ex vivo equine cartilage explant osteoarthritis model: a metabolomics and proteomics study. J Proteome Res 19(9):3652–3667
Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y (2014) Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci 34(18):6164–6176
Asano M, Furukawa K, Kido M, Matsumoto S, Umesaki Y, Kochibe N, Iwakura Y (1997) Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J 16(8):1850–1857
Asano K, Arito M, Kurokawa MS, Omoteyama K, Okamoto K, Suematsu N, Yudoh K, Nakamura H, Beppu M, Kato T (2014) Secretion of inflammatory factors from chondrocytes by layilin signaling. Biochem Biophys Res Commun 452(1):85–90
Atmuri V, Martin DC, Hemming R, Gutsol A, Byers S, Sahebjam S, Thliveris JA, Mort JS, Carmona E, Anderson JE, Dakshinamurti S, Triggs-Raine B (2008) Hyaluronidase 3 (HYAL3) knockout mice do not display evidence of hyaluronan accumulation. Matrix Biol 27:653–660
Balazs EA (2004) Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int 12:278–289
Balazs EA, Denlinger JL (1993) Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl 39:3–9
Balazs EA, Watson D, Duff IF, Roseman S (1967) Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum 10(4):357–376
Band PA, Heeter J, Wisniewski HG, Liublinska V, Pattanayak CW, Karia RJ, Stabler T, Balazs EA, Kraus VB (2015) Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr Cartil 23(1):70–76
Bano F, Banerji S, Howarth M, Jackson DG, Richter RP (2016) A single molecule assay to probe monovalent and multivalent bonds between hyaluronan and its key leukocyte receptor CD44 under force. Sci Rep 6:34176
Bano F, Tammi MI, Kang DW, Harris EN, Richter RP (2018) Single-molecule unbinding forces between the polysaccharide hyaluronan and its binding proteins. Biophys J 114(12):2910–2922
Barallobre-Barreiro J, Radovits T, Fava M, Mayr U, Lin WY, Ermolaeva E, Martínez-López D, Lindberg EL, Duregotti E, Daróczi L, Hasman M, Schmidt LE, Singh B, Lu R, Baig F, Siedlar AM, Cuello F, Catibog N, Theofilatos K, Shah AM, Crespo-Leiro MG, Doménech N, Hübner N, Merkely B, Mayr M (2021) Extracellular matrix in heart failure: role of ADAMTS5 in proteoglycan remodeling. Circulation 144(25):2021–2034
Bauters D, Bedossa P, Lijnen HR, Hemmeryckx B (2018) Functional role of ADAMTS5 in adiposity and metabolic health. PLoS One 13(1):e0190595
Bexiga MG, Simpson JC (2013) Human diseases associated with form and function of the Golgi complex. Int J Mol Sci 14:18670–18681
Block JA, Oegema TR, Sandy JD, Plaas A (2010) The effects of oral glucosamine on joint health: is a change in research approach needed? Osteoarthr Cartil 18(1):5–11
Bonassar LJ, Jeffries KA, Paguio CG, Grodzinsky AJ (1995a) Cartilage degradation and associated changes in biochemical and electromechanical properties. Acta Orthop Scand Suppl 266:38–44
Bonassar LJ, Frank EH, Murray JC, Paguio CG, Moore VL, Lark MW, Sandy JD, Wu JJ, Eyre DR, Grodzinsky AJ (1995b) Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum 38(2):173–183
Bonassar LJ, Grodzinsky AJ, Srinivasan A, Davila SG, Trippel SB (2000) Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys 379(1):57–63
Bonnet F, Dunham DG, Hardingham TE (1985) Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J 228(1):77–85
Bonnevie ED, Bonassar LJ (2020) A century of cartilage tribology research is informing lubrication therapies. J Biomech Eng 142(3):031004
Brebion F, Gosmini R, Deprez P, Varin M, Peixoto C, Alvey L, Jary H, Bienvenu N, Triballeau N, Blanque R, Cottereaux C, Christophe T, Vandervoort N, Mollat P, Touitou R, Leonard P, De Ceuninck F, Botez I, Monjardet A, van der Aar E, Amantini D (2021) Discovery of GLPG1972/S201086, a potent, selective, and orally bioavailable ADAMTS-5 inhibitor for the treatment of osteoarthritis. J Med Chem 64(6):2937–2952
Brenneis C, Serruys B, Van Belle T et al (2018) Structural and symptomatic benefit of a half-live extended, systemically applied anti-ADAMTS-5 inhibitor (M6495). Osteoarthr Cartil 26:S299–S300
Brown MP, West LA, Merritt KA, Plaas AH (1998) Changes in sulfation patterns of chondroitin sulfate in equine articular cartilage and synovial fluid in response to aging and osteoarthritis. Am J Vet Res 59(6):786–791
Brown MP, Trumble TN, Plaas AH, Sandy JD, Romano M, Hernandez J, Merritt KA (2007) Exercise and injury increase chondroitin sulfate chain length and decrease hyaluronan chain length in synovial fluid. Osteoarthr Cartil 15(11):1318–1325
Browning JA, Saunders K, Urban JP, Wilkins RJ (2004) The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology 41(3–4):299–308
Buckwalter JA, Rosenberg LC (1988) Electron microscopic studies of cartilage proteoglycans. Electron Microsc Rev 1(1):87–112
Buckwalter JA, Rosenberg LC, Tang LH (1984) The effect of link protein on proteoglycan aggregate structure. An electron microscopic study of the molecular architecture and dimensions of proteoglycan aggregates reassembled from the proteoglycan monomers and link proteins of bovine fetal epiphyseal cartilage. J Biol Chem 259(9):5361–5363
Buschmann MD, Hunziker EB, Kim YJ, Grodzinsky AJ (1996) Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J Cell Sci 109(Pt 2):499–508
Calabro A, Midura R, Wang A, West L, Plaas A, Hascall VC (2001) Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthr Cartil 9(Suppl A):S16–S22
Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr, Kubalak S, Klewer SE, McDonald JA (2000) Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 106(3):349–360
Campbell MA, Handley CJ, Hascall VC, Campbell RA, Lowther DA (1984) Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys 234(1):275–289
Caon I, Parnigoni A, Viola M, Karousou E, Passi A, Vigetti D (2021) Cell energy metabolism and hyaluronan synthesis. J Histochem Cytochem 69(1):35–47
Carney SL, Billingham ME, Muir H, Sandy JD (1984) Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Orthop Res 2(3):201–206
Chan DD, Li J, Luo W, Predescu DN, Cole BJ, Plaas A (2018) Pirfenidone reduces subchondral bone loss and fibrosis after murine knee cartilage injury. J Orthop Res 36(1):365–376
Chen L, Shi K, Andersen TL, Qiu W, Kassem M (2019) KIAA1199 is a secreted molecule that enhances osteoblastic stem cell migration and recruitment. Cell Death Dis 10(2):126
Chiusaroli R, Visentini M, Galimberti C, Casseler C, Mennuni L, Covaceuszach S, Lanza M, Ugolini G, Caselli G, Rovati LC, Visintin M (2013) Targeting of ADAMTS5′s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthr Cartil 21(11):1807–1810
Chockalingam PS, Sun W, Rivera-Bermudez MA, Zeng W, Dufield DR, Larsson S, Lohmander LS, Flannery CR, Glasson SS, Georgiadis KE, Morris EA (2011) Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthr Cartil 19(3):315–323
Chowdhury B, Hemming R, Hombach-Klonisch S, Flamion B, Triggs-Raine B (2013) Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction. J Biol Chem 288:520–528
Chua JS, Kuberan B (2017) Synthetic Xylosides: probing the glycosaminoglycan biosynthetic machinery for biomedical applications. Acc Chem Res 50(11):2693–2705
Clarkin CE, Allen S, Kuiper NJ, Wheeler BT, Wheeler-Jones CP, Pitsillides AA (2011) Regulation of UDP-glucose dehydrogenase is sufficient to modulate hyaluronan production and release, control sulfated GAG synthesis, and promote chondrogenesis. J Cell Physiol 226(3):749–761
Clement-Lacroix P, Little CB, Smith MM, Cottereaux C, Merciris D, Meurisse S, Mollat P, Touitou R, Brebion F, Gosmini R, De Ceuninck F, Botez I, Lepescheux L, van der Aar E, Christophe T, Vandervoort N, Blanqué R, Comas D, Deprez P, Amantini D (2021) Pharmacological characterization of GLPG1972/S201086, a potent and selective small-molecule inhibitor of ADAMTS5. Osteoarthr Cartil:S1063–S4584
Clement-Lacroix P, Little CB, Smith MM, Cottereaux C, Merciris D, Meurisse S, Mollat P, Touitou R, Brebion F, Gosmini R, De Ceuninck F, Botez I, Lepescheux L, van der Aar E, Christophe T, Vandervoort N, Blanqué R, Comas D, Deprez P, Amantini D (2022) Pharmacological characterization of GLPG1972/S201086, a potent and selective small-molecule inhibitor of ADAMTS5. Osteoarthr Cartil 30(2):291–301
Culty M, Nguyen HA, Underhill CB (1992) The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol 116(4):1055–1062
Day AJ, Milner CM (2019) TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol 78-79:60–83
Day AJ, Sheehan JK (2001) Hyaluronan: polysaccharide chaos to protein organisation. Curr Opin Struct Biol 11(5):617–622
De Sousa EB, Dos Santos GC Jr, Duarte MEL, Neto MV, Aguiar DP (2017) Metabolomics as a promising tool for early osteoarthritis diagnosis. Braz J Med Biol Res 50(11):e6485
de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V (2005) Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem 94(5):954–966
DeAngelis PL, Papaconstantinou J, Weigel PH (1993) Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem 268(26):19181–19184
Degala S, Williams R, Zipfel W, Bonassar LJ (2012) Calcium signaling in response to fluid flow by chondrocytes in 3D alginate culture. J Orthop Res 30(5):793–799
Deroyer C, Charlier E, Neuville S, Malaise O, Gillet P, Kurth W, Chariot A, Malaise M, de Seny D (2019) CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis 10(2):103
Deutsch AJ, Midura RJ, Plaas AH (1995) Structure of chondroitin sulfate on aggrecan isolated from bovine tibial and costochondral growth plates. J Orthop Res 13(2):230–239
Di Federico E, Bader DL, Shelton JC (2020) 3D models of chondrocytes within biomimetic scaffolds: Effects of cell deformation from loading regimens. Clin Biomech (Bristol, Avon) 79:104972
Dick G, Grøndahl F, Prydz K (2008) Overexpression of the 3′-phosphoadenosine 5′-phosphosulfate (PAPS) transporter 1 increases sulfation of chondroitin sulfate in the apical pathway of MDCK II cells. Glycobiology 18(1):53–65
DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, Kim YJ, Grodzinsky AJ (2004) Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum 50(3):840–848
Ding QH, Qi YY, Li XM, Chen WP, Wang XH, Ji XW (2019) Knockdown of KIAA1199 suppresses IL-1β-induced cartilage degradation and inflammatory responses in human chondrocytes through the Wnt/β-catenin signaling pathway. Int Immunopharmacol 73:203–211
Doege KJ, Sasaki M, Kimura T, Yamada Y (1991) Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem 266(2):894–902
Doege KJ, Coulter SN, Meek LM, Maslen K, Wood JG (1997) A human-specific polymorphism in the coding region of the aggrecan gene. Variable number of tandem repeats produce a range of core protein sizes in the general population. J Biol Chem 272(21):13974–13979
Dowthwaite GP, Edwards JC, Pitsillides AA (1998) An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. J Histochem Cytochem 46(5):641–651
Dowthwaite GP, Ward AC, Flannely J, Suswillo RF, Flannery CR, Archer CW, Pitsillides AA (1999) The effect of mechanical strain on hyaluronan metabolism in embryonic fibrocartilage cells. Matrix Biol 18(6):523–532
Early JO, Fagan LE, Curtis AM, Kennedy OD (2021) Mitochondria in injury, inflammation and disease of articular skeletal joints. Front Immunol 12:695257
Edwards J, Schulze E, Sabokbar A, Gordon-Andrews H, Jackson D, Athanasou NA (2008) Absence of lymphatics at the bone-implant interface: implications for periprosthetic osteolysis. Acta Orthop 79(2):289–294
Esko JD, Selleck SB (2002) Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71:435–471
Faltz LL, Caputo CB, Kimura JH, Schrode J, Hascall VC (1979) Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem 254(4):1381–1387
Fasanello DC, Su J, Deng S, Yin R, Colville MJ, Berenson JM, Kelly CM, Freer H, Rollins A, Wagner B, Rivas F, Hall AR, Rahbar E, DeAngelis PL, Paszek MJ, Reesink HL (2021) Hyaluronic acid synthesis, degradation, and crosslinking in equine osteoarthritis: TNF-α-TSG-6-mediated HC-HA formation. Arthritis Res Ther 23(1):218
Ferencz B, Condac E, Poudel N, Munteanu MC, Sivasami P, Choudhury B, Naidu NN, Zhang F, Breshears M, Linhardt RJ, Hinsdale ME (2020) Xylosyltransferase 2 deficiency and organ homeostasis. Glycoconj J 37(6):755–765
Fernández-Martín S, González-Cantalapiedra A, Muñoz F, García-González M, Permuy M, López-Peña M (2021) Glucosamine and chondroitin sulfate: is there any scientific evidence for their effectiveness as disease-modifying drugs in knee osteoarthritis preclinical studies?-A systematic review from 2000 to 2021. Animals (Basel) 11(6):1608
Ferrari LF, Araldi D, Bogen O, Levine JD (2016) Extracellular matrix hyaluronan signals via its CD44 receptor in the increased responsiveness to mechanical stimulation. Neuroscience 324:390–398
Fink SP, Myeroff LL, Kariv R, Platzer P, Xin B, Mikkola D, Lawrence E, Morris N, Nosrati A, Willson JK, Willis J, Veigl M, Barnholtz-Sloan JS, Wang Z, Markowitz SD (2015) Induction of KIAA1199/CEMIP is associated with colon cancer phenotype and poor patient survival. Oncotarget 6(31):30500–30515
Fosang AJ, Rogerson FM (2010) Identifying the human aggrecanase. Osteoarthr Cartil 18(9):1109–1116
Fülöp C, Szántó S, Mukhopadhyay D, Bárdos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130(10):2253–2261
Funderburgh JL (2000) Keratan sulfate: structure, biosynthesis, and function. Glycobiology 10(10):951–958
Garantziotis S, Savani RC (2019) Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol 78-79:1–10
Gilbert SJ, Bonnet CS, Blain EJ (2021) Mechanical cues: bidirectional reciprocity in the extracellular matrix drives mechano-signalling in articular cartilage. Int J Mol Sci 22:13595
Glasson SS, Askew R, Sheppard B et al (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434:644–648
Gotoh M, Yada T, Sato T, Akashima T, Iwasaki H, Mochizuki H, Inaba N, Togayachi A, Kudo T, Watanabe H, Kimata K, Narimatsu H (2002) Molecular cloning and characterization of a novel chondroitin sulfate glucuronyltransferase that transfers glucuronic acid to N-acetylgalactosamine. J Biol Chem 277:38179–38188
Götting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K (2000) Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol 304(4):517–528
Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H (2006) Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290(1):H458–H452
Guilak F (1995) Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech 28(12):1529–1541
Habuchi H, Ushida T, Habuchi O (2016) Mice deficient in N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase exhibit enhanced liver fibrosis and delayed recovery from fibrosis in carbon tetrachloridetreated mice. Heliyon 2(8):e00138
Han J, Li D, Qu C, Wang D, Wang L, Guo X, Lammi MJ (2017) Altered expression of chondroitin sulfate structure modifying sulfotransferases in thearticular cartilage from adult osteoarthritis and Kashin- Beck disease. Osteoarthr Cartil 25(8):1372–1375
Harada H, Takahashi M (2007) CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem 282:5597–5607
Hardingham TE, Muir H (1972) The specific interaction of hyaluronic acid with cartilage proteoglycans. Biochim Biophys Acta 279(2):401–405
Hardingham TE, Muir H (1974) Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J 139:565–581
Hardingham TE, Bayliss MT, Rayan V, Noble DP (1992) Effects of growth factors and cytokines on proteoglycan turnover in articular cartilage. Br J Rheumatol 31(Suppl 1):1–6
Hascall VC (2019) The journey of hyaluronan research in the Journal of Biological Chemistry. J Biol Chem 294(5):1690–1696
Hascall VC, Heinegard D (1974) Hyaluronic acid in cartilage and proteoglycan aggregation. J Biol Chem 249(13):I4232–I4241
Hascall VC, Handley CJ, McQuillan DJ, Hascall GK, Robinson HC, Lowther DA (1983) The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys 224(1):206–223
Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW (2014) The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35:14–17
Hatano H, Shigeishi H, Kudo Y, Higashikawa K, Tobiume K, Takata T, Kamata N (2012) Overexpression of receptor for hyaluronan-mediated motility (RHAMM) in MC3T3-E1 cells induces proliferation and differentiation through phosphorylation of ERK1/2. J Bone Miner Metab 30(3):293–303
Hayer S, Steiner G, Görtz B, Reiter E, Tohidast-Akrad M, Amling M, Hoffmann O, Redlich K, Zwerina J, Skriner K, Hilberg F, Wagner EF, Smolen JS, Schett G (2005) CD44 is a determinant of inflammatory bone loss. J Exp Med 201(6):903–914
He Y, Makarczyk MJ, Lin H (2020) Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci 15:263
Heinegård D, Lohmander S, Thyberg J (1978) Cartilage proteoglycan aggregates. Electron-microscopic studies of native and fragmented molecules. Biochem J 175(3):913–919
Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S (2011) Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem 286(20):17435–17444
Hiraoka S, Furuichi T, Nishimura G, Shibata S, Yanagishita M, Rimoin DL, Superti-Furga A, Nikkels PG, Ogawa M, Katsuyama K, Toyoda H, Kinoshita-Toyoda A, Ishida N, Isono K, Sanai Y, Cohn DH, Koseki H, Ikegawa S (2007) Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med 13(11):1363–1367
Hirschberg CB, Robbins PW, Abeijon C (1998) Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem 67:49–69
Hollander JM, Zeng L (2019) The emerging role of glucose metabolism in cartilage development. Curr Osteoporos Rep 17(2):59–69
Huang W, Nagasaka M, Furukawa KS, Ushida T (2020) Local strain distribution and increased intracellular Ca2+ signaling in bovine articular cartilage exposed to compressive strain. J Biomech Eng 142(6):061008
Hulme CH, Peffers MJ, Harrington GMB, Wilson E, Perry J, Roberts S, Gallacher P, Jermin P, Wright KT (2021) Identification of candidate synovial fluid biomarkers for the prediction of patient outcome after microfracture or osteotomy. Am J Sports Med 49(6):1512–1523
Ilic MZ, Handley CJ, Robinson HC, Mok MT (1992) Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys 294(1):115–122
Inada R, Miyamoto K, Tanaka N, Moriguchi K, Kadomatsu K, Takeuchi K, Igarashi M, Kusunoki S (2021) Chondroitin sulfate N-acetylgalactosyltransferase-1 knockout shows milder phenotype in experimental autoimmune encephalomyelitis than in wild type. Glycobiology 31(3):260–265
Irie F, Tobisawa Y, Murao A, Yamamoto H, Ohyama C, Yamaguchi Y (2021) The cell surface hyaluronidase TMEM2 regulates cell adhesion and migration via degradation of hyaluronan at focal adhesion sites. J Biol Chem 296:100481
Iseki T, Rothrauff BB, Kihara S, Sasaki H, Yoshiya S, Fu FH, Tuan RS, Gottardi R (2019) Dynamic compressive loading improves cartilage repair in an in vitro model of microfracture: comparison of 2 mechanical loading regimens on simulated microfracture based on fibrin gel scaffolds encapsulating connective tissue progenitor cells. Am J Sports Med 47(9):2188–2199
Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K, Ando K, Yamashita T, Ishiguro N, Kadomatsu K (2010) N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci 30(17):5937–5947
Izumikawa T, Kitagawa H (2010) Mice deficient in glucuronyltransferase-I. Prog Mol Biol Transl Sci 93:19–34
Jackson CG, Plaas AH, Sandy JD, Hua C, Kim-Rolands S, Barnhill JG, Harris CL, Clegg DO (2010) The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthr Cartil 18(3):297–302
Jadin L, Wu X, Ding H, Frost GI, Onclinx C, Triggs-Raine B, Flamion B (2008) Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? FASEB J 22(12):4316–4312
Johnson LA, Jackson DG (2021) Hyaluronan and its receptors: key mediators of immune cell entry and trafficking in the lymphatic system. Cell 10(8):2061
Jokela TA, Makkonen KM, Oikari S, Kärnä R, Koli E, Hart GW, Tammi RH, Carlberg C, Tammi MI (2011) Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1. J Biol Chem 286(38):33632–33640
Kan S, Duan M, Liu Y, Wang C, Xie J (2021) Role of Mitochondria in physiology of chondrocytes and diseases of osteoarthritis and rheumatoid arthritis. Cartilage 13(2_suppl):1102S–1121S
Karousou E, Kamiryo M, Skandalis SS, Ruusala A, Asteriou T, Passi A, Yamashita H, Hellman U, Heldin CH, Heldin P (2010) The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J Biol Chem 285(31):23647–23654
Kearns AE, Vertel BM, Schwartz NB (1993) Topography of glycosylation and UDP-xylose production. J Biol Chem 268:11097–11104
Kida D, Yoneda M, Miyaura S, Ishimaru T, Yoshida Y, Ito T, Ishiguro N, Iwata H, Kimata K (1999) The SHAP-HA complex in sera from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 26(6):1230–1238
Kido M, Asano M, Iwakura Y, Ichinose M, Miki K, Furukawa K (1999) Normal levels of serum glycoproteins maintained in beta-1, 4-galactosyltransferase I-knockout mice. FEBS Lett 464(1–2):75–79
Kiene LS, Homann S, Suvorava T, Rabausch B, Müller J, Kojda G, Kretschmer I, Twarock S, Dai G, Deenen R, Hartwig S, Lehr S, Köhrer K, Savani RC, Grandoch M, Fischer JW (2016) Deletion of Hyaluronan synthase 3 inhibits Neointimal Hyperplasia in Mice. Arterioscler Thromb Vasc Biol 36(2):e9–e16
Kim YJ, Grodzinsky AJ, Plaas AH (1996) Compression of cartilage results in differential effects on biosynthetic pathways for aggrecan, link protein, and hyaluronan. Arch Biochem Biophys 328(2):331–340
Kimura JH, Hardingham TE, Hascall VC, Solursh M (1979) Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem 254(8):2600–2609
Kimura JH, Hardingham TE, Hascall VC (1980) Assembly of newly synthesized proteoglycan and link protein into aggregates in cultures of chondrosarcoma chondrocytes. J Biol Chem 255(15):7134–7143
Kirk KM, Doege KJ, Hecht J, Bellamy N, Martin NG (2003) Osteoarthritis of the hands, hips and knees in an Australian twin sample–evidence of association with the aggrecan VNTR polymorphism. Twin Res 6(1):62–66
Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ (2004) Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37(5):595–604
Kitagawa H, Uyama T, Sugahara K (2001) Molecular cloning and expression of a human chondroitin synthase. J Biol Chem 276:38721–38726
Kitagawa H, Izumikawa T, Uyama T, Sugahara K (2003) Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J Biol Chem 278:23666–23671
Kitazawa K, Nadanaka S, Kadomatsu K, Kitagawa H (2021) Chondroitin 6-sulfate represses keratinocyte proliferation in mouse skin, which is associated with psoriasis. Commun Biol 4(1):114
Kjellén L, Lindahl U (2018) Specificity of glycosaminoglycan-protein interactions. Curr Opin Struct Biol 50:101–108
Knight MM, Bomzon Z, Kimmel E, Sharma AM, Lee DA, Bader DL (2006) Chondrocyte deformation induces mitochondrial distortion and heterogeneous intracellular strain fields. Biomech Model Mechanobiol 5(2-3):180–191
Knudson W, Aguiar DJ, Hua Q, Knudson CB (1996) CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res 228(2):216–228
Kobayashi T, Chanmee T, Itano N (2020) Hyaluronan: metabolism and function. Biomolecules 10(11):1525
Krolikoski M, Monslow J, Puré E (2019) The CD44-HA axis and inflammation in atherosclerosis: a temporal perspective. Matrix Biol 78-79:201–218
Kurtis MS, Tu BP, Gaya OA, Mollenhauer J, Knudson W, Loeser RF, Knudson CB, Sah RL (2001) Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res 19(6):1122–1130
Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ (2001) Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res 19(6):1140–1146
Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, Xue Y, Liu F, Genell C, Miller RE, Tran PB, Malfait AM, Maier CC, Matheny CJ (2015) Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthr Cartil 8:1254–1266
Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A (2013) Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J Biol Chem 288(1):205–214
Lauer ME, Loftis J, de la Motte C, Hascall VC (2015) Analysis of the heavy-chain modification and TSG-6 activity in pathological hyaluronan matrices. Methods Mol Biol 1229:543–548
Lee TH, Linstedt AD (1999) Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol Biol Cell 10(5):1445–1462
Lee RB, Wilkins RJ, Razaq S, Urban JP (2002) The effect of mechanical stress on cartilage energy metabolism. Biorheology 39(1–2):133–143
Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ (2005) Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52(8):2386–2395
Lee WJ, Tu SH, Cheng TC, Lin JH, Sheu MT, Kuo CC, Changou CA, Wu CH, Chang HW, Chang HL, Chen LC, Ho YS (2021) Type-3 Hyaluronan synthase attenuates tumor cells invasion in human mammary parenchymal tissues. Molecules 26(21):6548
Lewis A, Steadman R, Manley P, Craig K, de la Motte C, Hascall V, Phillips AO (2008) Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol 23(6):731–739
Li Z, Yao S, Alini M, Grad S (2007) Different response of articular chondrocyte subpopulations to surface motion. Osteoarthr Cartil 15(9):1034–1041
Li Y, Zhong G, Sun W, Zhao C, Zhang P, Song J, Zhao D, Jin X, Li Q, Ling S, Li Y (2015) CD44 deficiency inhibits unloading-induced cortical bone loss through downregulation of osteoclast activity. Sci Rep 5:16124
Li K, Zhang C, Qiu L, Gao L, Zhang X (2017) Advances in application of mechanical stimuli in bioreactors for cartilage tissue engineering. Tissue Eng Part B Rev 23(4):399–411
Li J, Ahata E, Wang Y (2019) Golgi structure and function in health, stress, and diseases. Results Probl Cell Differ 67:441–485
Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW (2011) Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol 128(2):403–411
Lin H, Zhou J, Shen L, Ruan Y, Dong J, Guo C, Chen Z (2014) Biotin-conjugated anti-CD44 antibody-avidin binding system for the improvement of chondrocyte adhesion to scaffolds. J Biomed Mater Res A 102(4):1140–1148
Lintuluoto M, Horioka Y, Hongo S, Lintuluoto JM, Fukunishi Y (2021) Molecular dynamics simulation study on allosteric regulation of CD44-Hyaluronan binding as a force sensing mechanism. ACS Omega 6(12):8045–8055
Lohmander LS, Shinomura T, Hascall VC, Kimura JH (1989) Xylosyl transfer to the core protein precursor of the rat chondrosarcoma proteoglycan. J Biol Chem 264:18775–18780
Lord MS, Melrose J, Day AJ et al (2020) The Inter-α-Trypsin inhibitor family: versatile molecules in biology and pathology. J Histochem Cytochem 68:907–927
Luo N, Knudson W, Askew EB, Veluci R, Knudson CB (2014) CD44 and hyaluronan promote the bone morphogenetic protein 7 signaling response in murine chondrocytes. Arthritis Rheum 66(6):1547–1558
Magee C, Nurminskaya M, Linsenmayer TF (2001) UDP-glucose pyrophosphorylase: up-regulation in hypertrophic cartilage and role in hyaluronan synthesis. Biochem J 360(Pt 3):667–674
Maloney FP, Kuklewicz J, Corey RA, Bi Y, Ho R, Mateusiak L, Pardon E, Steyaert J, Stansfeld PJ, Zimmer J (2022) Structure, substrate recognition and initiation of hyaluronan synthase. Nature; Mar 30. Epub ahead of print
Markovitz A, Cifonelli JA, Dorfman A (1959) The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem 234:2343–2350
Maroski J, Vorderwülbecke BJ, Fiedorowicz K, Da Silva-Azevedo L, Siegel G, Marki A, Pries AR, Zakrzewicz A (2011) Shear stress increases endothelial hyaluronan synthase 2 and hyaluronan synthesis especially in regard to an atheroprotective flow profile. Exp Physiol 96(9):977–986
Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E (1998) Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys 350(1):61–71
Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S, Hombach-Klonisch S, Csoka AB, Stern R, Triggs-Raine BL (2008) A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet 17(13):1904–1915
Mason RM, Crossman MV, Sweeney C (1989) Hyaluronan and hyaluronan-binding proteins in cartilaginous tissues. CIBA Found Symp 143:107–116
Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, Dealy CN, Toole BP, Takeda J, Yamaguchi Y, Kosher RA (2009) Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 136(16):2825–2835
McCorry MC, Kim J, Springer NL, Sandy J, Plaas A, Bonassar LJ (2019) Regulation of proteoglycan production by varying glucose concentrations controls fiber formation in tissue engineered menisci. Acta Biomater 100:173-183.
Melching LI, Roughley PJ (1999) Modulation of keratan sulfate synthesis on lumican by the action of cytokines on human articular chondrocytes. Matrix Biol 18(4):381–390
Messam BJ, Tolg C, McCarthy JB, Nelson AC, Turley EA (2021) RHAMM is a multifunctional protein that regulates cancer progression. Int J Mol Sci 22(19):10313
Midura RJ, Calabro A, Yanagishita M, Hascall VC (1995) Nonreducing end structures of chondroitin sulfate chains on aggrecan isolated from Swarm rat chondrosarcoma cultures. J Biol Chem 270(14):8009–8015
Mikami T, Kitagawa H (2013) Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta 1830(10):4719–4733
Miller KL, Clegg DO (2011) Glucosamine and chondroitin sulfate. Rheum Dis Clin N Am 37(1):103–118
Miller RE, Tran PB, Ishihara S, Larkin J, Malfait AM (2016) Therapeutic effects of an anti-ADAMTS-5 antibody on joint damage and mechanical allodynia in a murine model of osteoarthritis. Osteoarthr Cartil 24(2):299–306
Mizumoto S, Yamada S (2021) Congenital disorders of deficiency in Glycosaminoglycan biosynthesis. Front Genet 12:717535
Mobasheri A, Carter SD, Martín-Vasallo P, Shakibaei M (2002a) Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int 26(1):1–18
Mobasheri A, Neama G, Bell S, Richardson S, Carter SD (2002b) Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell Biol Int 26(3):297–300
Moffatt P, Lee ER, St-Jacques B, Matsumoto K, Yamaguchi Y, Roughley PJ (2011) Hyaluronan production by means of Has2 gene expression in chondrocytes is essential for long bone development. Dev Dyn 240(2):404–412
Morales TI (1994) Transforming growth factor-beta and insulin-like growth factor-1 restore proteoglycan metabolism of bovine articular cartilage after depletion by retinoic acid. Arch Biochem Biophys 315(1):190–198
Morales TI, Hascall VC (1989) Factors involved in the regulation of proteoglycan metabolism in articular cartilage. Arthritis Rheum 32(10):1197–1201
Mörgelin M, Paulsson M, Heinegård D, Aebi U, Engel J (1995) Evidence of a defined spatial arrangement of hyaluronate in the central filament of cartilage proteoglycan aggregates. Biochem J 307(Pt 2):595–601
Moriarity JL, Hurt KJ, Resnick AC, Storm PB, Laroy W, Schnaar RL, Snyder SH (2002) UDP-glucuronate decarboxylase, a key enzyme in proteoglycan synthesis: cloning, characterization, and localization. J Biol Chem 277(19):16968–16975
Morla S (2019) Glycosaminoglycans and Glycosaminoglycan mimetics in cancer and inflammation. Int J Mol Sci 20(8):1963
Mukhopadhyay D, Asari A, Rugg MS, Day AJ, Fülöp C (2004) Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-alpha-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J Biol Chem 279(12):11119–11128
Murata M, Yudoh K, Shimizu H, Beppu M, Nakamura H, Kato T, Masuko K (2013) Layilin, a Talin-binding hyaluronan receptor, is expressed in human articular chondrocytes and synoviocytes and is down-regulated by interleukin-1β. Mod Rheumatol 23(3):478–488
Nakamura N, Yamakawa N, Sato T, Tojo H, Tachi C, Furukawa K (2001) Differential gene expression of beta-1,4-galactosyltransferases I, II and V during mouse brain development. J Neurochem 76(1):29–38
Nakazawa K, Takahashi I, Yamamoto Y (1998) Glycosyltransferase and sulfotransferase activities in chick corneal stromal cells before and after in vitro culture. Arch Biochem Biophys 359(2):269–282
Nieduszynski IA, Huckerby TN, Dickenson JM, Brown GM, Tai GH, Bayliss MT (1990) Structural aspects of skeletal keratan sulphates. Biochem Soc Trans 18(5):792–793
Nuwayhid N, Glaser JH, Johnson JC, Conrad HE, Hauser SC, Hirschberg CB (1986) Xylosylation and glucuronosylation reactions in rat liver Golgi apparatus and endoplasmic reticulum. J Biol Chem 261:12936–12941
Oegema TR Jr, Hascall VC, Dziewiatkowski DD (1975) Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem 250(15):6151–6159
Oegema TR Jr, Deloria LB, Sandy JD, Hart DA (2002) Effect of oral glucosamine on cartilage and meniscus in normal and chymopapain-injected knees of young rabbits. Arthritis Rheum 46(9):2495–2503
Ogino S, Nishida N, Umemoto R, Suzuki M, Takeda M, Terasawa H, Kitayama J, Matsumoto M, Hayasaka H, Miyasaka M, Shimada I (2010) Two-state conformations in the hyaluronan-binding domain regulate CD44 adhesiveness under flow condition. Structure 18(5):649–656
Ogston AG, Stanier JE (1950) On the state of hyaluronic acid in synovial fluid. Biochem J 46(3):364–376
Ohtake-Niimi S, Kondo S, Ito T, Kakehi S, Ohta T, Habuchi H, Kimata K, Habuchi O (2010) Mice deficient in N-acetylgalactosamine 4-sulfate 6-o-sulfotransferase are unable to synthesize chondroitin/dermatan sulfate containing N-acetylgalactosamine 4,6-bissulfate residues and exhibit decreased protease activity in bone marrow-derived mast cells. J Biol Chem 285(27):20793–20805
Oikari S, Makkonen K, Deen AJ, Tyni I, Kärnä R, Tammi RH, Tammi MI (2016) Hexosamine biosynthesis in keratinocytes: roles of GFAT and GNPDA enzymes in the maintenance of UDP-GlcNAc content and hyaluronan synthesis. Glycobiology 26(7):710–722
Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, Malmström A (2005) Regulation of the chondroitin/dermatan fine structure by transforming growth factor-beta1 through effects on polymer-modifying enzymes. Glycobiology 12:1277–1285
Onodera Y, Teramura T, Takehara T, Fukuda K (2015) Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio 5:476–484
Patwari P, Kurz B, Sandy JD, Grodzinsky AJ (2000) Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys 374(1):79–85
Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, Cole AA, Lark MW, Grodzinsky AJ (2003) Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum 48(5):1292–1301
Pereira J, Ottevaere I, Serruys B et al (2018) Pharmacokinetic and pharmacodynamic modelling of the novel anti-ADAMTS-5 nanobody M6495 using the neoepitope ARGS as a biomarker. Osteoarthr Cartil 26:S176
Peters A, Sherman LS (2020) Diverse roles for Hyaluronan and Hyaluronan receptors in the developing and adult nervous system. Int J Mol Sci 21(17):5988
Petrey AC, de la Motte CA (2014) Hyaluronan, a crucial regulator of inflammation. Front Immunol 5:101
Petrey AC, de la Motte CA (2019) Hyaluronan in inflammatory bowel disease: cross-linking inflammation and coagulation. Matrix Biol 78–79:314–323
Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM (2006) Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol 209(2):389–397
Plaas AH, Sandy JD (1993) A cartilage explant system for studies on aggrecan structure, biosynthesis and catabolism in discrete zones of the mammalian growth plate. Matrix 13(2):135–147
Plaas AH, Sandy JD, Kimura JH (1988) Biosynthesis of cartilage proteoglycan and link protein by articular chondrocytes from immature and mature rabbits. J Biol Chem 263(16):7560–7566
Plaas AH, Hascall VC, Midura RJ (1996) Ion exchange HPLC microanalysis of chondroitin sulfate: quantitative derivatization of chondroitin lyase digestion products with 2-aminopyridine. Glycobiology 6(8):823–829
Plaas AH, Wong-Palms S, Roughley PJ, Midura RJ, Hascall VC (1997) Chemical and immunological assay of the nonreducing terminal residues of chondroitin sulfate from human aggrecan. J Biol Chem 272(33):20603–20610
Plaas AH, West LA, Midura RJ (2001) Keratan sulfate disaccharide composition determined by FACE analysis of keratanase II and endo-beta-galactosidase digestion products. Glycobiology 11(10):779–790
Plaas A, Sandy JD, Liu H, Diaz MA, Schenkman D, Magnus RP, Bolam-Bretl C, Kopesky PW, Wang VM, Galante JO (2011) Biochemical identification and immunolocalization of aggrecan, ADAMTS5 and inter–trypsin-inhibitor in equine degenerative suspensory ligament desmitis. J Orthop Res 29:900–906
Pönighaus C, Ambrosius M, Casanova JC, Prante C, Kuhn J, Esko JD, Kleesiek K, Götting C (2007) Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J Biol Chem 282(8):5201–5206
Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 276(22):19420–19430
Quinn TM, Grodzinsky AJ, Buschmann MD, Kim YJ, Hunziker EB (1998a) Mechanical compression alters proteoglycan deposition and matrix deformation around individual cells in cartilage explants. J Cell Sci 111(Pt 5):573–583
Quinn TM, Grodzinsky AJ, Hunziker EB, Sandy JD (1998b) Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res 16(4):490–499
Ragan PM, Chin VI, Hung HH, Masuda K, Thonar EJ, Arner EC, Grodzinsky AJ, Sandy JD (2000) Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys 15; 383(2):256–264
Raman PS, Alves CS, Wirtz D, Konstantopoulos K (2011) Single-molecule binding of CD44 to fibrin versus P-selectin predicts their distinct shear-dependent interactions in cancer. J Cell Sci 124(Pt 11):1903–1910
Ratcliffe A, Hughes C, Fryer PR, Saed-Nejad F, Hardingham T (1987) Immunochemical studies on the synthesis and secretion of link protein and aggregating proteoglycan by chondrocytes. Coll Relat Res 7(6):409–421
Reiprich S, Akova E, Aszódi A, Schönitzer V (2021) Hyaluronan synthases’ expression and activity are induced by fluid shear stress in bone marrow-derived mesenchymal stem cells. Int J Mol Sci 22(6):3123
Richardson C, Plaas A, Block JA (2019) Intra-articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum Dis Clin N Am 45(3):439–451
Riegger J, Baumert J, Zaucke F, Brenner RE (2021) The Hexosamine biosynthetic pathway as a therapeutic target after cartilage Trauma: modification of chondrocyte survival and metabolism by glucosamine derivatives and PUGNAc in an ex vivo model. Int J Mol Sci 22(14):7247
Rivinoja A, Pujol FM, Hassinen A, Kellokumpu S (2012) Golgi pH, its regulation and roles in human disease. Ann Med 44(6):542–554
Rodriguez E, Roland SK, Plaas A, Roughley PJ (2006) The glycosaminoglycan attachment regions of human aggrecan. J Biol Chem 281(27):18444–18450
Rosenberg L, Hellmann W, Kleinschmidt AK (1975) Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem 250(5):1877–1883
Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD (1990) Effects of tissue compression on the hyaluronate-binding properties of newly synthesized proteoglycans in cartilage explants. Biochem J 267(3):803–808
Sah RL, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD (1991) Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys 286(1):20–29
Salo J, Kaigle Holm A, Indahl A, Mackiewicz Z, Sukura A, Holm S, Jämsen E, Konttinen YT (2008) Expression of vascular endothelial growth factor receptors coincide with blood vessel in-growth and reactive bone remodelling in experimental intervertebral disc degeneration. Clin Exp Rheumatol 26(6):1018–1026
Sandy JD, Plaas AH (1986) Age-related changes in the kinetics of release of proteoglycans from normal rabbit cartilage explants. J Orthop Res 4(3):263–272
Sandy JD, Flannery CR, Neame PJ, Lohmander LS (1992) The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest 89(5):1512–1516
Sandy JD, Gamett D, Thompson V, Verscharen C (1998) Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J 335(Pt 1):59–66
Sandy JD, Thompson V, Doege K, Verscharen C (2000) The intermediates of aggrecanase-dependent cleavage of aggrecan in rat chondrosarcoma cells treated with interleukin-1. Biochem J 351(Pt 1):161–166
Santamaria S, Yamamoto K, Botkjaer K, Tape C, Dyson MR, McCafferty J, Murphy G, Nagase H (2015) Antibody-based exosite inhibitors of ADAMTS-5 (aggrecanase-2). Biochem J 471(3):391–401
Santamaria S, Fedorov O, McCafferty J, Murphy G, Dudhia J, Nagase H, Yamamoto K (2017) Development of a monoclonal anti-ADAMTS-5 antibody that specifically blocks the interaction with LRP1. MAbs 9(4):595–602
Sasaki K, Yoshida H (2019) Golgi stress response and organelle zones. FEBS Lett 593(17):2330–2340
Sato T, Gotoh M, Kiyohara K, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, Yada T, Inaba N, Togayachi A, Kudo T, Asada M, Watanabe H, Imamura T, Kimata K, Narimatsu H (2003) Differential roles of two N-acetylgalactosaminyl-transferases, CSGalNAcT-1 and a novel enzyme CSGalNAcT-2 Initiation and elongation in synthesis of chondroitin sulfate. J Biol Chem 278:3063–3071
Sauerland K, Plaas AH, Raiss RX, Steinmeyer J (2003) The sulfation pattern of chondroitin sulfate from articular cartilage explants in respo2 to mechanical loading. Biochim Biophys Acta 1638(3):241–248
Sauerland K, Wolf A, Schudok M, Steinmeyer J (2021) A novel model of a biomechanically induced osteoarthritis-like cartilage for pharmacological in vitro studies. J Cell Mol Med 25(24):11221–11231
Scavenius C, Poulsen EC, Thøgersen IB, Roebuck M, Frostick S, Bou-Gharios G, Yamamoto K, Deleuran B, Enghild JJ (2019) Matrix-degrading protease ADAMTS-5 cleaves inter-α-inhibitor and releases active heavy chain 2 in synovial fluids from arthritic patients. J Biol Chem 294(42):15495–15504
Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL (2007) Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum 56(3):882–891
Schneckmann R, Suvorava T, Hundhausen C, Schuler D, Lorenz C, Freudenberger T, Kelm M, Fischer JW, Flögel U, Grandoch M (2021) Endothelial Hyaluronan synthase 3 augments postischemic arteriogenesis through CD44/eNOS signaling. Arterioscler Thromb Vasc Biol 41(10):2551–2562
Senol O, Gundogdu G, Gundogdu K, Miloglu FD (2019) Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol 38(5):1351–1360
Sherpa MT, Kiwamoto T, Matsuyama M, Tsunoda Y, Yazaki K, Yoshida K, Nakajima M, Matsuno Y, Morishima Y, Ishii Y, Hizawa N (2022) Has2 regulates the development of Ovalbumin-induced airway remodeling and steroid insensitivity in mice. Front Immunol 12:770305
Shimbo M, Suzuki R, Fuseya S, Sato T, Kiyohara K, Hagiwara K, Okada R, Wakui H, Tsunakawa Y, Watanabe H, Kimata K, Narimatsu H, Kudo T, Takahashi S (2017) Postnatal lethality and chondrodysplasia in mice lacking both chondroitin sulfate N-acetylgalactosaminyltransferase-1 and -2. PLoS One 12(12):e0190333
Shimizu H, Shimoda M, Mochizuki S, Miyamae Y, Abe H, Chijiiwa M, Yoshida H, Shiozawa J, Ishijima M, Kaneko K, Kanaji A, Nakamura M, Toyama Y, Okada Y (2018) Hyaluronan-binding protein involved in Hyaluronan depolymerization is up-regulated and involved in Hyaluronan degradation in human osteoarthritic cartilage. Am J Pathol 188(9):2109–2119
Showiheen SAA, Sun AR, Wu X, Crawford R, Xiao Y, Wellard RM, Prasadam I (2019) Application of metabolomics to osteoarthritis: from basic science to the clinical approach. Curr Rheumatol Rep 21(6):26
Sikes KJ, Renner K, Li J, Grande-Allen KJ, Connell JP, Cali V, Midura RJ, Sandy JD, Plaas A, Wang VM (2018) Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. J Orthop Res 36(10):2622–2632
Silbert JE, DeLuca S (1968) Formation of chondroitin sulfate by a microsomal preparation from chick embryo epiphyseal cartilage. Biochem Biophys Res Commun 31(6):990–995
Smith RL, Carter DR, Schurman DJ (2004) Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res (427 Suppl):S89–S95
Sodhi H, Panitch A (2020) Glycosaminoglycans in tissue engineering: a review. Biomolecules 29; 11(1):29
Somemura S, Kumai T, Yatabe K, Sasaki C, Fujiya H, Niki H, Yudoh K (2021) Physiologic mechanical stress directly induces bone formation by Activating Glucose Transporter 1 (Glut 1) in Osteoblasts, inducing signaling via NAD+-Dependent Deacetylase (Sirtuin 1) and Runt-Related Transcription Factor 2 (Runx2). Int J Mol Sci 22(16):9070
Song Z (2013) Roles of the nucleotide sugar transporters (SLC35 family) in health and disease. Mol Asp Med 34(2-3):590–600
Southan J, McHugh E, Walker H, Ismail HM (2020) Metabolic signature of articular cartilage following mechanical injury: an integrated transcriptomics and metabolomics analysis. Front Mol Biosci 7:592905
Spicer AP, Kaback LA, Smith TJ, Seldin MF (1998) Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem 273(39):25117–25124
Stampoultzis T, Karami P, Pioletti DP (2021) Thoughts on cartilage tissue engineering: a 21st century perspective. Curr Res Transl Med 69(3):103299
Stanton H, Rogerson FM, East CJ et al (2005) ADAMTS-5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434:648–652
Stoolmiller AC, Horwitz AL, Dorfman A (1972) Biosynthesis of the chondroitin sulfate proteoglycan. Purification and properties of xylosyltransferase. J Biol Chem 10; 247(11):3525–3532
Sugumaran G, Coburn JN, Silbert JE (1986) Simultaneous sulfation of endogenous chondroitin sulfate and chondroitin-derived oligosaccharides. Studies with separate 4-sulfating and 6-sulfating microsomal systems. J Biol Chem 261(27):12659–12664. 45–48
Sugumaran G, Katsman M, Silbert JE (1992) Effects of brefeldin A on the localization of chondroitin sulfate-synthesizing enzymes. Activities in subfractions of the Golgi from chick embryo epiphyseal cartilage. J Biol Chem 267:8802–8806
Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA (2013) TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem 288(1):412–422
Swann DA, Radin EL, Nazimiec M, Weisser PA, Curran N, Lewinnek G (1974) Role of hyaluronic acid in joint lubrication. Ann Rheum Dis 33(4):318–326
Swann DA, Powell S, Broadhurst J, Sordillo E, Sotman S (1976) The formation of a stable complex between dissociated proteoglycan and hyaluronic acid in the absence of a link protein. Biochem J A157(2):503–506
Szafranski JD, Grodzinsky AJ, Burger E, Gaschen V, Hung HH, Hunziker EB (2004) Chondrocyte mechanotransduction: effects of compression on deformation of intracellular organelles and relevance to cellular biosynthesis. Osteoarthr Cartil 12(12):937–946
Takemura M, Itoh H, Sagawa N, Yura S, Korita D, Kakui K, Kawamura M, Hirota N, Maeda H, Fujii S (2005) Cyclic mechanical stretch augments hyaluronan production in cultured human uterine cervical fibroblast cells. Mol Hum Reprod 11(9):659–665
Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J 278(9):1419–1428
Tobisawa Y, Fujita N, Yamamoto H, Ohyama C, Irie F, Yamaguchi Y (2021) The cell surface hyaluronidase TMEM2 is essential for systemic hyaluronan catabolism and turnover. J Biol Chem 297(5):101281
Toole BP (2000) Hyaluronan is not just a goo! J Clin Invest 106(3):335–336
Tortorella MD, Burn TC, Pratta MA et al (1999) Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284:1664–1666
Triggs-Raine B, Natowicz MR (2015) Biology of hyaluronan: insights from genetic disorders of hyaluronan metabolism. World J Biol Chem 6(3):110–120
Tsiganos CP, Hardingham TE, Muir H (1972) Aggregation of cartilage proteoglycans. Biochem J 128(4):121P
Tsuchiya S, Ohashi Y, Ishizuka S, Ishiguro N, O’Rourke DP, Knudson CB, Knudson W (2020) Suppression of murine osteoarthritis by 4-methylumbelliferone. J Orthop Res 38(5):1122–1131
Tsuji S, Nakamura S, Yamada T, de Vega S, Okada Y, Inoue S, Shimazawa M, Hara H (2021) HYBID derived from tumor cells and tumor-associated macrophages contribute to the glioblastoma growth. Brain Res 1764:147490
Turley EA, Noble PW, Bourguignon LY (2002) Signaling properties of hyaluronan receptors. J Biol Chem 277(7):4589–4592
Uhlin-Hansen L, Yanagishita M (1993) Defferential effect of brefeldin A on the biosynthesis of heparan sulfate and chondroitin/dermatan sulfate proteoglycans in rat ovarian granulosa cells in culture. J Biol Chem 268:17370–17376
Vaupel P, Multhoff G (2021) Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 599(6):1745–1757
Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, Plaas A (2011) Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor β1 (TGFβ1) signaling. J Biol Chem 286(29):26016–26027
Vertel BM, Walters LM, Flay N, Kearns AE, Schwartz NB (1993) Xylosylation is an endoplasmic reticulum to Golgi event. J Biol Chem 268:11105–11112
Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, Maroudas A, TeKoppele JM (2001) Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol 20(7):409–417
Victor XV, Nguyen TK, Ethirajan M, Tran VM, Nguyen KV, Kuberan B (2009) Investigating the elusive mechanism of glycosaminoglycan biosynthesis. J Biol Chem 284(38):25842–25853
Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, Heldin P, Hascall VC, De Luca G, Passi A (2011) Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem 286(10):7917–7924
Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A (2012) Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287(42):35544–35555
Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A (2014) Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta 1840(8):2452–2459
Wang SX, Laverty S, Dumitriu M, Plaas A, Grynpas MD (2007) The effects of glucosamine hydrochloride on subchondral bone changes in an animal model of osteoarthritis. Arthritis Rheum 56(5):1537–1548
Wang A, de la Motte C, Lauer M, Hascall V (2011) Hyaluronan matrices in pathobiological processes. FEBS J 278(9):1412–1418
Wang VM, Bell RM, Thakore R, Eyre DR, Galante JO, Li J, Sandy JD, Plaas A (2012) Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res 30(4):620–626
Wang Y, Li Q, Liu F, Jin S, Zhang Y, Zhang T, Zhu Y, Zhou Y (2018) Transcriptional activation of glucose transporter 1 in orthodontic tooth movement-associated mechanical response. Int J Oral Sci 15; 10(3):27
Wang G, Kostidis S, Tiemeier GL, Sol WMPJ, de Vries MR, Giera M, Carmeliet P, van den Berg BM, Rabelink TJ (2020) Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler Thromb Vasc Biol 40(2):350–364
Watanabe Y, Takeuchi K, Higa Onaga S, Sato M, Tsujita M, Abe M, Natsume R, Li M, Furuichi T, Saeki M, Izumikawa T, Hasegawa A, Yokoyama M, Ikegawa S, Sakimura K, Amizuka N, Kitagawa H, Igarashi M (2010) Chondroitin sulfate N-acetylgalactosaminyltransferase-1 is required for normal cartilage development. Biochem J 432(1):47–55
Weber JF, Perez R, Waldman SD (2015) Mechanobioreactors for cartilage tissue engineering. Methods Mol Biol 1340:203–219
Weigel PH (2015) Hyaluronan synthase: the mechanism of initiation at the reducing end and a Pendulum model for Polysaccharide Translocation to the cell exterior. Int J Cell Biol 2015:367579
Weigel PH (2019) Discovery of the liver Hyaluronan Receptor for endocytosis (HARE) and its progressive emergence as the multi-ligand scavenger receptor Stabilin-2. Biomolecules. 9(9):454
Weigel PH, DeAngelis PL (2007) Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282(51):36777–36781
Weigel JA, Weigel PH (2003) Characterization of the recombinant rat 175-kDa hyaluronan receptor for endocytosis (HARE). J Biol Chem 278(44):42802–42811
Weigel PH, Hascall VC, Tammi M (1997) Hyaluronan synthases. J Biol Chem 272(22):13997–13400
West LA, Roughley P, Nelson FR, Plaas AH (1999) Sulphation heterogeneity in the trisaccharide (GalNAcSbeta1, 4GlcAbeta1, 3GalNAcS) isolated from the non-reducing terminal of human aggrecan chondroitin sulphate. Biochem J 342:223–229
Windhaber RA, Wilkins RJ, Meredith D (2003) Functional characterization of glucose transport in bovine articular chondrocytes. Pflugers Arch 446(5):572–577
Wu J, Qu Y, Zhang YP, Deng JX, Yu QH (2018) RHAMM induces progression of rheumatoid arthritis by enhancing the functions of fibroblast-like synoviocytes. BMC Musculoskelet Disord D19(1):455
Wu ZY, He YQ, Wang TM, Yang DW, Li DH, Deng CM, Cao LJ, Zhang JB, Xue WQ, Jia WH (2021) Glycogenes in Oncofetal Chondroitin Sulfate biosynthesis are differently expressed and correlated with immune response in placenta and colorectal cancer. Front Cell Dev Biol 9:763875
Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA (2003) Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis 62(12):1227–1229
Yada T, Gotoh M, Sato T, Shionyu M, Go M, Kaseyama H, Iwasaki H, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Watanabe H, Narimatsu H, Kimata K (2003) Chondroitin sulfate synthase-2. Molecular cloning and characterization of a novel human glycosyltransferase homologous to chondroitin sulfate glucuronyltransferase which has dual enzymatic activities. J Biol Chem 278:30235–30247
Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y (2017) A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem 292(18):7304–7313
Yao Q, Parvez-Khan M, Schipani E (2020) In vivo survival strategies for cellular adaptation to hypoxia: HIF1α-dependent suppression of mitochondrial oxygen consumption and decrease of intracellular hypoxia are critical for survival of hypoxic chondrocytes. Bone 140:115572
Yingsung W, Zhuo L, Morgelin M, Yoneda M, Kida D, Watanabe H, Ishiguro N, Iwata H, Kimata K (2003) Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J Biol Chem 278(35):32710–32718
Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S (2013a) KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A 110(14):5612–5617
Yoshida H, Nagaoka A, Nakamura S, Sugiyama Y, Okada Y, Inoue S (2013b) Murine homologue of the human KIAA1199 is implicated in hyaluronan binding and depolymerization. FEBS Open Bio 3:352–356
Yoshihara Y, Plaas A, Osborn B, Margulis A, Nelson F, Stewart M, Rugg MS, Milner CM, Day AJ, Nemoto K, Sandy JD (2008) Superficial zone chondrocytes in normal and osteoarthritic human articular cartilages synthesize novel truncated forms of inter-alpha-trypsin inhibitor heavy chains which are attached to a chondroitin sulfate proteoglycan other than bikunin. Osteoarthr Cartil 16(11):1343–1355
Yoshino Y, Shimazawa M, Nakamura S, Inoue S, Yoshida H, Shimoda M, Okada Y, Hara H (2018) Targeted deletion of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization/KIAA1199/CEMIP) decreases dendritic spine density in the dentate gyrus through hyaluronan accumulation. Biochem Biophys Res Commun 503(3):1934–1940
Yung S, Thomas GJ, Davies M (2000) Induction of hyaluronan metabolism after mechanical injury of human peritoneal mesothelial cells in vitro. Kidney Int 58(5):1953–1962
Zhang K, Wang L, Liu Z, Geng B, Teng Y, Liu X, Yi Q, Yu D, Chen X, Zhao D, Xia Y (2021a) Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels (Austin) 15(1):339–359
Zhang W, Yin G, Zhao H, Ling H, Xie Z, Xiao C, Chen Y, Lin Y, Jiang T, Jin S, Wang J, Yang X (2021b) Secreted KIAA1199 promotes the progression of rheumatoid arthritis by mediating hyaluronic acid degradation in an ANXA1-dependent manner. Cell Death Dis 12(1):102
Zhao M, Yoneda M, Ohashi Y, Kurono S, Iwata H, Ohnuki Y, Kimata K (1995) Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J Biol Chem 270(44):26657–26663
Zheng L, Zhang Z, Sheng P, Mobasheri A (2021) The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev 66:101249
Zhuo L, Hascall VC, Kimata K (2004) Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem 279(37):38079–38082
Zimmer BM, Barycki JJ, Simpson MA (2021) Integration of sugar metabolism and proteoglycan synthesis by UDP-glucose dehydrogenase. J Histochem Cytochem 69(1):13–23
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Plaas, A.H.K., Moran, M.M., Sandy, J.D., Hascall, V.C. (2023). Aggrecan and Hyaluronan: The Infamous Cartilage Polyelectrolytes – Then and Now. In: Connizzo, B.K., Han, L., Sah, R.L. (eds) Electromechanobiology of Cartilage and Osteoarthritis. Advances in Experimental Medicine and Biology, vol 1402. Springer, Cham. https://doi.org/10.1007/978-3-031-25588-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-25588-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25587-8
Online ISBN: 978-3-031-25588-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)