Abstract
In the palliative management of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who are not candidates for a complete resection or full-dose radiotherapy, systemic treatment has seen important advances over the past several decades. In general, there are six major factors impacting on the decision-making process. Four of them belong to a class of continuous functions and include overall health status (from fitness to frailty), disease burden (from high to low), pace of the disease (from fast to slow), and expression of programmed-death ligand 1 (PD-L1, from high to low). In addition, there are two categorical variables including disease site (e.g., locoregional recurrence versus metastatic) and platinum-sensitivity or resistance depending on disease-free interval after previous platinum-based therapy with a usual cut-off of 6 months. Taking into account these six factors and local drug policies, healthcare professionals opt either for 1) chemotherapy with or without cetuximab or 2) immunotherapy with or without chemotherapy. In platinum-sensitive cases, level I evidence based on data from the EXTREME and Keynote-048 randomized trials supports the use of the following three regimens. Biochemotherapy combining platinum, 5-fluorouracil, and cetuximab (the so-called EXTREME regimen) is suitable for fit patients with low PD-L1 expression measured as combined positive score (CPS). Higher CPS is predictive for improved overall survival when replacing cetuximab with the immune checkpoint inhibitor pembrolizumab, an anti-PD-1 antibody (immunochemotherapy regimen). Further, Keynote-048 demonstrated activity of single-agent pembrolizumab in patients with high CPS values. The latter (third) treatment retained its efficacy in the elderly, suggesting possible advantage in less fit patients who otherwise receive best supportive care only or single-agent cytotoxic chemotherapy with dubious impact on survival. In selected patients, the TPEx regimen consisting of cisplatin, docetaxel, and cetuximab represents an alternative to EXTREME. Treatment choice can also be influenced by disease extension (site). Compared with disseminated cancer cases, presence of locoregional recurrence without distant metastases may have a negative predictive value for immune checkpoint inhibitors, while favouring biochemotherapy. If the tumour is deemed platinum-resistant, the only evidence-based systemic approach is monotherapy with either pembrolizumab or nivolumab, another anti-PD-1 antibody. Alternatively, being especially pertinent to resource-limited countries, a taxane with or without cetuximab can be prioritized. Obviously, the list of different treatment schedules is longer, but the level of supporting evidence is proportionally lower. One of modern approaches to multidisciplinary management of SCCHN patients is treatment sequencing. It should be understood as a deliberate process of treatment planning typically starting in the locally advanced setting and reaching beyond several treatment failures. This has been enabled by a growing portfolio of effective anticancer modalities complemented by progress in supportive care. Finally, all therapeutic interventions impact somehow on quality of life, either in a positive or negative way, and the choice of anticancer agents should therefore not be reduced to a simple estimate of survival benefit but should contain an adequate appraisal and understanding of individual patient’s situation comprising emotional and spiritual dimensions, cultural and financial aspects, and environmental, social, and educational contexts.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Treatment sequencing
- Conventional chemotherapy
- Cisplatin
- Carboplatin
- Targeted therapy
- Cetuximab
- Pembrolizumab
- Nivolumab
- Paclitaxel
- Docetaxel
Introduction
In squamous cell carcinoma of the head and neck (SCCHN), therapeutic decision making depends on several tumour-, patient-, and institution-related factors, the former being defined by a well-known categorization into localized (also known as early), locally (or locoregionally) advanced, and metastatic disease. According to the US Surveillance, Epidemiology, and End Results (SEER) Program data, about one third of newly diagnosed patients present with a localized tumour, almost half of them with locally advanced disease, and up to 20% may have distant dissemination. While cure rates of early disease surpass 80%, they almost halve in locally advanced SCCHN due to high rates of recurrences manifesting in about 60% of cases despite combined modality treatment instead of single-modality surgery or radiotherapy used in early disease [1, 2]. In oropharyngeal cancer, the decline in prognosis has been shown to be neutralized by human papillomavirus (HPV)-positivity [3]. On the other hand, even in developed countries, this favourable, viral-related subgroup represents only a minority of SCCHN [4]. Long-term survivorship is further halved in patients with distant metastases notwithstanding the introduction of targeted systemic agents and immunotherapy [5, 6]. The latter prognostic group consists of recurrent tumours as well, except for those that are salvaged with surgery or radiotherapy, particularly in the case of early larynx relapses and limited metastatic recurrences (oligorecurrences) of HPV-positive oropharyngeal cancer in the lungs [7, 8]. Relapses affect about 10-15% of patients with early disease, but they are up to 4 times more frequent in locally advanced disease [9,10,11,12]. Depending on primary tumour site, HPV-positivity in oropharyngeal cancer, primary treatment, and intensity of follow-up, the ratio between early, locoregional, and distant recurrences is roughly comparable with possibly a slight predominance of locoregional relapses [2, 13, 14]. According to disease stage at relapse, salvage surgery and/or (chemo)radiotherapy usually offered to the majority of patients without distant metastases yields five-year survival rates between 30 and 40% on average with even better outcomes after surgical resection [7, 14]. Importantly, these snapshot clinical scenarios need to be put in the context of gradual cancer progression towards more advanced stages, occurring at different rates in different individuals and both in the primary and recurrent disease settings. The respective treatment outcomes are summarized in Fig. 13.1.
In mucosal head and neck squamous cell carcinoma, survival outcomes differ according to clinical presentation. Disease progression is a continuous process, and recurrences are common. Apart from several exceptions, poorer prognosis correlates with more advanced stages both in the primary and recurrent settings. Long-term survival in local and regional stages corresponds to a period of at least 5 years, while in the metastatic setting we rely on the results of the Keynote-048 trial, as reported in 2019, with a median follow-up of 13 months in the immunochemotherapy arm and expect that mature data will probably show inferior outcomes as was the case of CheckMate-141
Similarly entangled are therapeutic strategies defining antitumoral management of each of these disease categories. In this chapter, we will put the first-line palliative treatment in broader context and try to decipher this entanglement. Figure 13.2 illustrates the development of anticancer modalities in two temporal axes. The horizontal axe signifies historical evolution covering the modern era since 1970s, when the current concept of multimodality approach was grounded. Being one of the typical examples of a patient’s journey through the diagnosis and treatment of SCCHN as indicated above, the vertical axe demonstrates treatment sequencing models in a patient with initially locally advanced disease that later recurs and requires systemic therapy. Until the beginning of the 20th century, management of head and neck cancer was governed by surgery, albeit with generally very poor results and cure rates as low as 5%. Afterwards, radiotherapy began to develop, first independently and even replacing surgery as the mainstay between the two world wars but then gradually complementing resection and laying thus the foundation for treatment sequencing [15]. In the 1970s, adjuvant curative radiotherapy and low-dose methotrexate became the standard of care options in the primary and recurrent disease setting, respectively [16, 17]. At present, following 50 years of evolving multimodality management, median overall survival of these patients has significantly improved with a major impact of concurrent curative chemoradiation and advances in palliative therapy. The choice of the first-line systemic approach has thus a profound influence on patient outcomes, and we will discuss the role of patient- and disease-related factors in the context of the cancer care continuum.
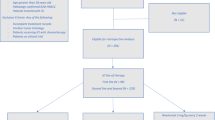
Evolution of treatment sequencing in mucosal head and neck squamous cell carcinoma. In any phase of the disease course, participation in well-designed clinical trials is recommended. Abbreviations: 5-FU, 5-fluorouracil; EXTREME, platinum/5-fluorouracil/cetuximab; TPEx, cisplatin/docetaxel/cetuximab; KN-048 combo, platinum/5-fluorouracil/pembrolizumab; anti-PD-1, anti-programmed cell death-1 (nivolumab or pembrolizumab)
Defining the First Line
First-Line Setting: Where it Begins and Ends
Candidates for first-line palliative treatment can be divided into two groups. The smaller one consists of those presenting with newly diagnosed SCCHN ineligible for locoregional treatment due to synchronous metastases, and the larger one of those with disease relapse after one or more previous locoregional interventions and with no further possibility of such therapy [18]. Recurrent SCCHN is not a homogenous entity but differs according to previous anticancer treatment and site of recurrence with important implications for the choice of first-line systemic regimens. Previous therapeutic attempts can be locoregional only, such as surgery and radiotherapy for early disease and surgery followed by radiotherapy for locally advanced disease, but can also involve systemic drugs. Since the first-generation of larynx preservation trials, platinum agents (cisplatin and carboplatin) are the most commonly used drugs in the locally advanced setting. They have become the cornerstone of induction chemotherapy and concurrent chemoradiotherapy [2, 19].
However, owing to cumulative toxicity of cisplatin, prior exposure can be considered a relative contraindication for its retreatment (i.e., after previous use in the primary setting) or rechallenge (i.e., after previous use in the recurrent and/or metastatic setting), especially if the total administered dose exceeds 300 mg/m2 with up to 200 mg/m2 being considered relatively safe [20,21,22]. Albeit less toxic, the use of carboplatin comes with lower efficacy [23]. Moreover, a short platinum-free interval portends poor prognosis. Time to progression or relapse of less than 6 months after termination of previous platinum-based regimen has been adopted in clinical trials and routine practice to identify cases resistant to platinum retreatment. Although longer periods of disease control are a prerequisite for the term “platinum-sensitivity”, they do no guarantee a therapeutic success. Historically, one of the reasons for this categorization was the urgent need to allow a rapid access to reasonably effective drugs, which were very limited, to as many patients as possible. Nevertheless, this population is quite heterogenous comprising also patients who progress during platinum treatment, those who have a probably persisting locoregional disease after primary treatment that visibly progresses only 6 months later, and those who maintain remission of the primary tumour but present with new distant metastases. Here, we remind that in accordance with disease kinetics and tumour doubling time being usually in the order of several months, such new metastases must have already been subclinically present during the primary treatment, and contrary to induction chemotherapy, concomitant potentiation of radiotherapy by platinum agents does not diminish distant failure [2, 24]. Thus, there are differences in terms of the type of previous administration but also dose.
Unfortunately, the topic of platinum-resistance is still far from being fully understood, and for example addressing local and systemic platinum-resistance, if there is such a distinction, merits special attention. In addition, determining the actual disease-free interval may be challenging. Primary response assessment after chemoradiotherapy is recommended at 3 months with no further imaging being required in the majority of patients in case of complete remission. Thus, it is not that uncommon that imaging at 6 months is performed if suspicious findings are detected already at 3 months. Subsequently, if a recurrence is confirmed at 6 months, its attribution to the designation “platinum-sensitive” may be problematic because its inception was earlier than thought. This type of diagnostic pitfall and the fact that the armamentarium of systemic therapy has broadened during the past 15 years advocate the pertinence of increasing the time span of platinum-resistance or introducing the term of “partial platinum-sensitivity/resistance” as in ovarian cancer [25].
Taken together, cisplatin ineligibility may be either due to toxicity reasons or treatment resistance. The most common alternative regimens comprise a carboplatin/5-fluorouracil doublet, sharing the same platinum resistance issues, and cetuximab [22]. Justified by a hypothetically increasing number of sensitive cells in a growing tumour that recurred, cetuximab may in principle be subjected to retreatment or rechallenge after previous failure (e.g., first in concomitance with curative radiotherapy and then in the palliative setting), but data are still limited [26]. Even less evidence exists for retreatment or rechallenge with the same class of immune checkpoint inhibitors, which currently dominate the recurrent and/or metastatic setting and are increasingly incorporated in ongoing clinical trials in the locally advanced setting. Here, some efficacy can be expected, but an off-immunotherapy period is obviously warranted [27, 28]. Disease-free interval prior to first-line palliative systemic treatment represents, therefore, a crucial indicator impacting on the drug choice. A short disease-free interval is a poor prognosticator, and the outcomes are almost uniformly worse than if the same treatment is given later. A cut-off of 6 months can still be reasonably used in clinical practice, but the relation is probably stochastic and not categorical.
As alluded to above, relapsing SCCHN differs according to the site of recurrence and can thus be classified into locoregional recurrence only, locoregional recurrence with metachronous (with respect to the primary tumour) metastases, and distant failure only. Intriguingly, locoregional relapse eligible neither for salvage surgery nor radiotherapy may not require the same systemic drugs as a widespread disseminated disease. In fact, the use of first-line immunotherapy, particularly as a single-agent regimen, is accompanied by an increased risk of progression in more than one third of patients and in some of them even in the form of hyperprogression, which is an abnormally accelerated tumour growth described in about one quarter of patients receiving immune checkpoint inhibitors and being more frequent in those presenting with locoregional recurrence relative to those with exclusively distant dissemination [29]. Keeping in mind the typical head and neck tumour location in a very sensitive area near vital structures, the increased risk of progression could explain a lack of survival benefit seen in subgroup analyses of the registration Keynote-048 trial in patients presenting with locoregional recurrence only [30]. On the other hand, this is exactly the group of patients in which a non-immunotherapy alternative for the first-line setting consisting of cetuximab/platinum/5-fluorouracil triplet seem to have a major effect (hazard ratio for death 0.65 [0.49, 0.87] in locoregional recurrence only versus 0.99 [0.72, 1.36] in metastatic tumours including also locoregional recurrences) [31]. Furthermore, a post-hoc pooled analysis of both arms of the TPExtreme trial (see below) showed a significantly improved progression-free survival in patients with a locoregional recurrence only [18].
About half of all patients starting with standard-of-care first-line treatment (see below) will also receive second-line therapy, where the drug choice is proportionally restricted. Importantly, the majority of these patients experience progression while on treatment, so there is no disease-free interval as could be the case after primary therapy. Although we might feel intuitively driven towards giving the most comprehensive therapy at the earliest possible opportunity, allowing thus the majority of patients to benefit from it, emerging evidence suggests that treatment sequencing may be the key of success. Illustrative to that are also the very recent results of three large randomized trials in first-line metastatic melanoma. Despite initial excitement and even FDA approval, they did not in the end confirm any significant clinical benefit of combining two of the most potent regimens, i.e., immune checkpoint inhibitors of programmed cell death-1 receptor (PD-1) or its ligand (PD-L1) with RAF and MEK inhibitors [32].
First-Line Treatment: Pros and Cons
Since 2008, the standard first-line treatment has been biochemotherapy according to the EXTREME trial combining the epidermal growth factor receptor inhibitor cetuximab with a platinum-doublet (cisplatin or carboplatin with 5-fluorouracil) in platinum-sensitive SCCHN patients. In comparison with the platinum-doublet alone, the EXTREME regimen significantly improved overall survival from 7.4 to 10.1 months, progression-free survival, and response rate, and all this without compromising quality of life [33, 34]. However, the regimen had several shortcomings including a high rate of severe acute adverse events observed in 82% of patients, poor long-term results with less than 5% of patients being alive at 5 years, absence of significant benefit in patients with distant dissemination according to a subgroup analysis, a lack of predictive biomarkers, and an inconvenient continuous administration of 5-fluorouracil [31, 33, 35]. Nonetheless, EXTREME dominated the first line for more than 10 years and withstood multiple challenges to be dethroned by other promising regimens.
Validating the first predictive molecular marker in SCCHN, the Keynote-048 trial introduced immunotherapy to the first line and demonstrated its superiority over EXTREME in patients with platinum-sensitive tumours marked positively for PD-L1 expressed as combined positive score (CPS). Immunochemotherapy (anti-PD-1 inhibitor pembrolizumab with a platinum doublet) significantly improved overall survival from 10.4 to 13.6 months and from 11 to 14.7 months in the in CPS ≥1 and CPS ≥20 subgroups, respectively. Immunotherapy alone proved such benefit only in the CPS ≥20 subgroup (10.7 versus 14.9 months) [36]. In the PD-L1 negative subgroup accounting for 15% of the study population, EXTREME defended its position. In the subgroup with low PD-L1 expression (CPS 1-19), immunotherapy should be combined with chemotherapy [37]. Furthermore, pembrolizumab prolonged median duration of response by more than 16 months, was substantially less toxic than EXTREME, and retained its efficacy in the elderly subgroup suggesting possible benefits in less fit patients as well [36, 38]. However, even this new schedule has its downsides. In comparison with EXTREME, both pembrolizumab alone and pembrolizumab with chemotherapy improved neither progression-free survival nor response rate, and progressions were more common, probably leading to a lack of overall survival benefit in locoregionally recurrent cases as mentioned above. In the immunochemotherapy arm, more treatment-related deaths than with EXTREME were noted, severe acute adverse events occurred in 74% of participants, and the inconvenient necessity of a continuous administration of 5-fluorouracil remained [36].
The third pivotal trial challenging EXTREME in patients with platinum-sensitive disease was TPExtreme (Fig. 13.3). Although the survival benefit of the better tolerated experimental TPEx arm (cetuximab/platinum/docetaxel) did not reach statistical significance, the trial provided us with valuable data that could improve the delivery of EXTREME, such as validation of biweekly administration of cetuximab in the maintenance phase, growth factor support to maximize dose intensity if tumour shrinkage is the main goal, or deintensification of cisplatin to decrease toxicity and subsequently enhance efficacy [18, 39]. Besides that, impressive outcomes were yielded in patients receiving second-line immunotherapy with overall survival reaching up to 21.9 and 19.4 months in the TPEx and EXTREME arms, respectively. Altogether, the TPEx regimen can be recommended as an alternative to EXTREME, but the eligibility criteria according to the study protocol are more restrictive (maximum age of 70 years, obligatory growth factor support and cisplatin use) [18].
In platinum-resistant disease, the current standard of care has been defined by two phase III trials, CheckMate-141 and Keynote-040, primarily focusing on the second-line setting but also including patients with confirmed progression within the first 6 months (22%) and between 3 and 6 months (15%) after primary treatment completion, respectively (Fig. 13.4). Both studies had a similar design exploring the anti-PD-1 inhibitors nivolumab and pembrolizumab, respectively, with almost the same comparator arms containing investigator’s choice between single-agent cetuximab, methotrexate, and docetaxel [40, 41]. For Checkmate-141, a subgroup analysis of overall survival in platinum-refractory patients confirmed the benefit in this difficult-to-treat population even at 2-year follow-up (median of 7.7 versus 3.3 months) [42]. Interestingly, another subgroup analysis performed in both trials suggested that single-agent docetaxel is more effective than monotherapies with either methotrexate or cetuximab and that it may even be as effective as immunotherapy. Nevertheless, such conclusions are speculative and biased by small numbers of patients in the respective analyses [43].
Decision-Making Factors
Some of them have already been addressed. Here, we will provide a summary, and interested readers are advised to refer to our previous publication presenting a decision-making algorithm [44]. Except for the first two, all factors are continuous variables ranging from minimum to maximum values.
Categorical Variables
Platinum Eligibility
In patients with platinum-sensitive tumours, three treatment options are supported by randomized data: biochemotherapy (the preferred EXTREME regimen or alternatively TPEx), immunochemotherapy (Keynote-048 combination regimen), and immunotherapy alone (pembrolizumab according to Keynote-048). Patients with platinum-resistant tumours should preferentially receive immunotherapy (nivolumab or pembrolizumab) or chemotherapy. For the latter option, no standard-of-care has been defined but taxanes (paclitaxel or docetaxel) with or without cetuximab can be recommended if immune checkpoint inhibitors are not accessible.
Reflecting compromised organ functions, patient’s general health status, previously administered dose, and some other specific situations, contraindications to cisplatin have been summarised elsewhere [22]. Contraindications to carboplatin are much less frequent, being mostly linked to impaired bone marrow capacity, hypersensitivity, first trimester of pregnancy, and lactation.
Disease Site
We have already indicated the caveat of locoregional recurrence in patients treated with immune checkpoint inhibitors and that distant dissemination may preclude efficacy of biochemotherapy [30, 31]. Another recent discovery points towards possibly restrained efficacy of immunotherapy in liver metastases owing to altered antitumour immunity and CD8+ T cell depletion [45].
Continuous Variables
Overall Health Status (From Fitness to Frailty)
In comparison to later stages of the disease course, treatment-naive patients are usually in a better overall condition, and their treatment tolerance and outcomes are superior. However, the majority of candidates for first-line systemic therapy present with a recurrence after multimodality management of locally advanced SCCHN and they may present with various adverse consequences thereof, especially if the disease relapses shortly after a platinum-based regimen.
Patient’s health status can be appraised at two levels. The first is more general and corresponds to the well-known performance status, being one of the most commonly used measures in clinical practice to estimate overall survival and treatment toxicity. However, it has several downsides. The correlation between toxicity and performance status pertains to conventional chemotherapy based on data from the 1980s. Thus, extrapolation to the current setting is problematic, primarily due to advances in supportive care and introduction of new medicines because targeted therapies, particularly modern immunotherapy with immune checkpoint inhibitors, might fit less to this model [46, 47]. Another disadvantage is that performance status is not an equal replacement of functional status comprising patient ability to complete activities of daily living (ADLs like washing, dressing, feeding, mobility etc.) and instrumental ADLs (IADLs like housework, shopping, taking medicines etc.), and it is even a less suitable surrogate of comorbidity scales. This holds true mainly for the elderly population, in which comorbidities rank among the most common indispositions followed by impaired IADLs, nutritional compromise, depression, cognitive dysfunction, impaired ADLs, and deteriorated performance status (grade ≥2 according to the Eastern Cooperative Oncology Group scale), the latter of which is found only in about 20% [48, 49]. Although performance status continues to be a widely accepted stratification factor for clinical trials and has real-world applicability in many young patients, it does not unfortunately play this role in the elderly.
Not only account elderly patients for the majority of cancer patients, more than half of them are frail or vulnerable and only less than one third fit [1, 50]. Comprehensive geriatric assessment (CGA) addresses the multifaceted health characteristics of elderly people summarized as biological age. In routine practice, geriatric screening tests (e.g., G8) are less time-consuming but still appropriate to select those who are not fit and require a full CGA to conclude on their biological age. Fit elderly persons should receive full-dose standard therapy because they derive the same anticancer benefit as their younger counterparts, albeit still with a potentially higher risk of toxicity due to physiological changes in metabolism; vulnerable patients may need alternative regimens or dose reductions and frailty precludes conventional treatment [50, 51]. Nevertheless, immune checkpoint inhibitors may still be a good option in frail or poor performance patients irrespective of age [38, 47].
The second level of health appraisal focuses specifically on comorbidities that besides their impact on general well-being, can also imply distinct contraindications for some drugs such as renal insufficiency for cisplatin, coronary artery disease for 5-fluorouracil, solid organ transplantation for immune checkpoint inhibitors, and many more [22, 52, 53]. In these cases, alternative regimens are required. Cisplatin/5-fluorouracil doublet may be replaced by carboplatin/5-fluorouracil in the EXTREME and Keynote-048 regimens. The TPEx schedule substitutes 5-fluorouracil for docetaxel, and treating physicians can opt either for cisplatin/docetaxel or carboplatin/docetaxel. If no third agent is added, both cisplatin/paclitaxel or carboplatin/paclitaxel are viable options [18, 33, 36, 54]. Instead of immune checkpoint inhibitor monotherapy in the second line, patients may receive single-agent taxane, methotrexate, or cetuximab [40, 41]. Every deviation entails changes in toxicity profile with some of them being also linked to decreased efficacy as in the above-mentioned cases of carboplatin or single-agent substitutions for immunotherapy [23, 40, 41].
Tumour Burden (From High to Low)
Mounting evidence suggests that increasing tumour size negatively correlates with response to immune checkpoint inhibitors and other types of immunotherapy. The underlying mechanism relates to local and systemic changes induced by large tumours leading to formation of a more immunosuppressive microenvironment [55]. Another implication of tumour volume is the corresponding probability to elicit symptoms. Here, three treatment characteristics have a key relevance, involving objective response rate, rate of progressive disease, and time to response. They inform us about the potential of a given systemic therapy to counteract increasing tumour size menacing to cause symptoms [56]. While a chemotherapy component is crucial to assure high response rates both in biochemotherapy and immunochemotherapy regimens, the immunotherapy component, either given alone or with chemotherapy, may have deleterious effects in terms of higher rates of progressions as we discussed earlier and probably also on time to response when given as monotherapy. Theoretically, due to its indirect action through mobilisation of immune cells, more time is needed to obtain tumour shrinkage with immunotherapy. This has been only partially reproduced in clinical practice so far because median time to response was comparable between immunotherapy and chemotherapy arms in CheckMate-141 and Keynote-048 but was longer in the pembrolizumab arm in Keynote-040 (4.5 versus 2.2 months) [36, 40, 41]. Of note, administration of only two doses of nivolumab prior to curative resection of locally advanced SCCHN (i.e., already at one month from nivolumab initiation) yielded radiographic tumour reduction from baseline in about 50% of patients [57].
Disease Pace (From Fast to Slow)
The speed of tumour-cell proliferation measured as tumour doubling time and the speed of tumour-cell shedding leading to formation of new regional or distant metastases are two principal events defining tumour kinetics. It ranges from indolent cases over faster progressing cancers to cases of hyperprogression [29, 56]. A fast growing disease needs a similar approach as large tumours aiming at high response rates, low rates of progression, and a short time to response, whereas in a slowly growing disease we may prioritize less intensive regimens similarly to small tumours (e.g., local ablation, immunotherapy alone) or even periods of watchful waiting [44]. Recently, we introduced the term argometastases delineating slowly developing distant metastases which can be cured with local ablation [58].
PD-L1 Expression (From High to Low)
Tumours exposing this ligand on cell surfaces derive better outcomes from immune checkpoint inhibitors. At present, this statement holds true for a survival advantage shown with pembrolizumab in the first-line setting according to Keynote-048 where the expression was measured as CPS, i.e., including also non-tumoural cells, mostly lymphocytes and macrophages [36]. In Keynote-040, better survival and response rate were associated with a higher PD-L1 expression measured solely on tumour cells as tumour proportion score (TPS) of 50% or more. Importantly, CPS was not predictive in Keynote-040, and no correlation with either CPS or TPS was found in CheckMate-141 [40, 41]. Nevertheless, PD-L1 expression remains the only validated molecular marker in recurrent and/or metastatic SCCHN warranting further research to improve its predictive value.
Treatment Sequencing
Acknowledging the continuous process of malignant development from early to advanced stages, both in the primary and recurrent disease settings, as well as the inherent propensity of SCCHN to relapse (Fig. 13.1), therapeutic decision making should always look farther into the natural disease course and respond to questions as of what will be the next best step in case of failure. Treatment sequencing is therefore not a series of ad hoc decisions each time a new treatment is required but a comprehensive pre-planned individualized analysis of anticancer management divided into several consequential therapeutic blocks that are employed at disease progression or relapse (Fig. 13.2). In SCCHN, it represents an emerging new concept evolving along with the introduction of new treatment options or combinations.
Illustrative to this is a sequential administration of immune checkpoint inhibitors in the recurrent and/or metastatic setting as opposed to the standard concomitant approach proposed by Keynote-048. Median progression-free survival of biochemotherapy is 5.6 months according to EXTREME [33]. If patients who progress receive single-agent nivolumab according to CheckMate-141, it takes about 3 months for the survival advantage of immunotherapy to manifest which is actually the time needed for a separation of survival curves between nivolumab and the comparator arm [40]. Taken together, patients starting biochemotherapy may be benefiting from immunotherapy after about 8 months. Interestingly, this seems to be the same period necessary for a separation of survival curves in the Keynote-048 trial. Moreover, median overall survival of concomitant immunochemotherapy is about 14 months [36]. In the TPExtreme trial, patients treated initially with biochemotherapy and then second-line immunotherapy had a median overall survival of almost 20 months [18]. There were such sequentially treated patients also in the standard arm of Keynote-048 but only about 25% [36]. In summary, these results suggest comparable or even better outcomes of a sequential versus concomitant approach. However, when translating them to clinical practice, further elements should be considered such as toxicity (not excluding immune-related adverse events), risk of progression (locoregional recurrence versus asymptomatic distant metastases or the presence of already symptomatic distant metastases), quality of life, and patient’s perspective.
Conclusions
Growing knowledge from clinical trials and the real-world setting help us understand the natural course of head and neck cancer, predictors of its outcome, and principles of treatment sequencing. The above mentioned six factors should all be integrated in the decision-making algorithm but some of them may be prioritized while others brought to background, particularly if conflicting results are yielded (e.g., high disease burden with high PD-L1 expression or a locoregional recurrence with high PD-L1 expression).
Treatment sequencing with deferral of immunotherapy to the second line should also be part of the decision-making algorithm but may still be difficult to explain to patients confronted with the general assumption about a universal benefit of immunotherapy. In these situations, presenting the therapeutic model as a comprehensive approach with clearly defined turning points and available options may help pave the way towards an optimal solution for each patient.
References
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site. 2020.
Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Low-dose versus high-dose cisplatin: lessons learned from 59 chemoradiotherapy trials in head and neck cancer. Front Oncol. 2019;9:86.
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50.
GLOBOCAN 2020. Available at: https://gco.iarc.fr/today/. Accessed 18 May 2022.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
Greil R, Rischin D, Harrington KJ, Soulières D, Tahara M, de Castro G, et al. Long-term outcomes from KEYNOTE-048: pembrolizumab (pembro) alone or with chemotherapy (pembro+C) versus EXTREME (E) as first-line (1L) therapy for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann Oncol. 2020;31:S660 (abstract 915MO).
Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means?. Laryngoscope. 2000;110(3 Pt 2 suppl 93):1–18.
Huang S, Waldron J, Xu W, Tong L, Ringash JG, Bayley AJ, et al. Potential cure in HPV-related oropharyngeal cancer with oligometastases. Int J Radiat Oncol Biol Phys. 2014:90:S180–1 (abstract 1055).
Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82.
Sigston E, de Mones E, Babin E, Hans S, Hartl DM, Clement P, et al. Early-stage glottic cancer: oncological results and margins in laser cordectomy. Arch Otolaryngol Head Neck Surg. 2006;132:147–52.
Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. 2019;20:1349–59.
Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. 2017;22:1056–66.
Huang SH, Perez-Ordonez B, Weinreb I, Hope A, Massey C, Waldron JN, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85.
Chang JH, Wu CC, Yuan KS, Wu ATH, Wu SY. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget. 2017;8:55600–12.
McGurk M, Goodger NM. Head and neck cancer and its treatment: historical review. Br J Oral Maxillofac Surg. 2000;38:209–20.
Vandenbrouck C, Sancho H, Le Fur R, Richard JM, Cachin Y. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39:1445–9.
Mead GM, Jacobs C. Changing role of chemotherapy in treatment of head and neck cancer. Am J Med. 1982;73:582–95.
Guigay J, Aupérin A, Fayette J, Saada-Bouzid E, Lafond C, Taberna M, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014–01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22:463–75.
Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a european organization for research and treatment of cancer phase III trial. EORTC head and neck cancer cooperative group. J Natl Cancer Inst. 1996;88:890–9.
Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28:2670–9.
Gebhardt C, Ascierto P, Atkinson V, Corrie P, Dummer R, Schadendorf D. The concepts of rechallenge and retreatment in melanoma: a proposal for consensus definitions. Eur J Cancer. 2020;138:68–76.
Szturz P, Cristina V, Herrera Gómez RG, Bourhis J, Simon C, Vermorken JB. Cisplatin eligibility issues and alternative regimens in locoregionally advanced head and neck cancer: recommendations for clinical practice. Front Oncol. 2019;9:464.
Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: raising the bar a step higher. Oral Oncol. 2020;101: 104492.
Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN, Neskey DM, et al. Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis. Oral Oncol. 2012;48:1076–84.
Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6:229–39.
Masuishi T, Tsuji A, Kotaka M, Nakamura M, Kochi M, Takagane A, et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J Cancer. 2020;123:1490–5.
Zaremba A, Eggermont AMM, Robert C, Dummer R, Ugurel S, Livingstone E, et al. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur J Cancer. 2021;155:268–80.
Cabezas-Camarero S, Cabrera-Martín MN, Merino-Menéndez S, Paz-Cabezas M, García-Barberán V, Sáiz-Pardo Sanz M, et al. Safety and efficacy of cetuximab-based salvage chemotherapy after checkpoint inhibitors in head and neck cancer. Oncologist. 2021;26:e1018–35.
Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–11.
Szturz P, Vermorken JB. Further clinical interpretation and implications of KEYNOTE-048 findings. Lancet. 2020;396:79.
European Medicines Agency. Assessment report for Erbitux EMEA/590803/2008. Available online: https://www.ema.europa.eu/en/documents/variation-report/erbitux-h-c-558-ii-0026-epar-asssessment-report-variation_en.pdf. Accessed 22 May 2022.
Callahan MK, Chapman PB. PD-1 or PD-L1 blockade adds little to combination of BRAF and MEK inhibition in the treatment of BRAF V600-mutated melanoma. J Clin Oncol. 2022;40:1393–5.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27.
Mesía R, Rivera F, Kawecki A, Rottey S, Hitt R, Kienzer H, et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21:1967–73.
Vermorken JB, Remenar E, Hitt R, Kawecki A, Rottey S, Knierim L, et al. Platinum-based chemotherapy (CT) plus cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neck cancer (R/M-SCCHN): 5-year follow-up data for the extreme trial. J Clin Oncol. 2014;32:5s (suppl; abstract 6021).
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study Lancet. 394;2019:1915–28.
Burtness B, Rischin D, Greil R, Soulières D, Tahara M, de Castro G, Jr et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40:2321–32.
Szturz P, Vermorken JB. Overcoming frailty in recurrent and metastatic head and neck cancer. Oral Oncol. 2020;109: 104636.
Szturz P, Vermorken JB. Revisiting EXTREME in the immuno-oncology era: how to improve its outcomes. Oncologist. 2021;26:899–901.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67.
Gillison ML, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Long-term outcomes with nivolumab as first-line treatment in recurrent or metastatic head and neck cancer: subgroup analysis of checkMate 141. Oncologist. 2022;27:e194–8.
Pai SI, Faivre S, Licitra L, Machiels JP, Vermorken JB, Bruzzi P, et al. Comparative analysis of the phase III clinical trials of anti-PD1 monotherapy in head and neck squamous cell carcinoma patients (CheckMate 141 and KEYNOTE 040). J Immunother Cancer. 2019;7:96.
Szturz P, Vermorken JB. Translating KEYNOTE-048 into practice recommendations for head and neck cancer. Ann Transl Med. 2020;8:975.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164.
Scott JM, Stene G, Edvardsen E, Jones LW. Performance status in cancer: not broken, but time for an upgrade? J Clin Oncol. 2020;38:2824–9.
Ahmed T, Lycan T, Dothard A, Ehrlichman P, Ruiz J, Farris M, et al. Performance status and age as predictors of immunotherapy outcomes in advanced non-small-cell lung cancer. Clin Lung Cancer. 2020;21:e286–93.
Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–7.
Extermann M. Evaluation of the senior cancer patient: comprehensive geriatric assessment and screening tools for the elderly. In: Schrijvers D, Aapro M, Zakotnik B, Audisio R, van Halteren H, Hurria A, editors. ESMO handbook of cancer in the senior patient. London: Informa Healthcare; 2010. p. 13–21.
Szturz P, Bossi P, Vermorken JB. Systemic treatment in elderly head and neck cancer patients: recommendations for clinical practice. Curr Opin Otolaryngol Head Neck Surg. 2019;27:142–50.
Perri F, Ionna F, Pavone E, Longo F, Caponigro F. Treatment approaches in elderly patients with head and neck cancer. Anticancer Agents Med Chem. 2013;13:1383–90.
Yuan C, Parekh H, Allegra C, George TJ, Starr JS. 5-FU induced cardiotoxicity: case series and review of the literature. Cardiooncology. 2019;5:13.
Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106.
Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the eastern cooperative oncology group. J Clin Oncol. 2005;23:3562–7.
Kim SI, Cassella CR, Byrne KT. Tumor burden and immunotherapy: impact on immune infiltration and therapeutic outcomes. Front Immunol. 2021;11: 629722.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13.
Ferris RL, Spanos WC, Leidner R, Gonçalves A, Martens UM, Kyi C, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer. 2021;9: e002568.
Szturz P, Vermorken JB. Steering decision making by terminology: oligometastatic versus argometastatic. Br J Cancer. 2022 [In print].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Szturz, P., Vermorken, J.B. (2023). Systemic Treatment Sequencing and Prediction of First-line Therapy Outcomes in Recurrent or Metastatic Head and Neck Cancer. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)