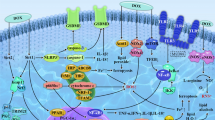
Abstract
Since the discovery of Doxorubicin (Dox), its safe use is under intense discussion due to its cardiotoxic side effects manifested in patients at different times during the treatment or even after years of treatment. Several therapeutic approaches to replace conventional Dox or use of other drugs in combination have exhausted the clinicians and researchers without much success. When the replacement strategies failed to show any rigor, a better understanding of Doxorubicin’s mechanism of action seemed like the only gateway to the discovery of a new targeted therapeutic approach. An increase in reactive oxygen species and the resultant oxidative stress as the mechanism of Dox-induced cardiomyopathy, proposed by us as well as others has gained some traction. However, this explanation has not been enough to alleviate concerns with Dox and we are still in search for a solution for its safe use. More recently, our laboratory and others have also shown the importance of nitrosative stress. Furthermore, we have shown that Vitamin C not only mitigates nitrosative stress but it also modulates Dox-induced cardiotoxic changes in isolated cardiomyocytes as well as in whole animals exposed to Dox. The present review chapter focusses on the mechanism of Dox-induced nitrosative stress and the role of Vitamin C in mitigating the cardiotoxic effects of Dox.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Arcamone F, Cossinellie G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C (1969) Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var.caesius. Biotechnol Bioeng 11:1101–1110
Lefrak EA, Pitha S, Rosenheim JA, Gottlieb A (1973) A clinico-pathologic analysis of adriamycin cardiotoxicity. Cancer 32:302–314
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900–905
Singal PK, Iliskovic N, Li T, Kumar D (1997) Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J 11:931–936
Singal PK, Olweny C, Li T (1999) Doxorubicin-induced cardiomyopathy: Correspondence. New Engl J Med 340:655
Lehenbauer Ludke AR, Al-Shudiefat AA, Dhingra S, Jassal DS, Singal PK (2009) A concise description of cardioprotective strategies in doxorubicin-induced cardiotoxicity. Can J Physiol Pharmacol 87:756–763
Chambers JT, Chambers SK, Kohorn EI, Carcangiu ML, Schwartz PE (1996) Uterine papillary serous carcinoma treated with intraperitoneal cisplatin and intravenous doxorubicin and cyclophosphamide. Gynecol Oncol 60:438–442
Weiss RB (1992) The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19:670–686
Van Tine BA, Agulnik M, Olson RD, Walsh GM, Klausner A, Frank NE, Talley TT, Milhem MM (2019) A phase II clinical study of 13-deoxy, 5-iminodoxorubicin (GPX-150) with metastatic and unresectable soft tissue sarcoma. Cancer Med 8:2994–3003
Ngan YH, Gupta M (2016) A comparison between liposomal and nonliposomal formulations of doxorubicin in the treatment of cancer: An updated review. Arch Pharm Pract 7:1–13
Zhao N, Woodle MC, Mixson AJ (2018) Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol 9:519
Chun C, Lee SM, Kim CW, Hong KY, Kim SY, Yang HK, Song SC (2009) Doxorubicin-polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials 30:4752–4762
Wu W, Chen H, Shan F, Zhou J, Sun X, Zhang L, Gong T (2014) A novel doxorubicin-loaded in situ forming gel based high concentration of phospholipid for intratumoral drug delivery. Mol Pharm 11:3378–3385
Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324:808–815
Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, Nagai R (2000) Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res 86:8–14
Ludke AR, Sharma AK, Akolkar G, Bajpai G, Singal PK (2012) Downregulation of vitamin C transporter SVCT-2 in doxorubicin-induced cardiomyocyte injury. Am J Physiol Cell Physiol 303:C645–C653
Pacher P, Obrosova IG, Mabley JG, Szabo C (2005a) Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 12:267–275
Pacher P, Schulz R, Liaudet L, Szabo C (2005b) Nitrosative stress and pharmacological modulation of heart failure. Trends Pharcol Sci 26:302–310
Szabo C (2009) Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol 156:713–727
Akolkar G, Bagchi AK, Ayyappan P, Jassal DS, Singal PK (2017) Doxorubicin induced nitrosative stress is mitigated by vitamin C via the modulation of nitric oxide synthases. Am J Physiol Cell Physiol 312:418–427
Akolkar G, da Silva DD, Ayyappan P, Bagchi AK, Jassal DS, Salemi VM, Irigoyen MC, Singal PK (2017) Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 313:H795–H809
Hare JM, Stamler JS (2005) NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest 115:509–517
Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P (2009) Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296:H1466–H1483
Lancaster JR (2006) Nitroxidative, nitrosative, and nitrative stress: Kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol 19:1160–1174
Weinstein DM, Mihm MJ, Bauer JA (2000) Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Therap 294:396–401
Pacher P, Szabo C (2006) Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol 6:136–141
Salvemini D, Doyle TM, Cuzzocrea S (2006) Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans 34:965–970
Turko IV, Murad F (2002) Protein nitration in cardiovascular diseases. Pharmacol Rev 54:619–634
Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L (2006) Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Rad Biol Med 41:886–895
Brown GC (2001) Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504:46–57
MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci 93:11853–11858
Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM (2001) Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-kappa B activation: role of H(2)O(2) and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide 5:504–513
Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA (2002) Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Brit J Pharmacol 135:581–588
Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P (2005) Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol 3:221–229
Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z (2014) Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 63:1381–1393
Bruckdorfer R (2005) The basics about nitric oxide. Mol Aspects Med 26:3–31
Bian K, Murad F (2014) What is next in nitric oxide research? from cardiovascular system to cancer biology. Nitric Oxide 43:3–7
Liu VW, Huang PL (2008) Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res 77:19–29
Bian K, Doursout MF, Murad F (2008) Vascular system: role of nitric oxide in cardiovascular diseases. J Clins Hypertens 10:304–310
Katsuki S, Arnold W, Mittal C, Murad F (1977) Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res 3:23–35
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Colasanti M, Suzuki H (2000) The dual personality of NO. Trends Pharmacol Sci 21:249–252
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Mocellin S, Bronte V, Nitti D (2007) Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev 27:317–352
Villanueva C, Giulivi C (2010) Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Rad Biol Med 49:307–316
Massion PB, Feron O, Dessy C, Balligand JL (2003) Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93:388–398
Ziolo MT, Kohr MJ, Wang H (2008) Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol 45:625–632
Singh S, Gupta AK (2011) Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother Pharmacol 67:1211–1224
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:837a–837d
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER (2001) L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276:40–47
Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24:413–420
Channon KM (2004) Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14:323–327
Knowles RG, Moncada S (1994) Nitric oxide synthases in mammals. Biomed J 298:249–258
Fleming I (2010) Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459:793–806
Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, Busse R (2003) AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. J Thromb Haemost 90:863–871
Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA Jr, Sessa WC (2003) Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem 278:44719–44726
Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, Heiss EH (2012) Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52:2082–2090
Xu L, Eu JP, Meissner G, Stamler JS (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279:234–237
Umar S, van der Laarse A (2010) Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 333:191–201
Gratton JP, Fontana J, O’Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC (2000) Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 275:22268–22272
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J et al (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293:2449–2452
Treuer AV, Gonzalez DR (2015) Nitric oxide synthases, S-nitrosylation and cardiovascular health: from molecular mechanisms to therapeutic opportunities (review). Mol Med Rep 11:1555–1565
Feron O (2006) Role of caveolin in angiogenesis and vasculogenesis processes: therapeutic implications in ischemic diseases and cancerology. Bull Mem Acad R Med Belg 161:290–291
Chen W, Xiao H, Rizzo AN, Zhang W, Mai Y, Ye ME (2014) Endothelial nitric oxide synthase dimerization is regulated by heat shock protein 90 rather than by phosphorylation. PLoS ONE 9:e105479
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS (2003) Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 92:88–95
Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N (2005) NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 288:F1144–F1152
Ziolo MT, Bers DM (2003) The real estate of NOS signaling: Location, location, location. Circ Res 92:1279–1281
Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B (2002) Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation 105:3011–3016
Hibbs JB Jr (1991) Synthesis of nitric oxide from L-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res Immunol 142:596–598
Rao VA, Zhang J, Klein SR, Espandiari P, Knapton A, Dickey JS et al (2011) The iron chelator Dp44mT inhibits the proliferation of cancer cells but fails to protect from doxorubicin-induced cardiotoxicity in spontaneously hypertensive rats. Cancer Chemother Pharmacol 68:1125–1134
Xia YF, Liu LP, Zhong CP, Geng JG (2001) NF-kappaB activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochem Biophys Res Commun 289:851–856
Kroncke KD, Fehsel K, Kolb-Bachofen V (1997) Nitric oxide: cytotoxicity versus cytoprotection–how, why, when, and where? Nitric Oxide 1:107–120
De Alba J, Cardenas A, Moro MA, Leza JC, Lorenzo P, Lizasoain I (1999) Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischemia-reperfusion. Gen Pharmacol 32:577–581
Murad F (2011) Nitric oxide: The coming of the second messenger. Rambam Maimonides Med J 2:e0038
Bryan NS, Bian K, Murad F (2009) Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci 14:1–18
Lima B, Forrester MT, Hess DT, Stamler JS (2010) S-nitrosylation in cardiovascular signaling. Circ Res 106:633–646
Irie T, Sips PY, Kai S, Kida K, Ikeda K, Hirai S, Moazzami K et al (2015) S-nitrosylation of calcium-handling proteins in cardiac adrenergic signaling and hypertrophy. Circ Res 117:793–803
Kouti L, Noroozian M, Akhondzadeh S, Abdollahi M, Javadi MR, Faramarzi MA, Mousavi S, Ghaeli P (2013) Nitric oxide and peroxynitrite serum levels in Parkinson’s disease: correlation of oxidative stress and the severity of the disease. Eur Rev Med Pharmacol Sci 17:964–970
Ben Anes A, Fetoui H, Bchir S, Ben Nasr H, Chahdoura H, Chabchoub E, Yacoub S, et al (2014) Increased oxidative stress and altered levels of nitric oxide and peroxynitrite in Tunisian patients with chronic obstructive pulmonary disease: Correlation with disease severity and airflow obstruction. Biol Trace Elem Res 161:2031
Islam BU, Habib S, Ahmad P, Allarakha S, Moinuddin AA (2015) Pathophysiological role of peroxynitrite induced DNA damage in human diseases: A special focus on poly (ADP-ribose) polymerase (PARP). Indian J Clin Biochem 30:368–385
Sayed-Ahmed MM, Khattab MM, Gad MZ, Osman AM (2001) Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol Toxicol 89:140–144
Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R (2000) Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res 87:241–247
Beltran B, Orsi A, Clementi E, Moncada S (2000) Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol 129:953–960
Iwakiri Y (2011) S-nitrosylation of proteins: A new insight into endothelial cell function regulated by eNOS-derived NO. Nitric Oxide 25:95–101
Ravi K, Brennan LA, Levic S, Ross PA, Black SM (2004) S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci 101:2619–2624
Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A et al (2003) Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107:896–904
Andreadou I, Sigala F, Iliodromitis EK, Papaefthimiou M, Sigalas C, Aligiannis N, Savvari P, Gorgoulis V, Papalabros E, Kremastinos DT (2007) Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol 42:549–558
Andreadou I, Mikros E, Ioannidis K, Sigala F, Naka K, Kostidis S, Farmakis D, Tenta R, Kavantzas N, Bibli SI, Gikas E, Skaltsounis L, Kremastinos DT, Iliodromitis EK (2014) Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J Mol Cell Cardiol 69:4–16
Oktem G, Uysal A, Oral O, Sezer ED, Olukman M, Erol A, Akgur SA, Bilir A (2012) Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol 64:471–479
Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43:521–531
Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA (2002) Modulation by peroxynitrite of akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277:32552–32557
Yang YM, Huang A, Kaley G, Sun D (2009) eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297:H1829–H1836
Gamez-Mendez AM, Vargas-Robles H, Rios A, Escalante B (2015) Oxidative stress-dependent coronary endothelial dysfunction in obese mice. PLoS ONE 10:e0138609
Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, Furutani E et al (2007) Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 116:506–514
Deng S, Kruger A, Schmidt A, Metzger A, Yan T, Godtel-Armbrust U, Hasenfuss G, Brunner F, Wojnowski L (2009) Differential roles of nitric oxide synthase isozymes in cardiotoxicity and mortality following chronic doxorubicin treatment in mice. Naunyn-Schmiedeberg’s Arch Pharmacol 380:25–34
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature 399:601–605
Aldieri E, Bergandi L, Riganti C, Costamagna C, Bosia A, Ghigo D (2002) Doxorubicin induces an increase of nitric oxide synthesis in rat cardiac cells that is inhibited by iron supplementation. Tox Appl Pharmacol 185:85–90
Ropelle ER, Pauli JR, Cintra DE, da Silva AS, De Souza CT, Guadagnini D, Carvalho BM et al (2013) Targeted disruption of inducible nitric oxide synthase protects against aging, S-nitrosation, and insulin resistance in muscle of male mice. Diabetes 62:466–470
Feng Q, Lu X, Jones DL, Shen J, Arnold AM (2001) Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation 104:700–704
Zhu J, Zhang J, Zhang L, Du R, Xiang D, Wu M, Zhang R, Han W (2011) Interleukin-1 signaling mediates acute doxorubicin-induced cardiotoxicity. Biomed Pharmacother 65:481–485
Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH (1999) Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res 84:21–33
Bowie AG, O’Neill LA (2000) Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol 165:7180–7188
Acknowledgements
Dr. P. K. Singal is the holder of the Dr. Naranjan S. Dhalla Chair in Cardiovascular Research supported by the St. Boniface Hospital Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Akolkar, G., Malik, A., Bagchi, A.K., Singla, D.K., Khaper, N., Singal, P.K. (2023). Role of Nitric Oxide Synthases in Doxorubicin-Induced Cardiomyopathy. In: Ray, A., Gulati, K. (eds) Nitric Oxide: From Research to Therapeutics. Advances in Biochemistry in Health and Disease, vol 22. Springer, Cham. https://doi.org/10.1007/978-3-031-24778-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-24778-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24777-4
Online ISBN: 978-3-031-24778-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)