Abstract
Background
Selective biomarkers may improve outcomes in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) treated with immune checkpoint inhibitor therapy. We investigated three independent biomarkers for association with efficacy in the randomized, phase III KESTREL study (NCT02551159) of first-line durvalumab monotherapy or durvalumab plus tremelimumab versus the EXTREME regimen: programmed cell death ligand-1 (PD-L1) immunohistochemistry, blood tumor mutational burden (bTMB) via circulating tumor DNA, and neutrophil-to-lymphocyte ratio (NLR).
Methods
Tumor or blood samples from patients enrolled in the KESTREL study were analyzed for PD-L1, bTMB, and NLR. Associations with overall survival (OS) or objective response rates (ORRs) were evaluated based on prespecified cut-offs for PD-L1 (tumor cell [TC] ≥ 50%/immune cell ≥ 25% or TC ≥ 25%), bTMB (≥ 16 mutations [mut] per megabase [Mb]), and NLR (≤ 7). Ad hoc analyses of exploratory cut-offs were performed.
Results
Prespecified or exploratory cut-offs for PD-L1 did not enrich for ORR or OS for durvalumab monotherapy or durvalumab plus tremelimumab versus EXTREME. In the bTMB ≥ 16 mut/Mb subgroup, OS hazard ratios (95% confidence interval) for durvalumab monotherapy and durvalumab plus tremelimumab versus EXTREME were 0.90 (0.48–1.72) and 0.69 (0.39–1.25), respectively. Complete response rates were 8.6% with durvalumab plus tremelimumab and 4.3% with EXTREME (≥ 16 mut/Mb subgroup). No improvement in OS was observed for durvalumab monotherapy or durvalumab plus tremelimumab versus EXTREME at prespecified or exploratory NLR cut-offs.
Conclusions
bTMB demonstrated potential utility for selecting patients with R/M HNSCC who benefited from durvalumab with or without tremelimumab versus EXTREME.
Trial registration ClinicalTrials.gov identifier NCT02551159.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) exhibits clinical and molecular heterogeneity and is associated with poor prognosis [1, 2]. High genomic instability and intratumoral genetic heterogeneity of HNSCC may explain treatment resistance and the tendency for locoregional recurrence in some patients [3, 4]. Moreover, survival outcomes in patients with HNSCC vary based on primary tumor location and human papillomavirus (HPV) status [1, 4, 5]. Immune checkpoint inhibitors (ICIs) are a treatment option for patients with R/M HNSCC; but only a subset of patients benefit from first-line treatment with ICIs [6, 7], as observed across many cancers [8]. Validated biomarkers that identify patients who are most likely to respond to ICIs may improve patient outcomes and avoid unnecessary toxicity and costs.
Several promising biomarkers, including programmed cell death ligand-1 (PD-L1), blood tumor mutational burden (bTMB) via circulating tumor DNA, and neutrophil-to-lymphocyte ratio (NLR), were identified in the EAGLE study (NCT02369874) of durvalumab with or without tremelimumab versus chemotherapy in patients with R/M HNSCC who progressed following definitive therapy [9, 10]. PD-L1 was further evaluated as a biomarker in the KESTREL study (NCT02551159) of durvalumab with or without tremelimumab versus the EXTREME regimen in patients with R/M HNSCC who had not received prior systemic therapy [11]. The primary objective of the KESTREL study was to assess the overall survival (OS) of durvalumab monotherapy versus the EXTREME regimen in patients with R/M HNSCC whose tumors expressed high levels of PD-L1 (PD-L1 tumor cell [TC] ≥ 50%/immune cell [IC] ≥ 25%) [11]. Secondary objectives included assessment of the efficacy of durvalumab monotherapy versus the EXTREME regimen in all randomized patients, and assessment of the efficacy of durvalumab plus tremelimumab combination therapy versus the EXTREME regimen in patients with PD-L1-high expression and in all randomized patients [11].
PD-L1 is the most widely used biomarker for selecting patients for ICI therapy in clinical practice and has been evaluated in phase III studies of patients with HNSCC [7, 10, 12, 13]. Long-term benefit was demonstrated for patients with platinum-refractory R/M HNSCC treated with nivolumab, irrespective of PD-L1 status, in the CheckMate 141 study (NCT02105636) [13]. In contrast, in the KEYNOTE-048 study (NCT02358031), survival benefit with first-line pembrolizumab versus chemotherapy was limited to patients with PD-L1-high expression (combined positive score [CPS] ≥ 20) [7, 14]. In the EAGLE study, median OS was longer for durvalumab monotherapy versus chemotherapy in patients with TC expression ≥ 25% versus < 25% [10]. However, unexpectedly, OS was also longer for durvalumab monotherapy versus chemotherapy in a small population of patients with TC expression < 1% [10]. Of 823 patients randomized in the KESTREL study (durvalumab monotherapy, n = 204; durvalumab plus tremelimumab, n = 413; EXTREME regimen, n = 206), 46.5% (n = 383) had tumors with PD-L1-high expression (PD-L1 TC ≥ 50%/IC ≥ 25%) [11]. The primary endpoint of the KESTREL study was not met; in patients with PD-L1-high expression, OS was comparable between durvalumab monotherapy and the EXTREME regimen (median OS, 10.9 vs. 10.9 months, respectively; hazard ratio [HR] = 0.96) [11]. In addition, no difference in OS was observed between durvalumab plus tremelimumab and the EXTREME regimen in patients with PD-L1-high expression (median OS, 11.2 vs. 10.9 months, respectively; HR = 1.05) and in all randomized patients (median OS, 10.7 vs. 10.3 months, respectively; HR = 1.04) [11]. Whether an alternative PD-L1 scoring algorithm could predict efficacy outcomes in patients enrolled in the KESTREL study remained to be assessed.
TMB has emerged as a promising biomarker for ICIs. In the US, patients with unresectable or metastatic solid tumors with TMB ≥ 10 mutations per megabase (mt/Mb), who have progressed following prior treatment and without alternative treatment options, are eligible for pembrolizumab treatment based on the results of the KEYNOTE-158 study (NCT02628067) [15, 16]. In R/M HNSCC, the EAGLE study showed a significant OS benefit for durvalumab with or without tremelimumab versus chemotherapy for patients with bTMB ≥ 16 mut/Mb (HR = 0.39 [confidence interval (CI) 0.20–0.76] and 0.38 [95% CI 0.19–0.78], respectively) [9]. The utility of bTMB ≥ 16 mut/Mb for predicting survival in patients with R/M HNSCC treated with these therapies in the front-line setting had not previously been determined.
NLR is a standard clinical assessment of tumor-induced inflammation based on blood cell counts. Although generally regarded as a prognostic marker of survival in HNSCC [17], it has gained interest as a predictive marker for ICIs [18]. High baseline NLR had a strong inverse relationship with OS and progression-free survival (PFS) in patients receiving anti-PD-1 therapy for metastatic head and neck cancer, regardless of PD-L1 expression [19]. A recent study found that low baseline NLR was associated with improved response in patients with R/M HNSCC receiving ICIs, and that on-treatment high NLR (≥ 4) had a significant negative correlation with OS and PFS [20]. These findings were further supported by the EAGLE study, which showed a statistically significant OS benefit in patients with R/M HNSCC and an NLR ≤ 7 who were treated with durvalumab monotherapy versus chemotherapy (HR = 0.75, 95% CI 0.57–0.97) [9]. As NLR is a simple, inexpensive, and routine assessment, it is of interest to assess its utility to select patients with R/M HNSCC for ICI therapy.
Herein, we analyzed data from the KESTREL study to evaluate the predictive utility of validated, prespecified cut-offs of PD-L1 expression, bTMB, and NLR. We further evaluated exploratory cut-offs for the selection of patients using these biomarkers in ad hoc analyses.
Methods
Patients
Patients with evaluable samples for biomarker analysis from the KESTREL study were included. Eligible patients were aged ≥ 18 years with histologically or cytologically confirmed R/M HNSCC (oral cavity, oropharynx, hypopharynx, or larynx) not amenable to local, curative therapy with surgery or radiation. Patients were eligible if they had not received prior systemic therapy for R/M disease, unless it was given as part of multimodal treatment for locally advanced or recurrent disease, and recurrence had occurred > 6 months from the last platinum dose.
The KESTREL study was conducted in accordance with the Declaration of Helsinki and was consistent with International Conference on Harmonization and Good Clinical Practice guidelines, and applicable regulatory requirements. Written informed consent from participants was obtained before performing any protocol-related procedures and use of biological samples.
Study design and treatment
The KESTREL study (NCT02551159) was a randomized, open-label, multicenter, global phase III study. The study was conducted at 197 sites in 23 countries. Patients were randomized 2:1:1 to durvalumab plus tremelimumab (concurrent durvalumab 1500 mg every 4 weeks [Q4W] and tremelimumab 75 mg Q4W for a maximum of 4 doses), durvalumab monotherapy (1500 mg Q4W), or the EXTREME regimen (cisplatin 100 mg/m2 of body surface area or carboplatin at an area under the curve of 5 mg/mL/min on Day 1, at the discretion of the investigator, and 5-fluorouracil 1000 mg/m2/day on Days 1 through 4 of every 3-week cycle, as well as cetuximab 400 mg/m2 on Day 1, followed by 250 mg/m2 every week). Randomization was stratified by tumor location (oropharyngeal or non-oropharyngeal), smoking history (> 10 or ≤ 10 pack-years), and PD-L1 status (positive or negative) at cut-off of TC ≥ 25%. Further stratification by HPV status (positive or negative) was performed for patients with oropharyngeal cancer.
Study objectives
Assessment of the efficacy of durvalumab monotherapy and durvalumab plus tremelimumab versus the EXTREME regimen in patients with PD-L1 TC ≥ 50%/IC ≥ 25% and in all randomized patients, were primary and secondary objectives of the KESTREL study [11]. In addition to the prespecified cut-off of TC ≥ 50%/IC ≥ 25% and stratification cut-off of TC ≥ 25%, an ad hoc analysis was performed to assess efficacy at exploratory cut-offs of PD-L1. Exploratory cut-offs were selected based on published reports for ICI treatment in R/M HNSCC [7, 12,13,14, 21].
A secondary objective of the KESTREL study was to assess efficacy in patients selected for high bTMB level at a prespecified cut-off of ≥ 16 mut/Mb. Efficacy was assessed in terms of OS and objective response rate (ORR), as defined previously [11]. In an ad hoc analysis, OS was also assessed at exploratory cut-offs of bTMB ≥ 8, ≥ 10, ≥ 12, ≥ 14, ≥ 18, and ≥ 20 mut/Mb to confirm the optimal cut-off.
Assessment of OS in patients selected for low NLR at a cut-off of ≤ 7 was a prespecified exploratory objective of the KESTREL study. An ad hoc analysis assessed OS in patients with baseline NLR ≤ 4 or ≤ 8, selected based on published reports for ICI treatment in R/M HNSCC [20].
Biomarker assessments
PD-L1 expression was determined using formalin-fixed, paraffin-embedded tumor tissue obtained from recently acquired (preferred) or archival samples (≤ 3 years before screening). PD-L1 testing was performed using the VENTANA PD-L1 (SP263) Assay (Roche Tissue Diagnostics, Tucson, AZ, USA) in a College of American Pathologists-accredited and Clinical Laboratory Improvement Amendments-certified central laboratory. Pathologists were trained by the manufacturer for validated scoring at the stratification cut-off of TC ≥ 25% and the primary endpoint population of TC ≥ 50%/IC ≥ 25%.
bTMB level was determined from baseline blood samples. Sampling ceased after a protocol amendment and, thus, was not continued throughout the duration of the study. Blood was collected in K2-EDTA tubes, processed for plasma collection, and stored at − 80 °C. bTMB was assessed using the GuardantOMNI assay, as previously described [22,23,24].
Absolute neutrophil count and absolute lymphocyte count were assessed according to local standards to derive NLRs.
bTMB and variant analysis
Cell-free DNA next-generation sequencing analysis was performed at Guardant Health, Inc. (Redwood City, CA, USA). The 2.145 Mb GuardantOMNI assay identifies single nucleotide variants (SNVs) and indels in 496 genes, copy number amplifications (106 genes), fusions (21 genes), microsatellite instability (MSI)-high status, and TMB [24, 25]. bTMB was reported as mut/Mb by the GuardantOMNI algorithm, which includes all somatic synonymous and non-synonymous SNVs and indels, excluding germline, clonal hematopoiesis of indeterminate potential, driver, and resistance mutations, with statistical adjustment for sample-specific tumor shedding and molecular coverage. Samples with low tumor shedding (all somatic mutations < 0.3% maximum somatic allele fraction) or low unique molecule coverage were identified as bTMB-unevaluable. Validation of bTMB has been previously described [23, 26].
Statistical analyses
Median OS values and 95% CIs were computed using the Kaplan–Meier method. OS HRs and 95% CIs were estimated using a stratified Cox proportional hazards model; ties were handled using the Efron approach, and 95% CIs were calculated using a profile likelihood approach. ORRs were calculated as the percent of patients with partial or complete responses of the total subgroup; 95% CIs were computed using exact binomial distribution. Statistical hypothesis testing for key secondary endpoints commenced only if the primary endpoint reached statistical significance. As the primary objective of the KESTREL study was not met [11], all statistics reported are descriptive.
Results
PD-L1
PD-L1 was evaluable in tumor samples from 820/823 (99.6%) of all randomized patients in the KESTREL study (Supplementary Table 1). PD-L1 prevalence was 31.1% (n = 256/823) at the TC ≥ 25% cut-off, consistent with the EAGLE study [10], and 46.7% (n = 383/820) at the TC ≥ 50%/IC ≥ 25% cut-off (subsubgroup for primary endpoint analysis; Supplementary Table 1). For the PD-L1 analysis, 51% of samples were recently acquired (< 3 months old) and 49% were archival.
Data from the primary analysis of the KESTREL study have previously been reported [11]. In the primary analysis, median OS was similar in patients with PD-L1 TC ≥ 50%/IC ≥ 25% in all treatment arms, and there was no significant OS benefit with durvalumab monotherapy versus the EXTREME regimen in this subgroup [11]. However, median OS and OS rates at 12, 18, and 24 months were numerically higher in patients treated with durvalumab monotherapy or durvalumab plus tremelimumab with TC ≥ 50%/IC ≥ 25% versus those with TC < 50% and IC < 25% (Supplementary Table 2) [11]. ORRs and complete response rates (CRRs) were similar in the TC ≥ 50%/IC ≥ 25% subgroup versus all randomized patients for durvalumab monotherapy (16.2% vs. 17.2% and 0 vs. 1.5%, respectively) and the EXTREME regimen (50.0% vs. 49.0% and 3.2% vs. 1.9%, respectively) [11]. The ORRs and CRRs were numerically higher in the TC ≥ 50%/IC ≥ 25% subgroup versus all randomized patients for durvalumab plus tremelimumab (25.3% vs. 21.8% and 5.3% vs. 3.9%, respectively) [11].
Median OS in patients with TC ≥ 25%, CPS ≥ 1, CPS ≥ 20, or IC ≥ 25% was similar across treatment arms (Fig. 1a–d). OS HRs across most TC or IC cut-offs showed no benefit for durvalumab monotherapy or durvalumab plus tremelimumab versus the EXTREME regimen (Supplementary Fig. 1). Median OS was generally similar in patients with TC < 25%, CPS < 20, or IC < 25% who were treated with durvalumab monotherapy, durvalumab plus tremelimumab, or the EXTREME regimen (data not shown). Median OS was longer for durvalumab plus tremelimumab and shorter for durvalumab monotherapy versus the EXTREME regimen in patients with CPS < 1 (data not shown).
In an ad hoc analysis of ORR at additional PD-L1 cut-offs, IC ≥ 50% showed the most enrichment, although this subgroup was small (Supplementary Table 3). In the durvalumab monotherapy arm, the highest ORR was observed in patients with IC ≥ 50% (23.8%; n = 5/21). At the cut-off of IC ≥ 50% in the durvalumab plus tremelimumab arm, 35.7% (n = 10/28) of patients had an objective response and 17.9% (n = 5/28) of patients had a complete response, whereas in the EXTREME regimen, 30.0% (n = 3/10) of patients had an objective response and no patients had a complete response. In the durvalumab plus tremelimumab arm, there appeared to be an overall numerical trend in increasing response rate with increasing TC or IC cut-offs (Supplementary Table 3).
bTMB
Blood samples for biomarker analysis were available for 73% (n = 598/823) of randomized patients. Variant landscape analysis in processed samples (n = 536) was reflective of HNSCC, with a high prevalence of TP53 (70%), PIK3CA (29%), KMT2D (26%), NOTCH1 (18%), FAT1 (18%), and LRP1B (18%) alterations (Supplementary Fig. 2). bTMB was determined for 56% (n = 461/823) of randomized patients, comprising the bTMB evaluable population (BEP; Supplementary Fig. 3). The prevalence of bTMB ≥ 16 mut/Mb was 26.0% (n = 120/461) in the BEP.
Baseline demographics and disease characteristics in the BEP were similar to all randomized patients [11], although some differences were apparent between the bTMB ≥ 16 mut/Mb and bTMB < 16 mut/Mb subgroups (Supplementary Table 4). The bTMB ≥ 16 mut/Mb subgroup was enriched for patients who had PD-L1 expression TC ≥ 25% or poorer performance status, and included fewer patients with oral cavity tumors, more patients with laryngeal tumors, and more patients with metastatic versus recurrent disease, compared with the bTMB < 16 mut/Mb subgroup (Supplementary Table 4).
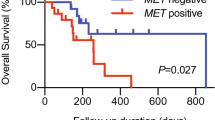
OS in the BEP was not consistent with the OS for all randomized patients (Fig. 2a) [11]. In patients treated with the EXTREME regimen, the median OS was 12.0 months in the BEP compared with 10.3 months in all randomized patients [11] and 9.1 months in patients with unknown bTMB results (Fig. 2b). Therefore, it is more appropriate to compare OS in the bTMB-selected populations comprising the BEP than to all randomized patients. For durvalumab monotherapy versus the EXTREME regimen, the OS HR was 0.90 (95% CI 0.48–1.72) in the bTMB ≥ 16 mut/Mb subgroup (Fig. 2c), compared with 1.16 (95% CI 0.87–1.54) in the BEP subgroup (Fig. 2a). The OS HR for durvalumab plus tremelimumab versus EXTREME was 0.69 (95% CI 0.39–1.25) in the bTMB ≥ 16 mut/Mb subgroup (Fig. 2c), compared with 1.22 (95% CI 0.96–1.57) in the BEP subgroup (Fig. 2a). In patients treated with the EXTREME regimen, OS was poorer in the bTMB ≥ 16 mut/Mb (median OS 7.2, 95% CI 4.3–16.5) versus the bTMB < 16 mut/Mb (median OS 13.0, 95% CI 10.2–14.7) subgroup (Fig. 2c, d). In a subgroup analysis of OS by demographic characteristics in patients with bTMB ≥ 16 mut/Mb, OS HRs generally favored durvalumab plus tremelimumab versus the EXTREME regimen, although patient numbers were low, 95% CIs were wide, and differences were not statistically significant (Supplementary Fig. 4). In an ad hoc analysis of increasing cut-offs of bTMB, OS HRs for durvalumab monotherapy or durvalumab plus tremelimumab versus the EXTREME regimen were lower for bTMB ≥ 16 mut/Mb, compared with other bTMB cut-offs, although 95% CIs were wide and overlapping (Supplementary Fig. 5).
Higher ORRs were observed for durvalumab plus tremelimumab, but not with durvalumab monotherapy or the EXTREME regimen in the bTMB ≥ 16 mut/Mb subgroup, compared with the bTMB < 16 mut/Mb subgroup (Table 1). In the durvalumab monotherapy arm, ORR was 10.3% (95% CI 4.3–27.6) in the bTMB ≥ 16 mut/Mb subgroup and 18.2% (95% CI 11.1–28.4) in the bTMB < 16 mut/Mb subgroup. In the durvalumab plus tremelimumab arm, ORR was 31.0% (95% CI 21.6–50.3) in the bTMB ≥ 16 mut/Mb subgroup and 16.9% (95% CI 12.1–23.2) in the bTMB < 16 mut/Mb subgroup. In the EXTREME arm, ORR was 43.5% (95% CI 24.5–69.4) in the bTMB ≥ 16 mut/Mb subgroup and 55.2 (95% CI 44.6–65.3) in the bTMB < 16 mut/Mb subgroup. The CRR was higher in patients with bTMB ≥ 16 mut/Mb treated with durvalumab plus tremelimumab (8.6%), compared with durvalumab monotherapy (0.0%) or EXTREME (4.3%).
NLR
NLR was evaluable for 100% (823/823) of all randomized patients, and the prevalence of NLR ≤ 7 was 68% (Supplementary Table 5). Median OS of patients with NLR ≤ 7 was longer than those with NLR > 7, irrespective of treatment arm (Fig. 3). In patients with NLR ≤ 7, OS HRs did not favor durvalumab monotherapy versus EXTREME (1.08 [95% 0.84–1.40]) or durvalumab plus tremelimumab versus EXTREME (1.00 [95% 0.80–1.26]).
In an ad hoc analysis, OS HR did not favor either ICI treatment arm in the NLR ≤ 4 subgroup (durvalumab monotherapy vs. EXTREME, 1.02 [95% CI 0.71–1.45]; durvalumab plus tremelimumab vs. EXTREME, 0.99 [95% CI 0.72–1.38]) or the NLR ≤ 8 subgroup (durvalumab monotherapy vs. EXTREME, 1.09 [95% CI 0.85–1.39]); durvalumab plus tremelimumab vs. EXTREME, 0.99 [95% CI 0.80–1.23]; Supplementary Table 6).
Discussion
The KESTREL study did not meet its primary objective of improved OS in patients with PD-L1-high expression treated with durvalumab monotherapy versus the EXTREME regimen [11]. The CheckMate 651 study also failed to demonstrate significant OS benefit with nivolumab plus ipilimumab versus the EXTREME regimen in patients with PD-L1-high tumors (CPS ≥ 20), albeit with numerical OS improvement [27]. Only the KEYNOTE-048 study showed prolonged OS with pembrolizumab monotherapy versus the EXTREME regimen in patients selected for high PD-L1 expression (CPS ≥ 20) [7, 14]. The CheckMate 651 and KESTREL studies both had higher frequencies of subsequent immunotherapy use in the control arm than the KEYNOTE-048 study, suggesting that subsequent immunotherapy use may have confounded OS analysis [7, 11, 27]. Other factors that may have contributed to the difference in outcomes between the KESTREL, CheckMate 651, and KEYNOTE-048 studies, include differences in study design, eligibility criteria, regional variations, and the type of PD-L1 assay used [7, 11, 27]. For example, the KESTREL study used an SP263 antibody clone [11], whereas the KEYNOTE-048 study used a 22C3 antibody clone [7]. Previous findings have shown modest agreement of SP263 and 22C3 antibody clones using the CPS algorithm (75% overall percent agreement) [28].
In the EXTREME arm of the KESTREL study, patients who received subsequent immunotherapy (24.3%) had longer survival compared with those who did not, and the predictive value of PD-L1 for OS benefit was likely impacted by the effect of subsequent immunotherapy use [11]. This is further supported by our finding that median OS and OS rates at 12, 18, and 24 months were numerically higher in patients treated with durvalumab monotherapy or durvalumab plus tremelimumab with TC ≥ 50%/IC ≥ 25% versus those with TC < 50% and IC < 25%. In order to remove the influence of subsequent therapy, we considered response rates. Our results showed that patients were more likely to have a complete response when treated with durvalumab plus tremelimumab versus the EXTREME regimen, across PD-L1 subgroups; however, this was not the case for patients treated with durvalumab monotherapy. No substantial improvement in OS or ORR was seen when alternative cut-offs were used.
bTMB appeared to be effective in enriching for response to durvalumab plus tremelimumab, with an ORR of 31.0% in the bTMB ≥ 16 mut/Mb subgroup versus 16.9% in the bTMB < 16 mut/Mb subgroup. In the durvalumab plus tremelimumab arm, CRR was 8.6% in the bTMB ≥ 16 mut/Mb subgroup, which may have contributed to the flattened, long tail (plateau) of the OS Kaplan–Meier curve observed for this subgroup. Moreover, our finding that ORRs were improved in the bTMB ≥ 16 mut/Mb subgroup with durvalumab plus tremelimumab, but not with durvalumab monotherapy or the EXTREME regimen, indicates that bTMB may be predictive of response to durvalumab plus tremelimumab therapy in patients with bTMB ≥ 16 mut/Mb. Recent findings have also shown higher ORR in patients with advanced or metastatic solid tumors and bTMB ≥ 10 mut/Mb treated with ipilimumab plus nivolumab (22.5%) versus nivolumab alone (15.6%) [29].
In the EXTREME arm, OS was lower in the bTMB ≥ 16 mut/Mb subgroup (7.2 months) compared with the bTMB < 16 mut/Mb subgroup (13.0 months). These findings suggest that patients with a lower bTMB may be more likely to benefit from treatment with chemotherapy. When compared to the bTMB < 16 mut/Mb subgroup, more patients treated in the bTMB ≥ 16 mut/Mb subgroup had a poorer performance status, and were more likely to have metastatic than recurrent disease, suggesting that patients in the bTMB ≥ 16 mut/Mb subgroup may have had a poorer prognosis. Unfortunately, the bTMB analysis was impacted by low sample ascertainment and sub-optimal sample collection, such that bTMB results were only available for 56% of patients. These factors led to small bTMB ≥ 16 mut/Mb subgroups (n = 23; EXTREME regimen) with wide CIs. However, an ad hoc analysis of multiple cut-offs confirmed that bTMB ≥ 16 mut/Mb was optimal for this disease setting.
In the EAGLE study, bTMB was predictive of survival for durvalumab monotherapy and durvalumab plus tremelimumab [9]. These findings differ from the KESTREL study, where bTMB appeared to be most effective in the durvalumab plus tremelimumab arm. The utility of bTMB as a biomarker for this combination was previously observed in the MYSTIC non-small-cell lung cancer study, wherein a greater OS benefit over chemotherapy was observed in patients with bTMB ≥ 20 mut/Mb treated with durvalumab plus tremelimumab (HR = 0.49) than for durvalumab alone (HR = 0.72) [30]. Similarly, in the DANUBE metastatic urothelial carcinoma study, the OS benefit of patients with bTMB ≥ 24 mut/Mb treated with durvalumab and tremelimumab (HR = 0.56) was considerably improved versus durvalumab alone (HR = 1.02) [31].
Overall, there is evidence supporting the relationship between TMB and clinical activity of anti-programmed cell death-1 (PD-1)/PD-L1 antibodies as monotherapy and in combination with anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibodies [32]. However, the clinical benefit of adding anti-CTLA-4 to anti-PD-1/PD-L1 therapy appears greatest in TMB-high tumors, which have increased tumor immunogenicity resulting from high expression of tumor neoantigens [32, 33]. It is possible that with a combination approach, anti-tumor immune responses are further enhanced by non-redundant targeting of the PD-1/PD-L1 and CTLA-4 signaling axes, or by specific tumor mutational features that benefit from CTLA-4 inhibition.
In contrast to previous observations from the EAGLE study [9], none of the NLR cut-offs assessed were predictive of OS benefit for durvalumab monotherapy or durvalumab plus tremelimumab versus the EXTREME regimen. However, subsequent therapy use may have confounded the survival results.
In summary, we investigated three independent selection methods to identify patients with R/M HNSCC most likely to benefit from treatment with durvalumab with and without tremelimumab. The results exemplify the imperfect nature of PD-L1 as a biomarker in HNSCC. bTMB showed promise as a biomarker of response and survival, particularly for the combination of durvalumab plus tremelimumab. This finding is consistent with our understanding of the mechanism of action of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies, and may be useful in the future for directing these types of therapies.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- BEP:
-
bTMB evaluable population
- bTMB:
-
Blood tumor mutational burden
- CI:
-
Confidence interval
- CPS:
-
Combined positive score
- CRR:
-
Complete response rate
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated antigen 4
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- HR:
-
Hazard ratio
- IC:
-
Immune cell
- ICI:
-
Immune checkpoint inhibitor
- MSI:
-
Microsatellite instability
- mut/Mb:
-
Mutations per megabase
- NLR:
-
Neutrophil-to-lymphocyte ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- PFS:
-
Progression-free survival
- Q4W:
-
Every 4 weeks
- R/M:
-
Recurrent or metastatic
- SNV:
-
Single nucleotide variant
- TC:
-
Tumor cell
- TMB:
-
Tumor mutational burden
References
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR (2020) Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6:92. https://doi.org/10.1038/s41572-020-00224-3
Cohen EEW, Bell RB, Bifulco CB et al (2019) The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 7:184. https://doi.org/10.1186/s40425-019-0662-5
Ausoni S, Boscolo-Rizzo P, Singh B, Da Mosto MC, Spinato G, Tirelli G, Spinato R, Azzarello G (2016) Targeting cellular and molecular drivers of head and neck squamous cell carcinoma: current options and emerging perspectives. Cancer Metastasis Rev 35:413–426. https://doi.org/10.1007/s10555-016-9625-1
Cancer Genome Atlas Network (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576–582. https://doi.org/10.1038/nature14129
Leemans CR, Snijders PJF, Brakenhoff RH (2018) The molecular landscape of head and neck cancer. Nat Rev Cancer 18:269–282. https://doi.org/10.1038/nrc.2018.11
Argiris A, Harrington K, Tahara M et al (2021) Nivolumab (N) + ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): final results of CheckMate 651. Ann Oncol 32:LBA36
Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394:1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7
Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2:e192535. https://doi.org/10.1001/jamanetworkopen.2019.2535
Wildsmith S, Li W, Wu S et al (2023) Tumor mutational burden as a predictor of survival with durvalumab and/or tremelimumab treatment in recurrent or metastatic head and neck squamous cell carcinoma. Clin Cancer Res 29:2066–2074. https://doi.org/10.1158/1078-0432.CCR-22-2765
Ferris RL, Haddad R, Even C et al (2020) Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 31:942–950. https://doi.org/10.1016/j.annonc.2020.04.001
Psyrri A, Fayette J, Harrington K et al (2023) Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol 34:262–274. https://doi.org/10.1016/j.annonc.2022.12.008
Ferris RL, Blumenschein G Jr, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867. https://doi.org/10.1056/NEJMoa1602252
Ferris RL, Blumenschein G Jr, Fayette J et al (2018) Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 81:45–51. https://doi.org/10.1016/j.oraloncology.2018.04.008
Burtness B, Rischin D, Greil R et al (2020) Efficacy of first-line (1L) pembrolizumab by PD-L1 combined positive score <1, 1-19, and ≥20 in recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): KEYNOTE-048 subgroup analysis. Cancer Res 80:LB-258. https://doi.org/10.1158/1538-7445.AM2020-LB-258
US Food and Drug Administration (2023) Keytruda (pembrolizumab): highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125514s128lbl.pdf. Accessed 21 June 2023
Marabelle A, Fakih M, Lopez J et al (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353–1365. https://doi.org/10.1016/S1470-2045(20)30445-9
Tham T, Bardash Y, Herman SW, Costantino PD (2018) Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Head Neck 40:2546–2557. https://doi.org/10.1002/hed.25324
Fukui T, Okuma Y, Nakahara Y et al (2019) Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer 20:208-214.e202. https://doi.org/10.1016/j.cllc.2018.04.021
Foster CC, Kochanny S, Khattri A et al (2018) Association of a baseline neutrophil-to-lymphocyte ratio (NLR) with progression-free and overall survival in head and neck cancer patients receiving anti-PD-1 therapy. J Clin Oncol 36:6038
Nenclares P, Gunn L, Soliman H et al (2021) On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J Immunother Cancer 9:e002718. https://doi.org/10.1136/jitc-2021-002718
Wildsmith S, Ye J, Franks A et al (2022) Association of PD-L1 expression on tumor and immune cells with survival in recurrent or metastatic head and neck squamous cell carcinoma and assay validation. Cancer Res Commun 2:39–48. https://doi.org/10.1158/2767-9764.Crc-21-0032
Si H, Kuziora M, Quinn KJ et al (2021) A blood-based assay for assessment of tumor mutational burden in first-line metastatic NSCLC treatment: results from the MYSTIC study. Clin Cancer Res 27:1631–1640. https://doi.org/10.1158/1078-0432.CCR-20-3771
Quinn K, Helman E, Nance T et al (2018) Development and analytical validation of a plasma-based tumor mutational burden (TMB) score from next-generation sequencing panels. Ann Oncol 29:131P
Helman E, Artier C, Vowles JV et al (2018) Analytical validation of a comprehensive 500-gene ctDNA panel designed for immuno-oncology and DNA damage research. Cancer Res 78:5603
Odegaard JI, Vincent JJ, Mortimer S et al (2018) Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res 24:3539–3549. https://doi.org/10.1158/1078-0432.CCR-17-3831
Artyomenko A, Sikora M, Lefterova M et al (2018) Microsatellite instability detection by targeted sequencing of cell-free DNA. Ann Oncol 29:1190P
Haddad RI, Harrington K, Tahara M et al (2023) Nivolumab plus ipilimumab versus EXTREME regimen as first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck: the final results of CheckMate 651. J Clin Oncol 41:2166–2180. https://doi.org/10.1200/JCO.22.00332
Scott M, Wildsmith S, Ratcliffe M, Al-Masri H, Scorer PW, Barker C, Rebelatto MC, Walker J (2018) Comparison of patient populations identified by different PD-L1 assays in head and neck squamous cell carcinoma (HNSCC). Ann Oncol 29:1051PD
Schenker M, Burotto M, Richardet M et al (2022) CheckMate 848: a randomized, open-label, phase 2 study of nivolumab in combination with ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. Cancer Res 82:CT022. https://doi.org/10.1158/1538-7445.AM2022-CT022
Rizvi NA, Cho BC, Reinmuth N et al (2020) Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 6:661–674. https://doi.org/10.1001/jamaoncol.2020.0237
Wildsmith S, Walker J, L’Hernault A et al (2020) 266 Tumour mutation burden (TMB) and efficacy outcomes in the phase III DANUBE study of advanced urothelial carcinoma (UC). J Immunother Cancer 8:A163–164
Osipov A, Lim SJ, Popovic A, Azad NS, Laheru DA, Zheng L, Jaffee EM, Wang H, Yarchoan M (2020) Tumor mutational burden, toxicity, and response of immune checkpoint inhibitors targeting PD(L)1, CTLA-4, and combination: a meta-regression analysis. Clin Cancer Res 26:4842–4851. https://doi.org/10.1158/1078-0432.CCR-20-0458
Rizvi NA, Hellmann MD, Snyder A et al (2015) Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. https://doi.org/10.1126/science.aaa1348
Acknowledgements
The authors would like to thank the patients who participated in the KESTREL study, their families, and the investigators and study site personnel. Medical writing support, under the direction of the authors, was provided by Sara Gibson, PhD, of CMC Connect, and Eilidh McLachlan, PhD, on behalf of CMC Connect, a division of IPG Health Medical Communications, and was funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines.
Funding
This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
TYS, SW, KH, JWe, SB, VB, NM, JWa, and AP contributed to the conception and design of the study. TYS, SW, JF, KH, MG, ST, JWe, SB, VB, KR, AL, and AP contributed to data acquisition. TYS, SW, JF, M-JA, ST, JWe, SB, J-PM, VB, BE, NM, JWa, AL, and AP analyzed and interpreted the data. SW and KH provided administrative, technical, or material support. SW, MG, NM, KR, and AP supervised the study. All authors contributed to the writing and reviewing of the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
TYS has received funding (to institution) from AstraZeneca, Bristol Myers Squibb, Cue Biopharma, Kura, Merck, Nanobiotix, Regeneron, and Roche; and honoraria as an advisor from Bayer, Cue Biopharma, Innate Pharma, IO Biotech, Merck, Nanobiotix, NEKTAR, Sanofi, and VIR. SW and NM are employees of and shareholders in AstraZeneca; and report published patent WO2021228988A1. JF has served in a consulting or advisory role for Bristol Myers Squibb, Innate Pharma, Merck, MSD, Rakuten, and Roche; and has received non-financial support from Bristol Myers Squibb and MSD. KH has received research funding from AstraZeneca, Boehringer Ingelheim, MSD, and Replimune, and received consultant/advisory/honoraria fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Codiak, Inzen, Merck Serono, Mersana Therapeutics, MSD, Pfizer, and Replimune. MG has received research funding from Agenus, Bristol Myers Squibb, Cullinan, Genentech, Genocea, Kura Oncology, LaRoche, NRG, and the University of Cincinnati; has served in a consulting or advisory role for Amgen, Aspyrian, AstraZeneca, Bayer, Bicara, BioMimetix, BioNTech AG, Bristol Myers Squibb, Celgene, Coherus, Debiopharm, Eisai, EMD Serono, Genocea, Gilead, Istari Oncology, iTeos, Ipsen, Kura Oncology, LLX Solutions, Merck, Mirati, Nektar, NewLink Genetics, NRG, OncLive Intellisphere, Roche, Roche Diagnostics GmbH, Seagen, Sensei, SQZ Biotech, Shattuck Labs, and TRM Oncology; has received honoraria from OncLive and Roche; and reports stock options in Sensei. M-JA has received honoraria from AstraZeneca, Bristol Myers Squibb, MSD, ONO, and Roche; and has served in a consultant or advisory role for Alpha, AstraZeneca, Bristol Myers Squibb, MSD, Novartis, Roche, and Takeda. ST has received grants from AstraZeneca during the conduct of the study; and grants and personal fees from Bayer, Chugai, Daiichi-Sankyo, Eisai, MSD, Novartis, and Taiho outside the submitted work. JWe has stock and other ownership interests in Achilles, Enfuego, Lyell, Nektar, Nuvalent, and Vesselon; has served in a consultant or advisory role for AbbVie, AstraZeneca, Azitra, BeiGene, Blueprint Medicines, Boehringer Ingelheim, EMD Serono, G1 Therapeutics, Genentech, Genmab, Jazz, Jounce, Lilly, Nanobiotix, Pfizer, Regeneron, Saatchi Wellness, and SDP Oncology; received research funding from Boehringer Ingelheim, Mirati, PDS Biotechnology, and Sumitomo Dainippon; received research funding (to institution) from Amgen, G1 Therapeutics, Immunicum, Inspirna, and Loxo/Lilly; and had travel, accommodations, or expenses paid by Mirati. J-PM has served as an advisory board member or speaker with honoraria (managed by institution) for ALX Oncology, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Cue, eTheRNA, Incyte, Innate, iTeos, Janssen, Merck Serono, NEKTAR, Novartis, Pfizer, and Roche; has received travel expenses from Amgen, Bristol Myers Squibb, MSD, and Pfizer; has served on a data safety monitoring board with honoraria for PsiOxus; has received funding (to institution) for research support from all aforementioned companies; and has received non-financial support from MSD. SB owns stock in Roche Holding AG and is an employee of Verana Health. VB is a freelance contractor for and shareholder in AstraZeneca. BE, JWa, KR, and AL are employees of and shareholders in AstraZeneca. AP has received research funding from Bristol Myers Squibb, DEMO, Kura Oncology, and Roche and received consultant/advisory/honoraria fees from AstraZeneca, Bristol Myers Squibb, HPVtRNA, Merck Serono, MSD, Nanobiotix, and Pfizer.
Ethical approval
The KESTREL study was conducted in accordance with the Declaration of Helsinki and was consistent with International Conference on Harmonization and Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca policy on Bioethics and Human Biological Samples. The final study protocol was reviewed and approved by an ethics committee or institutional review board. Written informed consent was obtained from participants before performing any protocol-related procedures.
Consent to participate
Written informed consent was obtained from participants before performing any protocol-related procedures.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seiwert, T.Y., Wildsmith, S., Fayette, J. et al. Outcomes in biomarker-selected subgroups from the KESTREL study of durvalumab and tremelimumab in recurrent or metastatic head and neck squamous cell carcinoma. Cancer Immunol Immunother 73, 70 (2024). https://doi.org/10.1007/s00262-024-03643-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03643-3