Abstract
This chapter aims to contribute to a comprehensive view of environmental radiobiology and discuss the effects of different kinds of ionizing radiation on ecosystems. The impact of ionizing radiation was considered on both organisms and the abiotic environment, assessing the fate of radionuclides in abiotic compartments (e.g., the movement through atmosphere, hydrosphere, and lithosphere) and in the trophic chains, with implications for human and non-human biota. The available methodologies for estimating radiation dose to biota were also addressed as well as the associated challenges. This chapter also focused on the impacts of ionizing radiation exposure on non-human biota from microorganisms to vertebrates, as well as on the basic concepts related to environmental radiobiology and the molecular effects associated with the exposure to different types of ionizing radiation. The particular context of Naturally Occurring Radioactive Material (NORM) contamination was also tackled, as well as its effects on non-human biota.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Radioecology
- Biota
- Radionuclides
- Radiation dosimetry
- Effects
- Radioresistance/radiosensitivity
- Transfer
- Uptake
- Biomarkers
At the end of this chapter, the reader should be able to:
-
Know the basic concepts associated with environmental radioactivity
-
Know the challenges involved in measuring impacts of radiation in the environment
-
Know the methodologies and tools available to measure the dose and effect at the level of the individual, population, and ecosystem
-
Know the effects of ionizing radiation in living organisms from microorganisms to vertebrates
-
Know the basic molecular effects associated with high and low Linear Energy Transfer (LET) radiation
-
Understand the concept of radiosensitivity and its relation with organism’s complexity and life stage
-
Understand the mechanisms underlying microbial tolerance and/or resistance to radionuclides and metals
-
Understand the complexity of natural environments and the consequent limitations of laboratory studies
-
Understand the particularities associated with NORM contamination
9.1 Introduction
Environmental radiobiology refers to the study of the effects of radiation on ecosystems and species that are part of various habitats, collectively known as “the environment.” The discipline is part of Radioecology which is a broad area of research, covering the transfer, uptake and effects of radionuclides in the environment. Radioecology includes, for example, the speciation of radionuclides in environmental media, the transfer of radionuclides through the different environmental compartments and exposure of wildlife to ionizing radiation and its consequences. While this chapter focuses predominantly on the biological and ecological impacts of radiation on non-human species—since transfer is a key aspect of wildlife dosimetry—the environmental behavior of key radionuclides is briefly covered in Sect. 9.2.
It is important to understand that the basic mechanisms that lead to effects in humans, discussed in earlier chapters, also occur in non-human biota, but the effects of concern lie at higher levels of organization, such as the population or ecosystem. For example, a harmful mutations induced by radiation exposure may lead to cancer on humans, but in the environment, where the sustainability of the population is a critical endpoint, low levels of carcinogenic mutations are unlikely to impact the overall population. This means that the tools and techniques needed to document and evaluate radiobiological effects in natural populations, and ultimately in ecosystems, are much more complex to those used in human radiobiology.
A key issue is the importance and the difficulty of conducting good experiments in field situations, particularly at environmentally relevant concentrations and with proper controls. Single species studies in the laboratory have an important role in determining high and low dose effects, understanding mechanisms and testing resistance. But results can be misleading if they are extrapolated to environmental conditions, with lower doses, chronic exposures, and a variety of confounding factors such as genetics, age, life stage, predation, availability of resources, as well as the interaction with other stressors and difficulties to make a proper dosimetry [1].
Another important issue is how to measure impacts on ecosystems. Several robust biomarkers are available to determine impacts at the level of the gene, cell, tissue, organ, and organism. These are discussed in Sects. 9.3 and 9.4 of this chapter. Population level markers are also available including population numbers, mortality and morbidity, fecundity and population growth rate, but at the level of the ecosystem, the complexity makes it very difficult to assess ecosystem health following radiation exposure, including effects on functions and services. The importance of legacy sites is discussed in Sect. 9.4, as natural labs like, for example, “Radioecological observatories” (https://radioecology-exchange.org/content/radioecological-observatories) where all the mechanisms of effect from populations to ecosystems can be deeply studied. Other approaches include measurements of biodiversity index and the use of drone technologies to monitor ecosystem change at the gross level, for example, forest cover and diversity, lake eutrophication, or extreme habitat change.
9.2 Behavior and Fate of Radioelements in the Environment
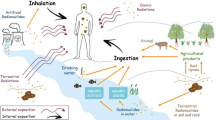
Transfer of anthropogenic radionuclides through food chains has been studied since the time of atmospheric weapons testing and has been supported by data from nuclear power generation and accidents, as well as studies of the behavior of naturally occurring radionuclides (NORs). While there is a wealth of data on the transfer of radionuclides through human food chains, there has been less focus on wildlife and especially organisms that are not common sources of food for humans such as insects and invertebrates. While much of the focus in studying the environmental impacts of radiation has been on the uncertainties in effects measurement, it is important to stress that there are also uncertainties in dosimetry, and especially from internal radionuclides. Hence, knowledge of the factors influencing the behavior of radionuclides in the environment will be fundamental to support dosimetry and exposure assessments. This includes information on the behavior of naturally occurring radionuclides, which is needed both to calculate background doses to organisms, and thus put anthropocentric exposures into perspective, as well as to assess doses in areas with enhanced levels of natural radioactivity.
9.2.1 Naturally Occurring Radionuclides
Naturally occurring radionuclides (NORs) include the radionuclides 14C, 3H, and 40K and also radionuclides that arise from three decay chains: the uranium (238U), the thorium (232Th), and the actinium (235U) decay chains [2] (Figs. 9.1 and 9.2). When they are contained in or released from processing materials they are defined as NORM [3]. Uranium and thorium are both metals belonging to the heavy actinide series, giving rise to long and complex decay chains that contain important radionuclides in the context of environmental radiation exposure (Fig. 9.1). Key radionuclides include isotopes of radon (222Rn with a half-life of 3.8 days; 220Rn with a half-life of 55 s), radium (226Ra half-life of 1602 years, 223Ra half-live of 11.43 days; 228Ra with a half-life of 5.7 days), and polonium (210Po with a half-live of 138 days, 216Po with a half-life of 0.145 s, and 212Po with a half-life of 299 ns). Compared to typical exposures from accidents such as Chernobyl and Fukushima, which are predominantly beta and gamma-emitting radionuclides, NORM exposures are often characterized by high levels of alpha emitters.
9.2.2 Radionuclide Interaction with Water, Air, Soil, and Biota
Radionuclides in the environment can be distributed through the Earth’s atmosphere, hydrosphere, and lithosphere (Fig. 9.2). The behavior and fate of radionuclides in the environment depend on physical and chemical properties of radionuclides, the location and the type of emission source, and the environmental conditions [4]. Radionuclides undergo chemical reactions that affect their distribution and retention time. Organisms interact with the nonliving environment and can be exposed to the radionuclides. In order to estimate the doses received by an organism, the activity concentration of radionuclides in the organism’s habitat is calculated.
The natural environment is a highly complex system in which elements flow and circulate through the spheres of the Earth. To simplify the study of radionuclides, the environment can be divided compartments such as air, surface and groundwater, sediment, soil, and biota. Compartments are usually chosen so that they are distinguishable by spatial boundaries [5]. In each compartment, there are certain processes that have the greatest influence on behavior, so simplifications are made by only taking into account the key interactions that are important to consider for the radionuclide in question. As such, an environmental compartment can be chosen so that it is a volume of medium within which it is assumed that system parameters are constant and chemical concentrations do not vary spatially [6]. For example, in the air compartment, the aerosol formation and particle deposition process of emitted radioactive iodine (e.g., 131I) are key processes to consider, while in the soil compartment, the association with organic matter has been considered the process that determines the largest share of the fate of iodine. Assumptions can be made so that only the key reactions and dynamics are taken into account.
In general, the first step in studying the behavior of the radionuclide in the environment is to obtain knowledge of the location and properties of the emission source. Knowing where the radionuclides come from and in what form they occur can already reveal much information about where the radionuclides will be transported to. For example, the radioactive uranium released from nuclear explosions may end up in very different locations than uranium in nuclear waste dumped into the sea or uranium brought to the surface during the mining of uranium-bearing ores [7]. In addition to the location, the type of emission source should be considered. Anthropogenic emissions of radionuclides result from human activities. These radionuclides are released into the environment at a certain point in time. Unlike anthropogenic emission sources, natural emission sources from the subsurface have been present since the creation of the Earth. Uranium and thorium ores, for example, can be considered as diffuse sources of radionuclides in the Earth’s crust. If groundwater near a uranium deposit flows in a particular direction toward areas where drinking water is extracted, it may behave as a point source. Anthropogenic radionuclide sources, such as nuclear weapon tests and nuclear power plant accidents, release radionuclides at high temperatures and pressures in a certain area over a relatively short period of time and can therefore, be considered a point source. Depending on the weather conditions, the radionuclides can be further dispersed as clouds, with the emission spreading diffusely rather than being a point source. Other point sources, such as the emission of nuclear waste dumped in the ocean, release radionuclides diffusely over a large waterbody. Radionuclides that are dispersed without a specific point of discharge and over a long period of time may be considered as a diffuse source. Agricultural practices, for example, often require high levels of fertilizers, which end up in water bodies through various diffuse processes. Phosphate rock in fertilizers can contain small amounts of naturally occurring radionuclides such as uranium, thorium, and radium. Human activities can enhance the release of radionuclides.
The study of the fate of radionuclides is complicated by the property of radioactive decay. Radioactive decay changes the type of radionuclide, thereby altering its physicochemical properties and potentially altering the fate of the entity. That is, when a radionuclide decays, the daughter element often has very different chemical properties than the parent element [8]. If the parent element is a solid and its daughter is a gas, the parent may partition into other compartments, such as air or water. For example, in the natural uranium (238U) decay series, radon (222Rn) is formed after the decay of radium (226Ra). Radium is an alkaline metal that can be present in a mineral structure within the parent rock or in the pore water as an ionic salt, while radon is an inert gas. If the released radon is captured in a closed space such as the basement of a building or a cave, it can be inhaled by an organism. The gaseous 222Rn decays further releasing alpha and beta particles and eventually decays into stable solid 206Pb. The latter is a metal chemically toxic for organisms. When radionuclides are the stressors of concern, both chemical- and radiation-induced effects on organisms are expected.
Once the radionuclide is emitted, its chemical speciation determines how the radionuclide reacts with components in the environment. It is important to keep in mind that radionuclides are not only physical entities, but also have chemical characteristics [9]. For a more detailed discussion of the importance of the chemical characteristics of radionuclides, the reader is referred to the text by Whicker and Schultz [10]. Radionuclides can occur in various chemical forms or species that have different mobility. The following examples of species are for thorium (Th). Radionuclides such as Th can occur in elemental form (e.g., Th0), but these are very rare in the environment. They can be present as free ions in water (e.g., Th4+). However, dissolved Th is almost always complexed in natural water [11]. Free ions can be bound to inorganic or organic molecules in either the solid or dissolved phases, such as thorium hydroxyl complexes Th(OH)40, Th(OH)3+, Th(OH)22+, ThOH3+, Th(SO4)2+, Th(HPO4)32−, Th-oxalate and Th-EDTA complexes. Radionuclides can also be components of a mineral, such as thorianite (ThO2), and thorite (ThSiO4). The thermodynamic properties of various species can be used to compute liquid-solid equilibria relations. These theoretical calculations reveal much about the possible conditions for and the extent of mobility of radionuclides [11]. The thermochemical data and adsorption results from laboratory experiments help to explain the behavior of radionuclides, such as Th in natural waters, sediments, and wastes.
In general, the total sum of chemical species can be expressed as [9]:
where (MS) is the total sum of species present; (M)n+/− the element present as positively or negatively charged free ion (n+/−); (MmLm)n+/− an element complexed by any kind of ligand, L, such as an oxide, organic, or any other form, negatively or positively charged; (MmA) an element adsorbed onto a surface or trapped in a crystal lattice, or in an amorphous structure, A; m is the number of M or L molecules in the complex; and n+/− is the number of charges.
The fraction of the different chemical species in this formula, that are present in the environment, will depend on the source of the radionuclide and the physicochemical conditions of its surroundings. Parameters such as pH, redox state, ionic strength and the presence of complexing ligands will influence the proportions of each chemical species present.
Some chemical species of radionuclides undergo chemical reactions that influence their mobility or retention. The main chemical reactions determining speciation are adsorption and desorption processes, ion exchange and dissolution reactions, precipitation and co-precipitation, complexation to inorganic and organic ligands [12] and redox reactions. For a detailed explanation of the mechanisms of these reactions, please refer to a course on aquatic chemistry such as Langmuir [8] or Sparks [13].
Of particular interest when studying the behavior of radionuclides are the chemical reactions at the solid–water interface, such as complexation with ligands and adsorption to mineral surfaces. These reactions will largely determine whether the radionuclide is mobile and potentially available for the biota to take up. A dissolved species can associate with an ion or molecule ligand and form a complex [8]. For example, Th is a complex-forming actinide metal for which the chemical speciation of the cation changes with the pH. The multivalent Th cations tend to form strong hydroxyl (OH) complexes. Only in acid waters, the OH concentration is low enough so that competition with ligands is minimal. In these conditions, it is easier for ligands to displace OH and complex it. Complexation with carbonates, humic materials, or other ligands increases the solubility of the Th species and thus the mobility in the environment. An adsorbed species can associate with charged surfaces or broken bonds of minerals. For example, Th adsorbs onto clays, oxides and organic matter in soils and sediments. The adsorption of Th increases if the pH increases from acid to neutral conditions [11]. Sorption processes increase the retardation of Th and thus decrease its mobility in the environment. In general, Th in the soil compartment will remain strongly adsorbed onto soil constituents so that contamination of groundwater through the transport of Th from soil to groundwater will not occur in most soils [14]. Certain microorganisms (Pseudomonas aeruginosa) present in soils may enhance the dissolution of Th by producing chelating agents that can form soluble complexes with this radionuclide [15]. This is not the only way for microorganisms to influence the speciation and mobility of radionuclides. They can also, for example, change their redox state, immobilize them by processes such as biosorption, biomineralization, and precipitation [16]. In the water compartment, soluble Th ions will hydrolyze at a neutral pH forming complexes with OH. The Th-hydroxyl complexes can in turn be absorbed on suspended particles in the water. Although dissolved Th tends to form strong complexes, facilitating its transport, Th concentrations in natural waters—with pH between 5 and 9—remain limited by the scarcity of the element, small solution rates and insolubility of Th-bearing minerals [11]. In groundwaters at mining facilities, Th concentrations may be higher due to the more acidic conditions which cause the leaching of Th.
A common approach to quantify the mobility and availability of radionuclides in the environment is to estimate the ratio between the activity concentrations of the radionuclide in two chosen compartments or trophic levels [9, 17]. The radionuclide retention on the solid phase is estimated by determining a partitioning coefficient. The coefficient describes the partitioning of a radionuclide between the solid and aqueous phases and takes no explicit account of sorption mechanisms [18]. It is assumed that an equilibrium exists between the dissolved and sorbed amount of radionuclides and that exchange is reversible [19]. This simplification relates the concentration of a radionuclide in water to the amount of radionuclide adsorbed:
where Maq and Mads are the aqueous and adsorbed species, respectively.
A solid-liquid distribution coefficient (Kd) is derived from the ratio of radionuclide concentrations in the solid phase to that in solution and is calculated as:
where Aint is the initial radionuclide activity (Bq), Aeq is the equilibrated radionuclide activity (Bq) in the aqueous phase, V is the volume of the liquid phase (L), and m is the mass of solid phase (kg).
The adsorption of radionuclides onto soil particles is often expressed as a Kd value. The Kd is determined by adding a known amount of sorbent (i.e., clay, oxide, soil) to a solution with an initial radionuclide concentration, and after equilibration and phase separation (e.g., by ultracentrifugation or a dialysis membrane), radionuclide concentration in the aqueous phase at equilibrium is measured.
In case of radiocesium (e.g., 134Cs and 137Cs), for example, the CsKd value is obtained by the ratio of the total radiocesium activity concentration in the solid phase and in liquid phase after a chosen time of contact between the two phases. The experimental design must be carefully thought out, as parameters such as contact time, radionuclide concentration, solid to liquid volume, and the ion composition of the aquatic phase affect the Kd value. Radiocesium dissolves well in water, so that radiocesium exists in the aqueous phase only as a free ionic species. Only one metal species of Cs should be considered, which simplifies the study of adsorption equilibria. Moreover, radiocesium cations can be directly adsorbed from solution by an organism, because the cations have no tendency to form soluble complexed species [20]. Thus, the CsKd value can be determined in a relatively simple manner and it can provide useful information about the radiocesium accessible to the organism for uptake [18].
However, caution must be taken in interpreting a Kd value, as it may change over time [18]. On the one hand, the Kd changes in a short term, because an equilibrium is not always reached instantaneously, as for example for radioactive isotopes of iron [21]. On the other hand, the Kd changes in long term, because adsorbed radionuclides, such as 137Cs, can migrate deeper into structures of minerals so that it is no longer available and becomes fixed. Kd values are often determined by short-term laboratory experiments lasting several hours or days. However, Kd values can also be determined in the field, where the results depend on the time elapsed since the contamination occurred and this gives a more reliable picture of the long-term fate of the radionuclides. The time effect was studied in a laboratory study [22] with soils showing that CsKd values of mineral soils with 5% clay minerals can increase from 30 to 1000 L/kg in 40 days and 200 to 5000 L/kg in 415 days for peaty soils with 10% clay minerals. In this example, the CsKd of the mineral soil increases by a factor of 30 over a relatively short period of time, and the CsKd of an organic soil increases accordingly but over a much longer period of time. Laboratory results of CsKd values can only partly explain the reduction in Cs soil-to-plant transfer in the field. A study after the Chernobyl accident [23] shows that 137Cs soil-to-plant concentration ratios, that were initially elevated, were reduced by more than 50 times in the following years. This trend was explained by an initial step of radionuclide release from fuel particles into soil aqueous phase, followed by a reduced transfer attributed to the progressive fixation of 137Cs by soil minerals, referred to as “aging effect” that makes 137Cs gradually less available for uptake by the plant.
In many cases, the factors that influence the transfer of radionuclides to biota are similar for humans and include soil and water chemistry, speciation of radionuclides, as well as biokinetics (biological and ecological half-lives) and interactions between radionuclides and stable elements. For example, the soil-to-plant transfer of 137Cs, is influenced by clay content and K levels in the soil, and radiostrontium (90Sr) by Ca levels. Another example, is the uptake of U to fish and other aquatic organisms, that is are dependent on pH and carbonate concentrations, which change the availability and complexation of this element [24]. In contrast to Cs, radionuclides such as U exist as several species in the environment. The bioavailability of different U species in soil to ryegrass was studied in a laboratory pot experiment [25], which showed that speciation has an important influence on the uptake of U by grass. From the results, it was concluded that the uranyl-cation (UO22+) and uranyl-carbonate complexes (e.g., UO2CO3(aq), UO2(CO3)34– and (UO2)2CO3(OH)3–) together with uranyl-phosphate (UO2PO4–) are the forms that are most readily taken up by ryegrass and thus are more bioavailable compared to other uranyl-phosphate complexes (e.g., UO2HPO4) and the hydroxy- (e.g., UO2(OH)2(aq) and UO2OH+) and sulfate-complexes (e.g., UO2SO4(aq) and UO2(SO4)2–). As demonstrated in the previous examples, some species are not available for uptake by biota. Hence, a value other than the total concentration in the compartment should be used to estimate the bioavailability of a given radionuclide and, the exposure of biota through ingestion of radionuclides should only be estimated from the activity concentrations of the bioavailable species [17].
Internal exposure and toxic effects of radionuclides require that an organism takes up the radionuclide, and for chemically available species to be taken up by biota, the radionuclide must be able to cross cell membranes [26]. To investigate whether this exposure will occur through ingestion, it is important to know whether this contaminant is a source for ingestion by biota. A radionuclide’s potential for biota uptake in soil and sediments is defined by its bioavailability or bioaccessibility. There is a slight difference between the bioavailability and bioaccessibility of pollutants in sediment and soil. This difference has implications for the design of experimental set-ups, but also for the interpretation of results. The bioaccessible fraction is the species in the environment, which are available to cross an organism’s membrane if the organism has access to the radionuclide in the longer term [26]. The bioavailable fraction is freely available to cross an organism’s membrane from the medium the organism inhabits at a given time. For example, technetium (Tc) may be highly mobile in aqueous solution at oxidation state +7 (i.e., Tc(VII)), but strongly absorbed and retarded in the subsurface at oxidation state +4 (i.e., Tc(IV)) [27]. Technetium is used in nuclear medicine for diagnosis and is emitted in the environment from the nuclear fuel cycle. Technetium exists primarily in two stable oxidation states as Tc(VII) or as Tc(IV), and the two species can have a different fate when released to the environment. While TcO4− in solution is bioavailable, TcO2·nH2O is expected to be adsorbed at low concentrations and precipitated at high concentrations. The species TcO2·nH2O can become available for uptake when oxidized by air and is thus bioaccessible.
Besides the speciation of radionuclides, the extent to which radionuclides can be transferred to different compartments is influenced by competition between ions. On the one hand, stable isotopes of the radionuclides may compete for adsorption to the solid phase or uptake by biota. For example, radionuclides such as 3H, 40K, 48Ca, 54Mn, 60Co, 65Zn, and 131I, are isotopes of essential biological nutrients [10]. Therefore, their uptake and retention characteristics are largely controlled by the flux of these essential nutrients through biological processes. On the other hand, elements that are chemically similar to the radionuclides may compete. Certain radionuclides behave in the environment in a similar way to essential elements for biota, due to their chemical properties. For example, 137Cs and 90Sr have similar chemical properties and follow the same transfer and cycling processes in the environment as the macronutrients potassium (K) and calcium (Ca), respectively. The tendency of these radionuclides to accumulate in the biota is reduced if there is an abundance of the analogous element in the environment [10]. Conversely, the accumulation of the radionuclide in the biota increases when there is a scarcity of the analogue element. For example, low concentrations of K and Ca in the soil can result in increased uptake of radionuclides by plants, as they find it more difficult to discriminate between nutrients and radionuclides under these stressful conditions [20]. As mentioned earlier, the long-term bioavailability of 137Cs and many other radionuclides depend heavily upon ecosystem characteristics, and in particular, soil properties [10]. Soils and sediments of high clay content can effectively immobilize 137Cs by chemical binding. In such systems, the soil acts like a sink for 137Cs and in time very little of the nuclide is available for biological incorporation. Other systems have sandy soils with a low cation exchange capacity, and larger quantities of 137Cs can be recycled through the biota of such systems for long periods of time [9].
In summary, depending on their speciation, radionuclides can be transferred in the biosphere from the emission source to different compartments until they reach an equilibrium or final sink, or they can be recycled within the environment.
9.2.3 Radionuclide Transfer and Exposure
Information on the uptake of radionuclides to biota is vital for calculating dose to the organisms, since both external and internal irradiation contributes to exposure. Soil and sediment dwelling organisms often have high external dose rates by virtue of their habitat, but also internal exposure from ingested radionuclides. Many field studies on radiation effects in wildlife are flawed due to underestimation of the internal dose, reporting only ambient air dose rates [28]. This is particularly important for 〈- (e.g., Ra) and ®-emitting (e.g., Sr) radionuclides, for which internal exposure is the greatest contributor to dose, but also internal contributions from radiocesium or radium, for example, can make a significant contribution to the overall dose.
There are a number of programs available for estimating the dose to biota. These are usually based on rather simplistic geometry and homogeneous internal distribution, but the basic principles are similar to those used for human dosimetry. They can also be adapted to give organ specific doses. For example, the ERICA Assessment Tool can calculate doses to a wide range of reference animals and plants, as well as user constructed organisms (see Box 9.1).
Box 9.1 The ERICA Assessment Tool
The ERICA Assessment Tool is a free to download, computer software system for assessing the risks of ionizing radiation to terrestrial, freshwater and marine biota (https://erica-tool.com/). The system is based on the three tier ERICA Integrated Approach that was originally developed as part of the ERICA EURATOM project [29] (see also https://wiki.ceh.ac.uk/display/rpemain/ERICA).
The ERICA Tool includes various components, all of which are linked to internationally recognized programs and databases. These include
-
Modelling transfer of radionuclides through the environment: links to IAEA Wildlife Transfer Database (WTD) and IAEA handbooks [30]; https://www.wildlifetransferdatabase.org/.
-
Methodology for estimating dose rates to biota from internal and external distributions of radionuclides: ICRP biota DC software version 1.5.1 for the calculation of dose conversion coefficients (DCC) [31].
-
Risk characterization in order to evaluate the significance of the dose rates received by organisms, including comparison with background radiation doses, screening values [32], Environmental Media Concentration Limits (EMCL) [33], derived consideration reference levels (DCRL) and biological effects (FREDERICA database, https://www.frederica-online.org/mainpage.asp).
The tool contains data on concentration ratios and DCC for all radionuclides in publication 107 [34], and in addition to a selection of pre-created reference organisms, allows users to create their own assessment organism.
The ERICA tool has been updated since its original release, and the current version, ERICA Tool 2.0 (beta version released in November 2021—https://erica-tool.com/the-erica-assessment-tool-has-been-updated-to-version-2-0/) includes updates on concentration ratios, as well as new approaches for calculation of dose contribution from short-lived progeny, noble gases radon and thoron [35,36,37].
Internal and external exposures are determined from specific dose conversion factors (DCC) combined with using field measurements of concentration activities or default concentration ratios (CR). The CR represents the activity concentration of radionuclides in biota (fresh and dry weight in animals and plants, respectively) and the activity concentration in soil (dry weight, upper 10 cm), water, or air for a given radionuclide [38]. The tool also allows the calculated exposures to be compared to background radiation or screening values.
The calculation of external dose rates takes account of the occupancy of the organism (i.e., percentage of time spent in, on, or above soil, sediment, or water) and is determined by:
-
DR—dose rate (Gy/unit of time)
-
DCC—dose conversion coefficient
-
Cmedia (Bq/kg or Bq/L)
Internal doses
-
DR—dose rate (Gy/unit of time)
-
DCC—dose conversion coefficient
-
Corganism (Bq/kg)
There are several other simplifications to the approach, including assumptions on habitat ranges and feeding habits of biota [38]. CR are lacking for many organisms and radionuclides; however, the tool provides default CR based on available data and assumptions (e.g., similar taxonomy or chemical behavior to other organisms or radionuclides).
Uncertainties in dose estimates can be reduced if field measurements are available, but determination of internal concentrations of radionuclides can also be challenging, as organisms may be too small for direct radiochemical analyses, or it can be difficult to distinguish between radionuclides internalized in animal tissues, from those adsorbed to the body segment or cuticle. Efforts have been made to compare ERICA default CRs with field measurements at Chernobyl, showing a relatively good agreement between the CR values calculated for many organisms [39]. However, it was concluded that such similarity may have resulted from the broad range of estimated CR values available [40].
In soil, Beaugelin-Seiller [41] concluded that DCC values are highly dependent on factors such as the porosity and soil water content, the body size of the organisms within other factors. For ®-emitters, the difference in DCC values recorded reached a factor of 3, between dry and saturated soil conditions. The calculation of doses in organisms under exposures to NORM is also highly dependent on assumptions of equilibrium that must be made for several radionuclides from the 238U decay series [42]. Usually a 100% equilibrium is assumed, although different equilibrium percentages are also accepted for radon, as it can escape to the atmosphere.
The positioning of organisms in the trophic chains and the composition of their diets may be determinant for the magnitude of exposures. In a coastal sand dune system, under a long-term contamination through atmospheric deposition and sea-to-land transfer of radionuclides at Sellafield nuclear reprocessing site (West Cumbria, England), Wood and collaborators [43] recorded high activity concentrations of 137Cs, 238Pu, 239+240Pu, and 241Am in soil detritivorous (e.g., Collembola and Isopoda) when compared with predators (e.g., Coleoptera larvae). Within the same trophic level, these authors also found significant differences in the whole-body activity concentrations of different invertebrate groups. Size also influences the internal doses to organisms. Dose calculations for two benthic invertebrates, the larval midge Chironomus tetans and the amphipod Hyalella azteca, based on estimations from NORM activity concentrations in sediments impacted by uranium mining demonstrated that the smaller amphipod, received a greater dose of alpha irradiation. This reflected the high content of ingested radionuclides within the gastrointestinal tract and that as diameter of the gastrointestinal tube decreases, the assessment factor (AF) for ingested alpha-emitters increases, as more alpha-particles are expected to reach the tissues of the organisms [42]. Therefore, it was suggested that the contribution of sediment within the gastrointestinal tract for the calculation of internal doses must be considered, and not only the activity concentrations of radionuclides recorded in external sediments.
In the case of accidents, there is also a need to account for historical dose and radionuclide decay, since observed effects may be a legacy of high levels of exposure after the accident. These high exposures can also be a source of confounding factors, since the initial damage may lead to indirect ecosystem changes (such as the replacement of pine trees by less sensitive species) [44]. While much of the focus in studying the environmental impacts of radiation has been on the uncertainties in effects measurement, it is important to stress that there are also uncertainties in dosimetry.
9.3 Impacts of Ionizing Radiation on Non-human Biota
Following the discovery of X-rays by Wilhelm Roentgen in 1895 and of radioactivity by Henri Becquerel in 1896, studies on its effects started immediately. The detonation of the atomic bombs over Hiroshima and Nagasaki in 1945 raised the concern about the health impacts of radioactive contamination and the behavior of radionuclides in the environment [45]. Therefore, a great number of studies using a variety of plants and animals have been performed since then.
The first harmful effects caused by the exposure to ionizing radiation occur at the molecular and cellular level. If these effects are severe enough, they can impact tissues, organs, individual organisms, populations, and entire communities. However, even though an individual organism may suffer from severe damage at the molecular and cellular level, it does not necessarily mean that entire populations and communities will be affected [46]. It seems that individual organisms are able to sustain a certain level of effects before they are reflected at a population level [46]. However, when an effect is seen at the population level or at higher levels of organization (i.e., communities or ecosystems), it means that effects at individual organisms are expected to be occurring (Fig. 9.3) [45].
There can be two types of effects caused by ionizing radiation. They can be stochastic or non-stochastic (deterministic). Stochastic effects are effects that occur by chance and the higher the dose the higher the probability of its occurrence. However, the severity of those effects is not dependent on radiation dose. The main stochastic effects related to ionizing radiation exposure are cancer and genetic damage/alterations (i.e., mutations) [47]. For non-human biota, stochastic effects that occur at germinal cells will be the ones that will have a higher impact, as they will have a higher probability of being inherited and, therefore, of affecting the next generations, impacting populations and communities [47]. Deterministic effects depend on time of exposure, doses and type of radiation. They are adverse tissue reactions that result from the damage or killing of many cells in an organ or tissue. The severity of these effects increases with dose when radiation levels reach a threshold, below which harmful effects to tissues/organs do not occur. The deterministic effects that are most important at a population level are mortality (which affects density, age distribution, and death rate), fertility (birth rate) and fecundity (which affects birth rate, age distribution, size of the population) [45] (Fig. 9.3). As for other stressors (i.e., chemicals), exposure to ionizing radiation can be acute or chronic. Acute exposures are short-term exposures to relatively high doses of radiation that usually last minutes or hours. Chronic exposures are long-term exposures or lifetime exposures to usually low doses of ionizing radiation. Doses in acute exposures are often reported as total absorbed doses, whereas for chronic exposures doses are often reported as dose rates (i.e., mGy/day, Gy/year, or mGy/h) [46, 48]. For a given dose of ionizing radiation, acute exposure induces higher injury than chronic exposure [46]. The higher the dose the lower the ability of cells to correctly and rapidly repair the damage and also the lower the ability of healthy cells to divide and regenerate the damaged tissue [46]. Depending on the dose received by cells or organisms, several types of effects can occur, namely genetic damage, DNA lesions that can induce teratogenic effects (malformations) on embryos when occurring in germinal cells (i.e., gametes), cell transformation in somatic cells and cell death (Fig. 9.3). In some cases, DNA damage can be so severe that it becomes incompatible with the survival of the cell or of the entire organism. Depending on the kind of cells that are affected (germ cell or somatic cells), there can be different consequences. Severe damage (i.e., DNA double strand breaks, gross mutation like duplications, deletions, translocations, and chromosome gain or loss) will cause cell death potentially leading to the death of the organism or, for example, to its sterility if it occurs in germ cells (Fig. 9.3). If the damage is not enough to cause cell death, it can cause cell transformation and cancer in somatic cells or it can affect the fitness of the organisms and entire populations if it affects germ cells. Mutations can cause a reduction in the production of viable embryos or viable gametes and also, they can be passed and accumulated throughout generations reducing the population’s fitness. Therefore, DNA alterations can have an important impact on fertility and fecundity and consequently in reproduction [46].
Also, there can be effects on the homeostasis of organisms (Fig. 9.3), namely depression of the immune system, alterations in normal metabolism, oxidative stress, and disturbances in the endocrine system [49]. The majority of the studies performed so far are focused on the determination of the acute effects of high doses of radiation, and only few studies are focused on chronic exposures to low doses of ionizing radiation.
The younger the organisms (namely fetuses and embryos) the more sensitive they are to the deleterious effects of radiation exposure. This is due to the higher sensitivity of cells that frequently undergo mitosis (which occurs frequently in young organisms for each tissue/organ as it is part of the growing process). Also, tissues/organs that have the ability to regenerate or that are constantly producing new cells like the hepatic tissue, the skin, the bone marrow, germinal cells, and gut lining are more sensitive to radiation (Fig. 9.3). The higher the cell division rate in an organism the more sensitive it will be to radiation’s harmful effects.
Regarding the sensitivity of parameters like mortality and reproduction, in general the reproductive capacity is a more sensitive parameter to the effects of radiation exposure both for terrestrial and aquatic invertebrates and vertebrates, than life expectancy (mortality) [45]. Negative effects on reproduction rate can occur at less than 10% of the radiation dose required to induce direct mortality in mammals [45].
All organisms evolved in the presence of radiation, being cosmic radiation or natural radiation emitted by NORs present in the earth crust [50]. The studies performed so far, on the effects of ionizing radiation, showed that there is a considerable variation in the response of organisms from the same or different species, due to intra- and interspecies variability in sensitivity. In general, it is widely accepted that mammals are the most sensitive organisms, followed by birds, fish, and reptiles and that invertebrates and other less complex organisms have the highest radiation resistance (Fig. 9.4) [46, 50]. However, it has to be noted that most of the knowledge gathered so far comes from laboratory exposures of specific strains of these organisms and that results may differ significantly from what happens to their wild counterparts.
9.3.1 Basic Molecular Effects of Low and High Linear Energy Transfer (LET) Radiation
The majority of the existing studies on the effects of ionizing radiation in cells are focused on DNA as the main target, making it clear that there is a cause-effect relationship between DNA damage with cytotoxicity and mutagenicity associated with ionizing radiation exposure. However, the cascade of molecular effects that lead to the induction of biological effects in exposed organisms is complex and involves, firstly, the interaction of radiation with water molecules and structural and functional biological molecules inside the cells. This interaction will induce the formation of ions, radical species, and excited molecules that will move from the site where they were formed to other cell compartments, causing damage to other biological molecules. This will trigger several signaling cascades, activating cell responses that will change the normal metabolic state of the cell, including changes in gene expression, enzyme recruitment and activities, DNA methylation patterns, and other stress-induced signaling events. When DNA is damaged, the cell cycle is interrupted allowing for DNA integrity check. DNA can be damaged directly through direct ionization or indirectly through the attack of free radicals that are formed when radiation interacts with water molecules of the cell [51]. Given the high content of water in cells, IR interacts with water in a process called radiolysis, generating free radicals as H∙ or OH∙, which trigger a cascade of events giving rise to other ROS as hydrogen peroxide and the superoxide anion [52] and references quoted. If not neutralized these products may diffuse within cells, as well as between cells, affecting other biomolecules such as DNA, proteins, and lipids, both in target and non-target cells (i.e., cells not directly irradiated) [53, 54]. Regarding DNA, ROS may oxidize bases or cause single and double strand breaks (SSB and DSB) [55]. Also, post-irradiation DNA lesions can be formed as a consequence of the attempt of the cell to repair sugar and base residues, which can be converted to SSBs (Single Strand Breaks) and DSBs (Double Strand Breaks) [51]. If DNA is correctly repaired, the cell will continue its cycle normally, if not, the cell can undergo transformation as mutations and chromosome aberrations may occur or if the damage is too severe, programmed cell death (apoptosis) will occur. The repairability of the damage and the repair accuracy will depend on damage severity and complexity. Low LET (beta particles, gamma and X-rays) and high LET (alpha particles and neutrons) radiation exposure can cause several types of DNA damage that are usually repairable, like SSBs, abasic and apurinic and apyrimidinic sites and DSBs (Fig. 9.5). However, the fraction of irreparable DNA damage depends strongly on LET. High and low LET radiation exposure can cause complex DNA damage, but this type of damage is more frequently associated with high LET radiation. Complex DNA damage is composed by closely spaced DNA lesions that form clusters [51]. Clusters contain two or more DNA lesions of the same or different origins, close to each other and on opposite strands (bistranded lesions). These lesions can be DSBs or non-DSBs oxidative clustered DNA lesions like SSBs, oxidized base lesions, and oxidized apurinic/apyrimidinic sites (AP sites) [51] (Fig. 9.5). These clustered lesions have a high mutagenic and carcinogenic potential since they are considered repair-resistant or even unrepairable due to the relative inefficiency of DNA repair systems to process such closely spaced and complex lesions. As there are several DNA repair systems in the cells and each of them is specialized in the processing of specific lesions, when several types of lesions are closely spaced in the DNA molecule, the different repair systems cannot act properly, retarding the repair and often generating other lesions. High LET radiation is mostly associated with the generation of DSB’s clustered DNA lesions and low LET radiation to non-DSB’s oxidative clustered DNA lesions [51], but this is not completely clear and needs further studies. High LET radiation is also associated with increased frequency of chromosome aberrations, and also to a high frequency of unrejoined DSBs and consequently with a higher cell killing efficiency, as unrejoined DSBs are a cause of cell death.
9.3.2 Effects on Microorganisms
Microorganisms, including fungi, can be seen as good indicators of the ecosystem’s “health.” They include ubiquitous and taxonomically diverse microorganisms that play important key roles on diverse ecosystems’ function. Specifically, with regard to radiation, microorganisms play a very important role in the health of these systems and in their cleaning and decontamination.
9.3.2.1 An Overview on Microbial Radiobiology: Radioresistance and Radiotolerance
Microorganisms play a key role in the biogeochemical cycle of elements. In soils, they are important for organic matter turnover and maintenance of soil structure and fertility. As such, changes in the structure of microbial communities, by either metals or radionuclides, can have indirect effects on the above processes. Prokaryotes (bacteria and Archaea) have dominated a large part of the history of our planet, occupying virtually every “inhabitable” niche on earth. To be able to do that they have adapted to withstand large ranges in: (1) temperature, e.g., the hot temperatures found in hot springs and fumaroles, and the contrasting cold temperatures found on sea ice and polar regions, (2) pressure, e.g., deep sea, (3) salinity, e.g., hypersaline lakes, (4) pH, e.g., acid mine drainage sites, and (5) radiation, e.g., naturally occurring (deserts and high mountains, mining sites) and from nuclear contaminated sites [56]. Microorganisms that have adapted to such environments are referred to as extremophiles or polyextremophile (the latter being capable of withstanding different extreme conditions simultaneously), and these conditions are a requirement for their normal metabolic and biochemical operation. Most of these microorganisms belong to the domains Bacteria and Archaea although some fungal species have also been described. To survive these harsh conditions, extremophiles produce various primary and secondary metabolites, such as extremolytes, enzymes, and pigments [57]. Extremolytes, for example, are known to protect extremophiles cell structures and macromolecules from their harsh environments by forming protective water layers (e.g., ectoine), which is a co-solvent that shields proteins and cell membranes from UV light, heat, and dryness [58] around them or acting as chemical scavengers (e.g., carotenoids), protecting cells and their structures from UV radiation and oxidative stress [58]. Ultimately, the exceptional properties of these biomolecules find possible applications in various industrial sectors, in human healthcare, and well-being [59].
With regard to radioactively contaminated sites, microorganisms play an essential role on the mobility, toxicity, and distribution of radionuclides, through processes that include reduction, uptake, and accumulation by the cells, biosorption, and biomineralization with phosphates and carbonates [16].
Culture dependent and culture-independent approaches have shown the effects of long-term exposure to metals or radionuclides on individual species and on microbial communities. In addition, they have allowed those specific genes and cell functions mostly affected by radiation and metals to be identified, thus contributing to a better understanding of the molecular mechanisms behind microbial metal/radioresistance. Furthermore, the acquisition of genetic determinants by horizontal gene transfer contributes to shape microorganisms and microbial communities occupying these sites. More recently, refined metagenomic approaches focusing on prokaryotic communities have been employed and are expected to shed more light on the cells’ strategies to overcome radiation stress to remain operational.
The following section addresses in more detail some of the mechanisms that contribute to the survival and maintenance of microorganisms in these environments. We will end by referring to the impact of more recent methodologies, such as metagenomics and other omics technologies, and their contribution to clarify aspects such as the impact of these contaminants on the microorganisms and communities that exist in these sites.
9.3.2.2 Mechanisms Underlying Microbial Radiation Resistance: Cell Damage and Repair
It has been reported that when radiosensitive microorganisms are subjected to multiple high IR exposures, their resistance increases [60]. This was recently demonstrated by experimental evolution, where populations of Escherichia coli very resistant to IR were generated in the laboratory, after 100 selection cycles, and to which the dose needed to kill 99% of the population increased from 750 Gy to about 3000 Gy [61]. Likewise, radioresistant species can become even more resistant with repeated exposure [62]. This “memory” adaptation is associated with smooth genetic alterations that affect DNA repair and metabolic functions. During this process of adaptation, other physiological characteristics of the microorganisms are profoundly affected as, for example, growth which is slowed down, because the microorganism must direct its energies to other processes, such as effectively repairing the damaged DNA.
The association between genome size and radiosensitivity between taxa has long been suggested. For instance, for the same chronic exposure to IR, fungi, for which genome sizes range between 12 and 20 Mbp, suffer more DSBs per unit time than bacteria with their smaller genomes (3–6 Mbp). However, this is not true for Shewanella oneidensis and Deinococcus radiodurans whose genomes are practically the same size, but while the former is killed after exposure to a radiation dose causing one DSB, the latter manages to recover from hundreds of DSBs. This is probably due to the fact that D. radiodurans has up to ten identical copies of its genome per cell and uses this genetic information to repair its DNA. In addition, there is also evidence for the interference of non-enzymatic antioxidants such as manganese complexes, which protect proteins from IR-induced oxidation, facilitating the maintenance of cell homeostasis and DNA repair. Although in many radioresistant bacteria and yeasts, the most common DNA DSB repair pathway is similar to homologous recombination (HR),Footnote 1 in fungi, non-homologous end joining (NHEJ)Footnote 2 is the preferred, as in other eukaryotes, despite being error-inducing. Melanin pigments also seem to be involved in protection against multiple stressors, including IR as it can act as an oxygen radical scavenger [62].
Radioresistant microbial extremophiles have developed strategies to survive and withstand dose rates that to the majority of organisms, including humans, would result in acute health effects [63]. It is believed that radioresistant microorganisms possess highly efficient processes to repair DNA damage. However, it has recently been demonstrated that the repair mechanisms and the proteins involved are common to those found in radiation sensitive microorganisms [64].
The genus Deinococcus is probably the most well studied and characterized and there is a great deal of information, to what its radioresistance is concerned. Metabolically active Deinococci vegetative cells can tolerate chronic radiation levels of more than 100 Gy/h, whereas other bacteria, Archaea, and fungi can be resistant to several kGy of acute IR. D. radiodurans exhibits resistance to acute IR up to 15 kGy, to 60 Gy/h of chronic radiation, and also to high levels of resistance to UV-C irradiation (100–295 nm), desiccation and oxidative stress. Thus, regarding the example of Deinococcus radiodurans, it can be argued that it efficiently and rapidly repairs DNA damage caused by IR. A number of genes have been identified whose expression is activated after irradiation, namely those encoding proteins associated with (1) efficient DNA repair, (2) protection against oxidation and (3) DNA supercoiling, which helps to maintain DNA integrity after irradiation [65]. More recently, it was demonstrated that in this organism, the adaptation to dryness and desiccation is at the basis of its radioresistance [64].
Nonetheless, it has been reported that Deinococcus’ ability to repair DNA damage results from a selective pressure other than ionizing radiation, because there are no terrestrial environments subjected to the levels of radiation it tolerates. Still, the information gathered, albeit with some degree of uncertainty, has contributed to a better understanding of the mechanisms of radioresistance in other organisms, making this an excellent model organism to unravel these mechanisms [66].
Studies have shown that the DNA repair systems used by D. radiodurans are less complex than those of radiation sensitive bacteria, namely Bacillus subtilis, a spore-former species and Escherichia coli. Transcriptomics studies revealed that in response to γ-radiation, specific genes involved in damage response are activated (ddrA, ddrB, and irrE (pprI)). PprI, for instance, regulates the expression of the recombinase recA and pprA, which is a protein involved in DNA ligation that is essential for the radiation resistance exhibited by D. radiodurans. Strains lacking pprI show impaired genome recovery [66]. Another important DNA repair system involves the synthesis of long and single-stranded overhangs, a process referred to as “Extended Synthesis-Dependent Strand Annealing” (ESDSA).Footnote 3 The process allows the reconstruction of a functional genome from the chromosome fragments produced by the exposure to radiation. Accordingly, the process is used by the RecFOR pathway to repair DNA double strand breaks. To support these observations, strains mutated in the genes involved in the RecFOR pathway are susceptible to γ-radiation [67].
Laboratory experiments with Escherichia coli, and other mesophilic bacteria, have shown that these may become resistant to the chronic exposure to IR just by adding Mn2+ and orthophosphate to its growth medium, which spontaneously form potent Mn-antioxidant complexes. Another important factor associated with radioresistance is cell density. For example, in D. radiodurans high cell concentrations seem to exert a protective effect against a radiation dose of 67 Gy/h [60]. Still, further and more complete studies are required until we know all the phenomena that contribute to the radioresistance exhibited by microorganisms. One thing is certain, it results from the interplay of several factors.
9.3.3 Multiomic Approaches Applied to the Study of Radioresistant Microorganisms
Undoubtedly, multi-omics approaches (genomics, transcriptomics, proteomics, and metabolomics) will shed light and will further contribute to our understanding of the mechanisms involved in microbial radioresistance and detoxification. In order to contribute to a better understanding of the mechanisms involved in uranium resistance/tolerance, a recent high-throughput proteogenomic study was applied to bacteria of the genus Microbacterium, isolated from Chernobyl U contaminated soils and from natural U rich soils. The approach allowed the identification of proteins involved in membrane transport (e.g., ABC transporters and efflux pumps), phosphate (e.g., phosphatases involved in biomineralization) and iron metabolism (e.g., siderophores), in addition to a large percentage of proteins of unknown function, which reveals the complexity of this mechanism [68]. Still, in another study carried out with a member of the genus Geobacter exposed to 100 μM U, proteins involved in DNA protection, in efflux pumps of the RND family and in oxidative stress responses (e.g., SOD and superoxide reductase), were also identified. Exploring these recent approaches will certainly allow us to gain knowledge that will contribute to clarify this complex intricate process. Furthermore, they will allow the selection for the best microorganism(s) with the potential to clean-up these contaminated sites by more eco-friendly processes. So far, in addition to the above study, genomic approaches proved useful in the identification of key genes and their respective products, encoded in the genomes of microorganisms resistant/tolerant to radionuclides/metals and which are, therefore, involved in the detoxification of this contaminants. With this approach, U-resistant bacteria of the genus Burkholderia and fungi of the genus Penicillium have been identified. Transcriptomics studies, by giving access to the analysis of gene expression and regulation, have gained relevance in the area of bioremediation. The information gathered from this comprehensive analysis, and also from future studies employing these methodologies, will surely shed light on the mechanisms of microbial resistance/tolerance to radionuclides/metals, while helping in the identification and selection of microorganisms that can be employed for bioremediation purposes of radionuclide/metals contaminated sites [69].
9.3.3.1 Contribution of Metagenomics Approaches to Understanding Microorganisms’ Radioresistance
Unlike most laboratory studies, environmental exposure to radionuclides, (e.g., NORM sites and nuclear power plant accident sites), includes different radiation types (α and β, as well as γ) combined with many other stressors (e.g., temperature, nutrients, toxic chemicals like metals, etc.) over long periods. Thus, in polyextremophiles, the response to the adaptation/resistance should be broader and involve an intricate crosstalk between the different cellular processes [70].
Culture-independent field studies have shown that radionuclide contaminated environments host a wide diversity of bacteria and that radionuclides strongly impact community function and structure. Recently, a metagenomics approach carried out in surface soil samples from Chernobyl and Fukushima, over a gradient of radionuclide concentrations (137Cs 1680—0.4 and 90Sr 209.1—1.9 kBq/kg), revealed that samples clustered according to the level of radiological contamination, irrespective of the collection site [71]. Nonetheless, a lower microbiota diversity was found in Chernobyl samples, which was expected as Chernobyl soils are more contaminated. The following were reported to be the most common phyla: Proteobacteria, Acidobacteria and Actinobacteria. Furthermore, as expected, the functions encoded by the genes identified seem to be related with stress, metal and radiation tolerance. For instance, genes involved in decontamination, DNA repair, information storage and processing, cellular processes and signaling and metabolism. A comprehensive listing of the function of the genes responsive to this type of contaminants has been recently reviewed by Hoyos-Hernandez and co-workers [71].
A similar approach was employed in a study performed by Theodorakopoulos and colleagues [72], in Chernobyl, which demonstrated the high diversity of bacteria in those contaminated sites. The same authors isolated cultivable bacteria of the genus Microbacterium that were employed in laboratory exposure studies, contributing to a better understanding of the mechanisms of tolerance to radionuclides/metals in those bacteria. The identified mechanisms involve biosorption, efflux and biomineralization [68].
Although further studies are required to better understand how radiological contamination exerts a selective pressure and how it shapes the structure of the microbial community, the sensitivity of the various organisms to radioactive contamination under environmental conditions generally exceeds the sensitivity of the same organisms to experimental laboratory exposures [62]. It is clear though that communities from soils of these contaminated sites have functional profiles that allow them to deal with this type of radiological and chemical contamination. Furthermore, these environments constitute a genetic pool from which the phylogenetic affiliation of cultivable and non-cultivable microorganisms can be determined, thus allowing the identification of new genes involved in the resistance to these contaminants, in addition to further contributing to clarify those mechanisms.
9.3.4 Effects on Plants
Plants are sessile organisms that cannot leave the surrounding environment if the ecological factors are not suitable for their growth. Thus, under unfavorable circumstances, plants have only the choice to perish or adapt to changing environments. The extreme physiological plasticity of plants allowed their diffusion in all ecosystems of the Earth and today we may have a comprehensive vision of the multitude of adaptations carried out by these organisms in diverse places. Indeed, plants such as other living organisms can adapt to cyclical natural disturbances over time, developing the capacity for endurance (resistance) and self-repair (resilience) in different ecosystems.
Laboratory and field studies showed that ionizing radiation may exert different effects on plant metabolism, growth and reproduction, depending on plant developmental stage at the time of exposure, plant physiological and morphological traits, as well as genetic characteristics [73, 74]. Moreover, depending on the dose or radiation type (low or high-LET), ionizing radiation induces detrimental outcomes at high doses, harmful consequences at intermediate levels and stimulatory effects at low doses.
In some cases, ionizing radiation exposure increases embryo lethality, induces dwarf architecture and modifies floral elements [74] and literature herein. Other studies indicated that some irradiated crops showed a taller architecture, increased yields and reproductive success and the ability to endure water shortage [75, 76]. As for many other organisms, within plant cells, the nucleus is considered the primary site of injury by ionizing radiation, which is responsible for random DNA damage and generates different kinds of mutations, such as deletions, base substitutions and chromosomal alteration [74, 77]. There is a direct relationship between the radiosensitivity of a plant and the average volume occupied by a chromosome in the cell nucleus. If the chromosome volume is large, the plant will be more sensitive and, therefore, the dose of ionizing radiation causing severe damages is less. Hence, polyploid species exhibit a minor sensitivity to radiation damage because gene redundancy protects polyploidy from the deleterious effect of mutations [78]. Besides plant cells, it is noteworthy that ionizing radiation may have different impacts on organs and tissues. Generally, more complex tissue architecture is less sensitive to damage; thus, young tissues are more vulnerable than old [73, 79]. At functional level, many studies have evidenced that radiation is dangerous for the photosynthetic apparatus. Generally, a decline of photosynthesis often implicates damage to photosystem II (PSII) and in particular to D1 protein, implicated in the right functioning of photosynthetic electron transport. Together with the impairment of PSII, a significant decrease of photosynthetic pigments and enzymes of the carbon assimilation cycle was also detected [73].
The majority of information on the impacts of radioactivity on plants comes from studies carried out by scientists after the nuclear disasters of Chernobyl (Ukraine) in 1986 and Fukushima (Japan) in 2011 [80].
Since 1986 the Chernobyl red forest has represented a living laboratory for biologists to study for long-lasting plant behavior in response to acute and chronic radioactive contamination. The name “Red Forest” comes from the ginger-brown color of the pine trees as a result of the high radiation levels immediately after the explosion of the nuclear plant. Studies continued in the post-accident period and enlarged the knowledge on the effects of acute and chronic radiation on plants [81]. Generally different plant species show diverse sensitivity to radiation, being shrubs more resilient than conifers. The sensitivity of the pine compared to other tree species was most apparent in the Chernobyl exclusion zone and trees showed dramatic alterations in the morphology of trunks and branches, indicating damage at meristems level [82]. Following the Fukushima accident, despite the much lower exposure levels, Japanese red pine (Pinus densiflora Siebold & Zucc.) and Japanese fir (Abies firma Siebold & Zucc.) species showed developmental anomalies similar to those observed in Chernobyl [83, 84]. However, it is uncertain if the aberrations observed in Chernobyl are due to direct effects of radiation on the trees or multiple stresses due to biotic and other abiotic factors.
It is noteworthy that the quantity of radionuclides absorbed by plants depends on their phenological stage and growth status which, in turn, varies with the pedo-climatic conditions and cultivation factors. Once deposited on the vegetation and in particular on the leaf surface, the radioisotopes are absorbed through stomata and then transported to the other organs including fruits, thus possibly entering the food chain through edible leaves and fruits [85].
Today the Red Forest remains one of the most contaminated sites globally, and the surrounding forest area also represents an area of active research and scientific interest because of the return of wildlife in the exclusion zone. Here, the understory vegetation and deciduous (silver birch) trees have reappeared, but radioactive dust still remains stored in plant biomass and soil, for the very slow matter cycle.
The occurrence of revegetation has proven to be remarkably resilient to the intense radiation around the nuclear disaster zone. The exclusion zone is now dominated by grasslands and shrublands, while the most representative trees are Scots pine and silver birch Betula pendula [74] and literature herein.
Recent studies suggest that plants subjected to not-lethal doses of ionizing radiation show an increased resistance to other environmental stresses. Two strategies have been hypothesized, namely the production of ROS-mediated cell signaling and/or a boost of secondary metabolites [86].
The resilience to radiation in plants of the Chernobyl exclusion zone and from most contaminated sites at Fukushima is due to different mechanisms to protect the genetic material, improving the plant radioresistance [80]. Generally, plants are more radioresistant than animals because they present integrated adaptation mechanisms at genetic, anatomical, and physiological levels.
At genetic level, mechanisms include the regulation of expression of some genes encoding for radical scavenging and DNA-repair enzymes, homologous and non-homologous recombination, and the activation of scavengers. The higher stability induced by polyploidy, typical among plant kingdom, enhances radioresistance thanks to the presence of several copies of the same genes, which may serve as additional wild type copies in the case of radiation-induced injuries [87]. At the structural and metabolism level, plant cells present some traits such as thickened cell walls, cuticles, pubescence, increased deposition of phenolic compounds around membranes [88, 89]. At the anatomical level, complex tissue organization is associated with high resistance to mutagenic effects and the capability to adopt repair mechanisms.
Non-lethal doses of ionizing radiation may also induce hormesis improving plant defense against stressors, through the stimulation of the production of antioxidant enzymes (SOD, CAT, APX) or morpho-anatomical and photosynthetic changes that favor plant growth and metabolism [74, 90, 91].
Radiation-induced hormesis is still an unclear phenomenon in plants because it strongly depends on species intrinsic characteristics. At present, further studies are in progress to understand if it is a sort of compensation to irradiation damage or a transitory change, not enough to induce permanent injuries.
9.3.5 Effects on Invertebrates
Invertebrates have been considered a relevant group of organisms for studying the effects of ionizing radiation, both focusing on mechanisms of action and on previewing impacts in natural communities. Several reasons can be enumerated for choosing aquatic and terrestrial invertebrates as model organisms for IR studies, namely:
-
1.
They have long served for providing insights into fundamental mechanisms of development, biomedical research (e.g., neurobiology, basic physiology, genetics, immunology, cancer biology), species diversification and genome evolution (e.g., Drosophila melanogaster, Caenorhabditis elegans; planarians and crustaceans) [92,93,94,95]; for studying the effects of ionizing radiation in neuronal function [96] and as model organisms in radiation hormesis studies [97].
-
2.
Due to their important role in food webs, transferring carbon from producers to higher trophic levels (i.e., cladocerans, copepods), as detritivores contributing for degradation of organic matter through comminution (e.g., oligochaetes) and turnover of microbial communities (i.e., bacterivorous nematodes).
-
3.
The role of some species as ecosystem engineers dynamically working the structure of soils and sediments (i.e. oligochaetes, polychaetes, ants) and the contribution for other soil and sediment functions.
-
4.
The sensitivity and the ease of culture for some invertebrate species under laboratory conditions, as well as proliferation, producing a great number of individuals for testing in complex experimental designs and without tight regulatory requirements.
Aquatic invertebrates as benthic organisms and invertebrates living burrowed in soils or dwelling at the surface are among the group of organisms that may receive the highest radiation doses, since these environmental compartments are relevant environmental sinks of radionuclides. The mechanisms of action and the subsequent effects of ionizing radiation in invertebrates have been addressed mainly since the seventies, with a limited number of species, through laboratorial exposures to gamma radiation of single species, frequently at high-dose rates, with few environmental relevance for chronic exposure scenarios [98]. Real conditions include exposures to industrial radionuclides in areas affected by nuclear accidents, nuclear power plants, or in nuclear test sites, as well as through exposures to natural occurring radionuclides (NORs), as those found in uranium mining areas. In the later areas, the effects of radionuclides, mainly alpha-emitters, cannot be distinguished from that of metals, also present at high levels in the affected environmental matrices. The same difficulty exists in areas of nuclear accidents as the Chernobyl exclusion zone, where the release of different artificial radionuclides has occurred, although data available for activity concentrations in biota are almost limited to 90Sr, 137Cs, and some few other radionuclides [38].
Invertebrates are among the least sensitive organisms to ionizing radiation [62, 99]. Cassidy and co-authors [100] suggested that the reasons for these differences in sensitivity, between organisms of different taxonomic groups, may include differences in DNA content, DNA repairing processes, and kinetics of cell cycle, within other aspects. The doses able to cause mortality or decrease life span are species dependent and frequently very high: as for example above 1000 Gy for Caenorhabditis elegans [101]. However, differences in sensitivity of different life stages were also reported (i.e., Johnson and Hartman [101]), with reproduction effects being seen at much lower doses (i.e., 4 mG/h for earthworm).
Ionizing radiation hormesis has been reported in a number of studies with invertebrates (dipterans, coleoptera), exposed to low doses from different sources (X-ray, gamma radiation, 137Cs) (see review by Vaiserman et al. [97]). Reduced mortality rates and long-life spans were highly dependent on the exposure conditions [102], for example, life-extended effects were only observed in house flies (Musca domestica) reared in groups, and thus under high locomotor activity and exposed to a 10 Gy dose. Several hypotheses were then postulated and tested to unveil the factors responsible for modulating radiation hormesis, using Drosophila melanogaster, as model species, as for example: increased IR resistance, IR-induced sterility in females, apoptosis induction and changes in DNA repair genes and life-stage differential sensitivity were some of the proposals [97] and references quoted herein. X-ray irradiation of D. melanogaster eggs with 0.75 Gy, decreased the amount of DNA segments, by cleavage of S1 nuclease sensitive sites (<3 kb), resulting in a great DNA stability, changing the repair and/or transcription processes and thus affecting lifespan and the resistance of adults to IR [103]. Based on all the studies conducted, the radiation hormesis model proposes that the exposure to low doses of IR could induce several adaptive responses, which in turn will prevent environmental-induced health effects [97].
At the cellular level, oxidative stress and the activation of oxidative stress-response mechanisms have been reported as the major indirect consequences of exposures to IR of aquatic and terrestrial invertebrates. Won and Lee [104] observed a significant increase in the activation of several enzymes, as for example, superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR) and glutathione-S-transferase (GST) in the marine copepod Paracyclopina nana, exposed to gamma radiation doses equal or greater than 10 Gy (at a dose rate of 2 Gy/min). However, in this study, no data from additional molecular parameters, as those related with DNA damage or lipid peroxidation were provided, preventing us to infer if the activation of these enzymes was sufficient or not to prevent cellular damages. A dose-dependent increase in ROS was also recorded in another marine invertebrate species, as for example, the copepod Tigriopus japonicus and the rotifer Brachionus koreanus, for a range of concentrations from 50 to 200 Gy (irradiated at a dose rate of 2 Gy/min) [105, 106]. Concomitantly, the antioxidant response system was activated, and GST and GR activities were significantly increased for the copepod, while for the rotifer the same was recorded for the activity of GST. A cellular and lipid peroxidation (LPO)-related ROS was dose-dependent overproduction was also recorded in the freshwater cladoceran Daphnia magna after 8-day exposure to a dose rate of 100 mGy/h of gamma radiation. The overproduction of mitochondrial ROS was significantly enhanced at 40 and 100 mGy/h [1]. Dose rates of the same order of magnitude (10.7 and 42.9 mGy/h) were also able to cause lipid peroxidation in daphnids, after both 24 and 48 h of exposure. However, at the highest dose rate tested (106 mGy/h), the same effect was only registered after 48 h of exposure [55]. This observation, which was consistent with other studies (i.e., Fuller et al. [107]), gave rise to the hypothesis that ROS may also act as a signaling molecule, requiring a certain level within the cell to activate antioxidant defense mechanisms. Neutral lipid catabolism was also observed in the nematode C. elegans independently of the different doses and dose rates tested (7 and 52 mGy/h), and this effect was associated with a reduced longevity, as lipid homeostasis is responsible for endocrine signaling of longevity [108]. In fact, the up-regulation of different hormone receptors in daphnids was suggested as a signal of disruption of normal endocrine functions in response to IR exposure [1].
Regarding the interaction of ROS with proteins, Won and Lee [104] registered an upregulation of the hsp gene in the copepod P. nana, which was interpreted as being related with a possible response to protect key proteins (probably those involved in DNA repair signaling pathways) through the synthesis of chaperones.Footnote 4 In the cascade of events promoted by ionizing radiation, Song and co-authors [1] recorded an enhanced expression of the Ube2 gene in D. magna, involved in the degradation of proteins, suggesting the activation of a mechanism responsible for the elimination of proteins damaged by ROS. A highly efficient antioxidant protection system may not be able to protect DNA from damage but it can delay protein carbonylation.Footnote 5 Therefore, protecting cellular components involved in the repair of DNA double-strand breaks (DSB) was proposed as a main factor to explain the resistance of bdelloid rotifers to ionizing radiation [109].
DNA damage is a frequently reported effect in invertebrates exposed to IR from different sources. These damages can be either caused indirectly, mediated by ROS, or by direct deposition of radiation energy in DNA [1, 104]. In response to DNA damage, the expression of genes related with DNA repair systems (e.g., p53, RAD50, Mre11 coding for the DSB repair protein, Ku70, Ku80, and DNA-PK) was recorded in different invertebrate species, frequently with a non-monotonic response, but always with a significant and differential expression at low and higher dose rates (at 4, 100, and 200 mG/h) [1, 55, 104, 106]. In summary, genes involved in nucleotide excision, base excision, homologous recombinant, and non-homologous recombination repair pathways have been found to be all involved in the response of cells to IR. At high IR dose/dose rates, ROS may also induce DNA methylation,Footnote 6 leading to the accumulation of damages, by silencing some genes. Song et al. [1] recorded an enhanced expression of DNA (cytosine-5)-methyltransferase 1 (Dnmt1), DNA cytosine-5 methyltransferase 3A2 (Dnmt3a2) genes, involved in maintenance of DNA methylation and in de novo DNA methylation, respectively, in D. magna.
The disruption of energy metabolism under the exposure to IR is another reported effect at the cellular level, once again in different invertebrate species [1, 55]. Direct interference with proteins of the electron transport chain, mitochondria ultrastructural changes caused by ROS and modulation of oxidative phosphorylation are within some of the mechanisms proposed, based on observations made in D. magna exposed to gamma radiation [55]. Genes encoding NADH dehydrogenase (Nd), succinate dehydrogenase subunit A (SdhA) of complex II, different cytochrome oxidase subunits (COX1, COX2, and COX3), cytochrome c oxidase copper chaperone (COX17) of complex IV and ATP synthase subunit mitochondrial (sun) of complex 4 were some of the genes involved in the electron transport chain found to be suppressed by gamma radiation [55]. At the end of the cascade of events triggered by gamma-radiation, the regulation of different apoptotic signaling pathways was observed in freshwater Cladocera, in parallel with DNA damage and regulation of repair mechanisms, cell cycle disruption and mitochondrial dysfunction [1, 55]. Although not significant, an increasing trend in apoptotic cell death with increasing dose rates of radiation was recorded in crustaceans, namely daphnids and in the Norway lobster (Nephrops norvegicus) cell cultures exposed to 60Co gamma-radiation [110]. Apoptosis is a downstream event, to oxidative stress and DNA damage occurrences, that is activated to eliminate damaged cells in an ultimate effort for protecting organisms.
The effects of ionizing radiation at the population level are poorly documented and it has been demonstrated that equal levels of effect at similar individual endpoints (e.g., growth or reproduction) may have different impacts on population dynamics [111]. Furthermore, it is still difficult to link the results of biomarkers of oxidative stress and genotoxic damage with phenotypic consequences (changes in morphology, growth, reproductive output, and viability of offspring) [112]. Data available allowed a tentative hierarchization of individual endpoints based on their radiosensitivity: mutation > reproduction > morbidity and mortality [113]. One step forward, modeling population responses it was shown that they differed depending on the affected individual reproduction endpoint (juvenile or adult survival, delay in maturity, or reduction in fecundity) [114]. Hatching was shown to be the most sensitive endpoint to chronic exposures to gamma radiation for aquatic invertebrates (EDR10Footnote 7 of 830 mGy/h for the polychaete worm Neanthes arenaceodentata) and fecundity for terrestrial invertebrates (EDR10 of 2600 mGy/h for Porcellio scaber). These species displayed similar EDR10 values for individual and population level endpoints (net reproduction rate). This was observed for the species that had a particularly sensitive individual endpoint.
The most concerning consequences of genotoxicity, that may support inferences about potential effects on natural populations, are those that affect the reproductive fitness of organisms. Reproduction has shown to be the most sensitive parameter in invertebrates (collembolans, worms, tardigrades, chironomids, and polychaetes) exposed to IR, when compared with survival or other endpoints at the individual level [109, 115,116,117,118,119,120]. It was suggested that the decrease in fecundity is not caused by the number of DNA DSB, but by the inactivation of the DNA repair systems [109]. In fact the incomparable ability of bdelloid rotifers to remain fertile, after extensive DNA damage, was attributed to high efficiency of repair systems and to mechanisms that protect proteins of these repair systems [121]. These authors also associated the resistance to ionizing radiation of these organisms with their resistance to desiccation resulting from their adaptation to ephemeral ponds. Desiccation, similarly to radiation, increases ROS production and DNA breakage.
Harrison et al. [112, 122], also working with the polychaeta N. arenaceodentata, hypothesized that chromosomal aberrations caused by gamma radiation doses of 2.0 and 4.0 Gy were responsible for gametal cell death and subsequent decreases in brood sizes of this species. In opposition, under laboratory conditions, significant effects were recorded in sperm quality, but not on sperm numbers, of males of the crustacean Echinogammarus marinus chronically exposed to doses rates of 1 and 10 mGy/day provided by the betta emitter 32P, for two weeks. Significant DNA damage was recorded in spermatozoa cells only at the highest dose rate. Furthermore, only a weak correlation was found between sperm quality parameters, fecundity, and embryo parameters analyzed [107]. Effects on ovary structure and oocyte development were also reported in the freshwater cladoceran D. magna in response to exposure to 1 and 100 mGy/h gamma radiation, dose rates.
Another possible cause of reproduction impairment in invertebrates, under exposure to ionizing radiation, may be related with the allocation of energy to molecular response mechanisms (e.g., activation of antioxidant defense system, DNA repair mechanisms) rather than to reproduction, with consequences on the fecundity of organisms [117].
An ED50Footnote 8 for reproduction of 21.9 Gy, one order of magnitude lower than that recorded for growth (144 Gy) was found for the collembolan Folsomia candida, under exposure to 137Cs gamma radiation at a constant dose rate of 8.3 Gy/min. Song et al. [1] also observed a non-monotonic reduction in the total number of offspring of the cladocera D. magna, concomitantly with no effects on survival, molting or ovulation frequency (at dose rates of 1 and 100 mGy/h). At the lowest dose, the effect on the cumulative reproduction output was mainly associated with an increase in the number of days needed to deliver four broods, while at the highest dose rate, the reproductive cycles were accelerated but the size of the broods was reduced. A similar observation was made by Parisot et al. [123] in the same organisms exposed to dose rates of 0.07–35.4 mGy/h of gamma-radiation, for 23 days. The same non-monotonic response was recorded D. magna representing 38 different genotypes collected in lakes located inside the Chernobyl exclusion zone with a range of dose rates between 0.1 and 181.2 mGy/h.
In a study conducted by Alonzo and collaborators [111], the freshwater species D. magna and the terrestrial earthworm Eisenia fetida, two species with different life history strategies (short lived/parthenogenic versus more long-term life/sexually reproducing hermaphrodite, respectively) were selected: (1) to model population growth in response to individual effects caused by the exposure to IR and (2) to investigate populations susceptibility using two different models to take into account single generation and multiple generation exposures. It was shown that in daphnids, the population growth was 1.5-fold more sensitive to changes in fecundity than in mortality. Daphnids population growth was also highly affected by delays in reproduction. Earthworms’ population growth was more sensitive to delays in reproduction, while effects in fecundity and mortality have a similar and lower impact on populations. Despite the different life strategies, the intrinsic rates of population increase were equivalent for both species, because the greater reproductive rate of daphnids is compensated by a shorter life span relative to earthworms.
After disturbances of great magnitude, the recovery of natural populations of cladocera may rely on the banks of resting eggs in the sediments of lentic systems. These resting eggs if irradiated may have its performance compromised, affecting the dynamic of the natural populations. Zadereev et al. [124] observed that although doses up to 100 Gy (variable dose rates) did not affect the survival and hatching of resting eggs of Moina macrocopa, the size and the structure of populations initiated from resting eggs exposed to this highest dose of gamma-radiation, were affected. Therefore, subsequent effects on the dynamic of the populations of this cladocera may be expected in lakes with highly contaminated sediments.
Under a real scenario of radionuclides contamination, no correlation was found between different reproduction endpoints (proportion of breeding females, fecundity, brood mass, maternal body mass) of the crustacean Asellus aquaticus, sampled at different lakes in the Chernobyl affected area, with the gradient of dose rates between 0.064 and 27.1 mGy/h registered at these ecosystems [107]. Also, upscaling to populations and communities, Murphy et al. [125] focused on the diversity of littoral macroinvertebrates communities at eight natural lakes in Belarus, with a range of external dose rates from 0.066 to 10.22 mGy/h once again did not find any correlation between population endpoints (abundance, taxon richness, Shannon-Wiener diversity index, and the Berger-Parker dominance index) and the range of external dose rates registered in the sampled lakes. This study suggested that the IR dose rates recorded had no detectable effects on the littoral macroinvertebrate communities of these lakes.
Impacts on natural populations of invertebrates may be also caused by other mechanisms rather than those affecting gamete production, eggs viability, fecundity, or reproduction delays. For example, the exposure of fourth-instar nymphs to IR from a 137Cs source up to doses of 12 Gy (at a dose rate of 0.25 Gy/min) has shown to affect the acoustic signaling of male crickets (Acheta domesticus) and subsequently their ability to find mates, due to morphological changes in their wings [126]. In fact, few is known about other direct and indirect effects that may affect the fitness individuals, its biotic relationships, and subsequently the dynamics of natural populations and communities at IR contaminated scenarios, rather than those effects identified based on commonly used biomarkers. The complexity of the biotic interactions, as well as the role of dominant abiotic factors determines the type and the impact of the indirect effects on ecosystems, whose responses can be unpredictable [98]. In a birch forest in South Urals, the contamination of litter with 90Sr (doses reaching up 70 Gy) compromised the development of pupae of tachinid flies (Tachinid sp.). This accounted for an increased survival of the host caterpillars of the gypsy moth (Lymantria dispar dispar L.) with increasing IR levels [127] in Geras’kin [98]. Møller et al. [128] also linked the reduction in the set of fruits produced by trees and bushes, at the Chernobyl exclusion zone with the local reduction of pollinator insects. The role of other biotic factors in the radiosensitivity of invertebrates also needs to be investigated, as it may be relevant under specific environmental or industrial scenarios. It was shown that the ability of marine mussels (Mytilus galloprovincialis) to respond to genotoxic induced effects by tritiated water, released by cooling operations of nuclear power plants, was limited by enhanced temperatures [129].
Invertebrates also have a key role in several ecosystem functions, as, for example, the degradation of wood, organic matter, and nutrients recycling. Mousseau et al. [130] conducted a study in forest areas within the Chernobyl exclusion zone, at different distances of the nuclear plant and with levels of background radiation differing by several orders of magnitude (range 0.09–240.25 mSv/h). A significant effect of background radiation in the mass loss of litter bags buried in the surface of the forest soils was registered. The mass loss of litter bags from the sites with high levels of background IR was 40% lower than that recorded at the sites with lower levels of radiation. However, no significant influence of the mesh size of litter bags was found, suggesting that decrease in the decomposition of litter at that site was not only caused by impacts on soil invertebrates’ communities, but also on soil microbiome. Soil invertebrates’ assemblages from pit falls and wood slices from the same area showed that the abundance of taxonomic groups displayed a different relationship with background radiation and with wood contamination with radionuclides, being positively, negatively, or not affected at all [131, 132]. This was consistent with a previous observation of a general loss of diversity in sub-surface and flying invertebrates with increasing concentration activities of 90Sr and 137Cs in the litter of forest sites within the Chernobyl exclusion zone [133], as well as by the apparent decrease in the feeding activity of these organisms measured by the bait-lamina test.Footnote 9 However, such changes were not followed by changes in total biomass of organisms. These results suggest that chronic environmental exposures to IR may exert their effects on natural communities, through structure and functional diversity simplification, with possible impacts on ecosystem’s functions.
In a first attempt to estimate risk limits for chronic g-radiation exposures, predicted no effect dose rates (PNEDR) of 10 mGy/h (0.24 mGy/day) for freshwater ecosystems and of 67 mGy/h (1.61 mGy/day) for terrestrial ecosystems were obtained, using assessment factors and species sensitivity distribution methods, respectively. The estimated values were found to be highly protective as they were about ×50 to ×100 times higher than the upper bound of the range of natural background concentrations and of the lower dose rates causing effects at contaminated sites [134]. Later, and by applying an assessment factor (AF) of 3 to the HDR5Footnote 10 estimated for invertebrates, a PNEDR of 170 mGy/h for IR was obtained. However, and considering that no sufficient data was available for applying probabilistic methods to estimate PNEDR for specific groups of organisms or for environmental compartments, Garnier-Laplace et al. [135] derived a generic HDR5 from a species sensitivity distribution using data from controlled laboratorial chronic exposures to low dose rates of gamma-radiation, and applied an AF of 5, obtaining a PNEDR of 1.5 mG/h which was considered to be protective for the conditions found at Chernobyl exclusion zone.
9.3.6 Effects on Vertebrates
9.3.6.1 Terrestrial Organisms
9.3.6.1.1 Mammals
Among all the vertebrates, mammals are organisms on which the effects of radiation exposure were most extensively studied in radiobiological experiments. Negative effects on these organisms, due to radiation exposure at high doses (i.e., 10–50 Gy), are primarily due to effects at the hematopoietic system and the gastro-intestinal mucosa [45, 46]. The time needed for death to occur varies widely within species. The dose of radiation needed to cause lethality, due to gastro-intestinal syndrome, to 50% of the exposed organisms (LD50) is approximately as follows for dog—8 Gy, mouse—12 Gy, rat—11 Gy, and rhesus monkey—9 Gy [46]. However, these values were estimated for particular species of these organisms, so there can be wide variations for other species. These variations are normally related with specific intestinal morphologies, which are related to diet (i.e., herbivores, carnivores, and omnivores). Regarding bone marrow damage, the weight of the animals receiving the dose appears to have a significant role in the bone marrow radioresistance, being weight inversely proportional to radiation sensitivity, as LD50 values are greater for smaller mammals (6–10 Gy approximately) than for larger ones (1.2–3.9 Gy). A reduction in life span is also related to the type of radiation to which animals are exposed, being high LET radiation more effective than low LET radiation. Also, acute exposures are substantially more effective by a factor of 7 in causing mortality than chronic exposures [46]. Significant life span shortening occurred in dogs and mice exposed to low LET radiation (gamma radiation) at dose rates between 100 and 1000 mGy/h and the same happened for mice exposed to neutrons (high LET radiation) at the same dose rates [136]. In general, a significant reduction in life span of several mammal species was observed at dose rates higher than 1000 mGy/h [50, 136, 137]. Chronic exposures of less than 100 mGy/h have a low probability of inducing significant effects on most terrestrial organisms [45, 46, 136]. Particularly, a dose rate of less than 40 mGy/h has a low probability of inducing effects on the fertility, fecundity, and the production of viable offspring of a mammalian population [45]. This is true for low LET radiation, however for high LET radiation, this dose rate value is lower, as this type of radiation has a much higher relative biological effectiveness (RBE) [45]. An experiment performed in mice irradiated with neutrons, at dose rates lower than 100 mGy/h for at least 475 days, led to a significant increase of mortality in mice in comparison with the control [136].
Reproduction is a more radiosensitive parameter than mortality, and effects of radiation may appear at radiation levels that apparently do not induce other observable responses. The magnitude of the effects depended also on the developmental stage in which the animal was irradiated [136]. A good example are mice, as the LD50 occurs at a radiation dose approximately between 6 and 10 Gy; however, at a radiation dose of 0.08 Gy, the production of oocytes was reduced to 50% in newborn mice (the most radiosensitive stage in mice) [45, 46]. However, this does not necessarily mean that there will be a decline in fecundity, since mice produce much more oocytes than the amount effectively used for reproduction, but there could be a reduction in the offspring [46]. In adult males, fertility is temporarily impaired after a 10 Gy exposure; however, in young mice (3–5 days old), it can cause permanent sterility. Mice in the second week after birth are also especially sensitive to the detrimental effects of radiation on reproduction [136]. The differences between males and females are mostly a consequence of the differences in the gametogenesis process. There are also differences between species, being mice one of the least radioresistant. Chronic irradiation affects mainly the time needed for oogonial cell division and the size of stem cell pool [46]. In males, the spermatogenic process is maintained, although at lower levels than unexposed organisms [46].
The developing embryo is particularly sensitive to radiation, due to the high number of cells proliferating, reducing fecundity and postnatal survival, potentially influencing population size [46]. Acute radiation exposure, before the implantation of the embryo, causes its early death and can also cause post implantation and postnatal death [46]. This has a good correlation with the occurrence of DNA damage in the form of chromosome aberrations in the blastomeres (cells that result from the cleavage during the early development of the embryo) [46]. Radiosensitivity is strongly influenced by cell cycle stages and mitotic cycle in the very early developmental stages [46]. During organogenesis, the most typical response to acute radiation is the occurrence of malformations (teratogenic effects), which can occur during embryonic and fetal growth and may or may not be fatal. The occurrence of teratogenic effects in a particular organ is related to a high level of cell proliferation in the precursor tissue [46]. Although this has been observed for the several species studied (mouse, hamster, cattle, pig, monkey rabbit, etc.), the responses to specific radiation doses will depend on the species and on its developmental stage at the time of exposure [46]. There are not many studies on the effects of chronic radiation exposure during organogenesis, however a study performed on mice showed doses of 0.01 Gy/day in pregnant mice 6–9 days after conception induce a significant impairment of the offspring’s learning ability [46]. Also, dose rates of 420 mGy/h reduced neonatal brain weight, with unknown effect at the functional and behavioral levels [45].
A direct relationship between DNA damage and radiation dose is expected at high doses of radiation; however below 100 mGy, it is not clear. In reindeer, a tenfold increase in the number of chromosome aberrations was observed at dose rates between 100 and 1000 mGy/h [136]. For rodent species acutely exposed to low LET radiation, mutations in the form of reciprocal translocations (exchange of DNA between homologous chromosomes) occur in stem cell spermatogonia when organisms are exposed to between 0.01 and 0.03 Gy at total doses from 3 Gy [46]. High LET radiation exposure (in the form of alpha particles emitted by 239Pu), delivered at a dose rate of 36 mGy/h significantly increased the occurrence of translocations and acentric fragments (chromosome fragments without a centromere) in spermatogonia and spermatocytes, respectively [46]. In primates, the dose interval is 0.01–0.078 Gy at doses from 1 Gy [46]. Translocations, ring chromosomes (aberrant chromosomes whose ends were broken and then fused together to form a ring) and dicentric chromosomes (the result of two broken chromosomes that fused together) are used for radiation dosimetry in human and non-human biota for a long time, as their frequency increases with radiation dose [138]. In rodents, this is more easily seen at total absorbed doses higher than 0.5 Gy, suggesting that their use as a biomarker of radiation exposure is more effective at high dose exposure than at low doses (below 100 mGy). Regarding carcinogenicity, there is a wide variation in the sensitivity for tumor formation among tissues and species. The induction of cancer, even at high radiation exposure doses (>100 mGy) will also vary according to the age of exposure. Dogs exposed to doses higher than 7 Gy showed soft tissue cancers when exposed in utero but not when exposed as young adults [46]. In rodent species, there were limited carcinogenic effects on animals that were exposed to doses between 0.1 and 1 Gy [138].
9.3.6.1.2 Birds
The effects of radiation exposure in birds are apparently similar to the ones observed in small mammals [45]. The LD50 for wild birds is in the same range as small mammals (5–12 Gy). For poultry, the LD50 determined experimentally for mortality is of 7–11 Gy in 3–4-day-old individuals when irradiation lasts for less than 1 h and of 12–20 Gy when irradiated for 24 h. Egg production is affected in white leghorn chicken at a total absorbed dose of 4–8 Gy and at higher doses, effects are more severe and long lasting [45]. A limited number of experiments performed in artificially incubated chicken embryos showed a LD50 of 12–13 Gy, which apparently indicates a higher radioresistance than adults [46]. In white leghorn chickens, eggs hatchability is affected at a total absorbed dose of 8 Gy, but the progeny is unaffected [48]. The International Commission on Radiation Protection also reported dose ranges for which long-term effects on developing embryos were reported (100–1000 mGy/day), reduced reproductive success (1–10 mGy/day) and increased morbidity (10–100 mGy/day) [139]. Recently, it was reported a decrease in species abundance at a dose range of (from 0.3 to 97 μGy/h) in the Fukushima exclusion zone, which is consistent with the dose ranges reported for increased morbidity and decreased reproductive success [140]. The existing knowledge on DNA damage/alterations on birds exposed to ionizing radiation results from the evaluation of effects of radioactive environmental contamination resulting from the Fukushima and Chernobyl accidents [141].
9.3.6.1.3 Reptiles and Amphibians
The information gathered so far for reptiles and amphibians suggest that their radiosensitivity is similar to that of mammals and birds. The LD50 values recorded for frogs, salamanders, turtles and snakes vary between 2 and 24 Gy [46]. The main cause of death identified was damage to the hematopoietic system [46]. In two separate experiments performed on lizards, two very different LD50 doses ranges were obtained (10–12 and 17–22 Gy). The possible reasons for this marked difference are associated with the fact that these values may vary according to radiation type and quality, the dose rate to which the organisms were exposed and their maintenance conditions at the laboratory [46]. An acute exposure to 50 Gy caused temporary sterility in males, but recovery was well in process after 48 days post irradiation and irradiation of gonads in males and females to an absorbed dose of 4.5 Gy leads to a substantial decrease in the production of offspring [46].
Regarding amphibians, different life stages showed different radiosensitivities. For adult toads, the LD50 value is of 24 Gy, for juveniles it is of 10 Gy and for tadpoles it is of 17 Gy [46, 139]. The life stage more sensitive to radiation exposure was the fertilized egg with an LD50/40 (LD50 after 40 days of exposure) of 0.6 Gy [33]. There is evidence that the exposure of male toads to 3–20 Gy caused a reduced survival and increased induction of abnormalities to the offspring [46, 139]. Although these LD50 values for amphibians seem slightly higher than the ones recorded for mammals, time after exposure optimal for the recording of LD50 values seem to be an important factor [33]. Reptiles and amphibians are poikilothermic organisms; therefore, their metabolism is quite variable and different from mammals and birds [33]. A study performed on 4 species of amphibians showed that if the assay period was extended a decrease in the LD50 to values that ranged between 0.8 and 7 Gy would be recorded [33].
Chronic irradiation exposure (5.5 years duration) of common side blotched lizard, western whiptail, long nosed leopard lizard and long nosed lizard showed that at ranges from 285 to 570 μGy/h, radiation exposure caused lack of reproduction, female ovaries regression and some degree of male sterilization [46].
Regarding the induction of DNA damage, it was observed by Ulsh and co-authors [142] that the exposure of turtles from the species Trachemys scripta to 0–8 Gy 137Cs gamma radiation, given at a dose rate of 0.55 Gy/h induced the occurrence of significant levels of chromosome translocations in lymphocytes. Studies on the induction of DNA alterations in amphibians and reptiles have been performed in Fukushima and Chernobyl exclusion zones, as well as in areas contaminated with NORM.
9.3.6.1.4 Aquatic Vertebrates
Among non-mammalian aquatic organisms, fish are the most sensitive to the exposure to ionizing radiation [45, 46]. Although these organisms are also poikilothermic (as amphibians and reptiles), and therefore, apparently more radioresistant than mammals, there is a substantial overlap in radiosensitivities [46]. Until now, there is no substantial data on effects of ionizing radiation on marine mammals, however, there is no reason to believe that their radiosensitivity is substantially different from that of terrestrial mammals. Data on acute exposures exist mainly for bony and freshwater fishes, with a small number of studies on cartilaginous and marine and anadromous species.
The LD50 determined for six marine species after 40–50 days of exposure was of 9–23 Gy [46, 139]. Fish developing embryos are, however, more sensitive than adults, as for silver salmon their LD50 after 50 days of exposure is of 0.30 Gy at hatching and 0.16 Gy at a post-hatching larval stage of 90 days [46]. A study performed on sharks (Triakis scyllium and Heterodontus japonicus) exposed to 20 Gy showed that mortality occurred after 20 days of exposure, due to hematopoietic and gastrointestinal damage [33]. This suggests that the radiosensitivity of cartilaginous fish may be similar to that of teleost fish.
Regarding reproduction, an acute exposure to 10 Gy reduced the total number of germ cells at all developmental stages of medaka fish (Oryzias latipes) [46]. A similar radiosensitivity was found in rainbow trout, with an induction of more than 50% sterility in organisms exposed late in embryonic development [46]. This leads to the conclusion that as in mammals, the newly hatched fry and the primordial gonads in fish embryos are more sensitive to the acute radiation exposure than in adult fish [46]. Irradiation of mature medaka fish at acute doses of 5–10 Gy only induced temporary sterility, being completely recovered at 60 days after irradiation [46]. On the other hand, chronic irradiation of males from the fish species Ameca splendens for 5.4 days at a dose rate from 137Cs gamma rays of 7300 mGy/h disrupted spermatogenesis and render the animals sterile at an accumulated dose of 9.7 Gy (8 weeks of exposure) [46]. There was 60–70% recovery, 236 days after irradiation [46]. Another freshwater fish, the guppy (Poecilia reticulata), when exposed to gamma dose rates from 1700 to 13,000 mGy/h showed a significant reduction in fecundity, but no negative effects on survival and sex ratio, as well as no significant higher incidence of abnormalities in the offspring were observed [33, 136]. The marine fishes Pleuronectes platessa and the eelpout (Zoarces viviparus) exposed to 240 and 2000 mGy/h gamma radiation, respectively, showed a significant reduction of testes when compared to the control [136].
There are some findings also on the effects of the exposure to ionizing radiation in the immune system of these organisms. A significant reduction in the humoral immune response in the rainbow trout (Oncorhynchus mykiss) exposed to tritium beta-particles for 20 days at a dose rate as low as 8.3–83 mGy/h during embryogenesis was evidenced through a reduction in antibody titer following a specific challenge [46].
Regarding DNA damage there are very few studies on which some conclusion can be taken on this matter. On a study on medaka fish, at larval stages, there was a significant induction of vertebral anomalies after irradiation at dose rates from 137Cs gamma rays higher than 18,000 mGy/h and also to beta particles from 3H at dose rates higher than 35,000 mGy/h [46]. There is also a report on the occurrence of minor morphological abnormalities in the operculum of salmons exposed to a gamma radiation dose rate of 200 mGy/h that may affect latter survival [136].
9.4 The Particular Case of NORM Contamination
Anthropogenic activities of concern related to the environmental release of natural uranium isotopes (mainly 238U and 235U) and other radionuclides from their decay chains, namely 226Ra and 223Ra, 222Rn, and 210Po, include mainly the production of phosphate fertilizers, uranium mining and milling and the incorrect disposal of tailings, uranium conversion and enrichment, the production of uranium fuel, production of coal, oil and gas, extraction of rare earths, extraction and purification of water, extraction of minerals for building materials and the generation of geothermal energy [3, 143]. All of these industrial activities increase the concentration of these elements in all environmental matrices, thereby posing a risk to human and non-human biota as many of them have not been regulated for NORM release [3, 143]. Another important issue is the fact that the contaminated areas that result from these anthropogenic activities do not only present high levels of certain natural radionuclides, like 226Ra, 222Rn, and 210Po but also other important stressors, namely metals like manganese, zinc, iron, aluminum, etc. [143]. These are usually multiple exposure scenarios, which contain several kinds of contaminants that may act synergistically and increase the risk of the occurrence of biological effects on human and non-human biota and even of modifying the susceptibility of cells/organisms to the biological effects of ionizing radiation exposure [144].
9.4.1 Chronic Exposure and Interaction with Uranium and Metals
The accumulation of small amounts of radionuclides and metal over long periods is translated in chronic exposure to radiation. Naturally contaminated sites harbor a diversity of microbial species that become resistant or tolerant to these contaminants by bioaccumulating radionuclides and metals either by biosorption to their cell surfaces and biomolecules or by internalization into their cells. Briefly, under environmental conditions, chronic IR effects are very complex, particularly when compared to those from laboratory exposures because (1) radiation emitted by the different radionuclides present has different biological effects, (2) radiation from the same location is absorbed differently by different microorganisms, (3) abiotic factors (e.g., temperature, nutrients, pH, other stressors) are present and can interfere with radiation, (4) cooperation/interaction between microbial communities, including diversity and/or abundance can all be modulated by radiation [62]. Regarding uranium, probably the most well studied radionuclide, and for which a lot of information is available, interaction with microbial cells involving solubility by biomineralization (bioprecipitation) depends on all the above factors and also on the presence of affinity groups generated by microorganisms’ cell metabolism, like hydroxides, phosphates, and carbonates. Uranium toxicity is both chemical and radiological. In the environment, uranium exists in its reduced insoluble form U(IV), and/or the oxidized form U(VI), which is soluble and toxic. Microorganisms interact with uranium by changing its redox state, aerobically, through oxidation (biolixiviation), or anaerobically by reduction. In order to do that, microorganisms need to be highly tolerant to uranium and to radiation. Other processes of microbial interaction with metals, involve biosorption, where contaminants passively concentrate through binding to cell structure constituents (e.g., lipopolysaccharides, teichoic acids, peptidoglycan), and biomineralization, which leads to the formation of biominerals using organic phosphate sources and phosphatases.
Unless disturbance occurs, NORM sites have a characteristic microbiome, which is specific for a given site, but may share common microbial genera and species, regardless of location and/or chemical contamination. It includes nitrate-reducing bacteria that tolerate acidic and low-nutrient conditions, while being highly resistant to metals. Members of the Proteobacteria (Alpha-, Beta-, Delta- and Gamma- proteobacteria), Acidobacteria, Actinobacteria, Bacteroidetes, and Firmicutes are generally associated with uranium transformation and are therefore found in these environments. Most represented bacterial genus include Geobacter, Thiobacillus, Arthrobacter, Bacillus, Actinobacteria, Desulfovibrio, and Microbacterium. Most of the studies are focused on bacteria and bacterial communities. Although little information exists regarding fungi, they are particularly resistant to radiation and thus play a role in the process of detoxification of radionuclides. For instance, an isolate of the genus Paecilomyces, was found to detoxify U(VI) through bioprecipitation of the metal, and the reduction was promoted by phosphate. Also, the yeast S. cerevisiae was able to reduce U(VI) toxicity by biomineralization [60].
Accordingly, the survival, abundance, and maintenance of a given species or community diversity depend on its adaptability to the existing conditions. Furthermore, several studies suggest that in those radionuclide-rich natural sites, resistance to high levels of chronic IR may occur among taxa that tolerate a wide range of environmental conditions and, therefore, have an advantage over other more sensitive species [62].
9.4.2 Effects of NORM and Metals on Eukaryotes
9.4.2.1 Invertebrates
There have been some studies in aquatic organisms, namely in Daphnia magna, Daphnia longispina, and Moinodaphnia macleayi at NORM sites [145, 146]. When testing several percentages of a uranium mine effluent containing metals and radionuclides from 238U and 235U decay chains, the Antunes et al. [145] study recorded an EC50Footnote 11 for daphnids immobilization at 50.4% for D. magna and at 28.4% for D. longispina, showing that D. magna was less sensitive than D. longispina. However, regarding fertility, D. magna was more sensitive than D. longispina, as this last species did not show significant effects in the offspring produced at effluent concentrations lower than 30.38%. Regarding M. macleayi, when a natural population of these organisms, living adjacent to a uranium mine in Australia, was challenged with a concentration of uranium ranging from 0 to 700 μg/L, it was shown that this population comparing to other populations tested, was the one that presented the highest sensitivity as it evidenced the lowest NOECs and LOECs.Footnote 12 It was shown that although this population lived in a water containing already considerable amounts of uranium, there was no tolerance to higher levels of uranium, when compared to the other tested populations. This probably shows that it was an already very stressed population that suffered “genetic erosion” [147] and because of that, it had lower capacity to deal with additional stresses, such as a single high dose of uranium.
When D. magna was exposed to uranium and to a uranium mine effluent [148, 149], significant genotoxic effects (DNA strand breaks) were detected in neonates and <5 days old daphnids after exposure to 55.3 μg/L of uranium and 2% of a uranium mine effluent. Moreover, in this same study, bystander effects, in the form of DNA damage, were detected in unexposed organisms when placed in contact with organisms directly exposed to uranium and to uranium mine effluent. In another paper [149], published by the same authors, on a transgenerational study performed on D. magna exposed to the same concentrations of uranium and uranium mine effluent as the study previously referred, it was observed that DNA damage was transmitted only to the first broods of the exposed organisms. By the third brood, DNA damage was no longer detected. This study showed that although short-term exposure to low concentrations of uranium and uranium mine effluent induces DNA damage to exposed organisms, it seems that it was not enough to significantly affect life history traits of D. magna populations in a long-term scenario. Nevertheless, the interpretation of these results is limited to the response observed for the endpoints here analyzed (DNA strand breaks). As such, other endpoints for genotoxicity assessment (i.e., mutation detection) and also the analyses of the epigenome of these organisms should be performed, as these molecular changes do not reflect a loss of DNA’s structural integrity [149].
As for terrestrial invertebrates, most of the studies conducted so far were on the annelid Eisenia andrei [150,151,152,153,154,155]. Gene expression alterations were reported in earthworms exposed to sludge from a uranium mine decantation pond. These genes were mainly related with metabolism, oxidoreductase activity, redox homeostasis, and response to chemical stimulus and stress [152]. In these studies, the occurrence of DNA damage in the form of DNA strand breaks and changes in cell’s DNA content in exposed organisms was also detected. Alterations in earthworm’s immune system were also reported, in terms of the frequency of each cell compartment, as it was observed a decrease in the number of effector cells (amebocytes) and an increase of the cells responsible for the maintenance of the organism’s homeostasis (eleocytes) [153, 154]. In parallel with a significant bioaccumulation of metals and radionuclides from uranium’s decay chain (238U, 234U, 235U, 226Ra, 230Th, and 210Pb), it was also observed a significant decrease in earthworms’ biomass, a reproduction inhibition, and significant histological alterations, namely in earthworm’s body wall (epidermis, circular, and longitudinal muscles) and gastrointestinal tract (chloragogenous tissue and intestinal epithelium) [153,154,155].
Under a real scenario of contamination, all of these effects may explain the lower biodiversity of soils contaminated with NORM, and the subsequent loss of their functions, if the contamination is perceived by the organisms. By using an avoidance assay (a standard ecotoxicological assay), to study earthworms’ behavioral responses to soils collected in a uranium mine area, it was shown that earthworms actively avoided several contaminated soils. Earthworm’s avoidance responses allowed it to discriminate highly to moderately toxic soils. On the other hand, on another study published by the same authors, using the analyses of oxidative stress enzymatic biomarkers (catalase, glutathione peroxidase) and lipid peroxidation biomarkers (through the quantification of thiobarbituric acid reactive substances), in earthworms exposed to soils nearby a uranium mine, showed no response for none of the biomarkers analyzed [150].
9.4.2.2 Vertebrates
Although there have been a wide number of studies performed on the effects of gamma radiation exposure on vertebrates, very few were performed so far for NORM exposure. Regarding aquatic vertebrates, fish have been the most used model organisms. On a study performed in former uranium mines from the Limousin region of France, where Rutilus rutilus specimens were caged on a pond contaminated with NORM and metals, immune, oxidative stress, biotransformation, neurotoxicity, and physiological parameters were measured [156]. The results obtained showed a stimulation of the immune parameters, the occurrence of oxidative stress and a decrease of acetyl choline esterase-AChE in the fish caged in the contaminated pond [156]. Zebrafish (Danio rerio) specimens exposed to uranium mill tailings leaching solution also showed alterations for the oxidative stress biomarkers used (superoxide dismutase—SOD, catalase—CAT, malondialdehyde—MDA and Na+–K+–ATPase) but specially for Na+–K+–ATPase and also evidenced that the organs most susceptible to oxidative stress were the gills [157]. In another study performed on a uranium milling operation in Northern Saskatchewan, Canada, Pimephales promelas specimens (adults and 5-day-old larvae) were exposed to contaminated water and contaminated sediment [158]. Results indicated effects on reproduction (reduced hatching) and larvae development (increase of skeletal deformities) and an increase in metal body burdens. However, the effects detected on the offspring, when considering the increase in egg production, were not significant in the level of deformities between treatments [158]. The effects on reproduction on the same species have already been observed under an exposure to effluent waters also from a uranium mining site in Saskatchewan, Canada. A significant decrease in eggs hatching time and hatching success was registered when early life stages of fathead minnows were exposed [159]. Nevertheless, metals and radionuclides are not the only stressor responsible for the effects caused by effluent waters from NORM sites. Lourenço et al. [160] performed an exposure of zebrafish eggs to a uranium mine effluent, barium chloride-treated mine effluent, and settling ponds sludge elutriates and showed that pH of the mine effluent strongly affected hatching success. After eliminating the effect of pH, this study also showed some teratogenicity associated with the uranium mine effluent, the occurrence of DNA damage, mainly associated with the exposure to treated mine effluent and sludge elutriates and mild effects on growth observed mainly on embryos exposed to the mine effluent and sludge elutriates. This study showed that the use of the Fish Embryo Toxicity Test (FET) test is suitable to test uranium mining wastes to determine and discriminate the risk of discharge. It also showed that the inclusion of the evaluation of genotoxicity endpoints in the FET test prevented the underestimation of risks, when only looking at chemical and radiological benchmark values defined by national and international directives, for the determination of risks, due to the chemical complexity of these wastes.
On what concerns amphibians, there are very few studies on these organisms as well. Marques and co-authors performed very important studies on amphibians, namely Pelophylax perezi exposed to NORM in situ. They have studied both tadpoles and adults and they have analyzed several endpoints, such as growth, survival, oxidative stress biomarkers (catalase, glutathione peroxidase, glutathione reductase, and lipid peroxidation through thiobarbituric acid reactive species (TBARS) quantification), gene expression alterations, histopathological changes, erythrocytic nuclear abnormalities and micronuclei, on organisms exposed to a uranium mine effluent in Portugal [161,162,163,164,165]. A study performed on 2008, on larvae and eggs [165] exposed to a uranium mine effluent, showed a decrease in larvae body length as well as a decrease in stimulus reactions, an increase in pigmentation along with tail deformities and metals bioaccumulation. The in situ exposure of tadpoles of the same species showed decreased survival and growth, a higher glutathione peroxidase activity and an increased lipid peroxidation [164] in organisms exposed in the mine effluent pond, when compared with organisms from a control pond. Although there may have been the influence of NORM and metals exposure, the studies also evidenced the effects of effluent’s acidity (typically seen in metal mining contexts), mainly in the growth and survival parameters and also in metal’s uptake. Another study, performed by the same authors [163], on adults living on the same uranium mine pond, analyzed gene expression changes using a technique called Suppressive Subtractive Hybridization (SSH). Significant changes in the expression levels of genes that play an important role in protecting cells against oxidative stress were shown, evidencing once again that oxidative stress response is very important in protecting cells and in maintaining DNA integrity on organisms exposed to NORMs and metals. Another study performed by this team on Pelophylax perezi adults inhabiting a uranium mining pond [162], showed significant metals bioaccumulation in the liver and the kidneys. Significant histopathological alterations in the liver, the lungs and in the kidneys, mainly in the form of a slight increase in melanomacrophagic centers, a dilatation of the renal tubules, a discrete thickening along with a slight hyperplasia of the alveolar septa and a slight hypoplasia of the goblet cells, were observed. The same animals living in the mine pond also displayed a significantly higher number of erythrocytic abnormalities (micronuclei and notched, kidney and lobed shaped nuclei) as well as a significantly lower frequency of immature erythrocytes. Both observations led to the belief that the removal and replacement of abnormal blood cells might be compromised.
There are a few studies published on the uptake of NORM by mammals that were performed mainly on former uranium mining areas, but very few examined the effects of that exposure. A study performed by Cleveland et al. [166] analyzed NORM uptake and histopathological alterations in liver and kidneys of rodents (Peromyscus maniculatus and P. boylii) inhabiting former uranium mines and observed that rodents bioaccumulated elements from 238U decay chain but without exceeding literature-based effects thresholds for small rodents. The authors also observed that there were some minor lesions in the tissues (liver and kidneys) analyzed that could not, however, be attributed to U mining activities. Lourenço and co-authors [167], captured mice (Apodemus sylvaticus) on the surroundings of a former uranium mining site and on a control area. DNA damage and bioaccumulation of metals and radionuclides were assessed, as well as the expression and the presence of single nucleotide polymorphisms on tumor suppressor genes. Results showed that cadmium and uranium were significantly bioaccumulated by exposed organisms. Organisms living in the former uranium mining area also evidenced significantly higher levels of DNA damage when compared with control organisms and also a higher expression of TP53 tumor suppressor gene and the presence of single nucleotide polymorphisms in Rb tumor suppressor gene. These effects can cause a disturbance in the genetic material of exposed organisms causing genetic instability and changes in the genetic pool of the population, potentially affecting the population’s fitness and stability. However, they cannot be attributed to any of the stressors in particular. It is known that uranium is genotoxic due to its chemical and radiological properties. Nevertheless, other metals present in uranium ore have shown greater genotoxic properties [151].
9.4.2.3 Plants
As for plants, there are a few studies already performed using soil/sludge or plant species collected directly from radium production industry storage sites, uranium rich regions, but mainly uranium mining sites and uranium milling tailings, that showed NORM bioaccumulation [168,169,170,171,172,173,174,175,176,177,178,179,180]. However, very few assessed the effects of that bioaccumulation. On a study performed by Evseeva et al. [170], Vicia cracca populations, inhabiting areas contaminated with uranium mill tailings and radium production wastes, were sampled and analyzed for the presence of chromosome aberrations, frequency of embryonic lethal mutations, seed germination and survival rate of seed sprouts. Results showed an increased frequency of embryonic lethal mutations, decreased seed germination, increased chromosome aberration counts and decreased survival rate of seed sprouts. The same authors [171], used Allium cepa specimens to determine the genotoxicity of an effluent from a radium production storage facility, through chromosome aberrations counting. Results showed a significant increase in chromosome aberration counts in the roots of exposed plants. Two studies [168, 179] using soils contaminated with metals and radionuclides from Portuguese former uranium mines were performed using Lactuca sativa and Zea mays as test species to determine the eco (through growth inhibition) and genotoxicity (mutation analysis through the Ames test) of amended and unamended mine soils. Studies showed genotoxicity of the unamended soils containing the highest levels of metals and radionuclides, a significant decrease in Lactuca sativa biomass and also a significant bioaccumulation of these elements. The soil amendment methodology used in these studies significantly decreased the levels of metals and radionuclides in soils leachates and the soil available fraction.
9.5 Exercises and Self-Assessment
-
Q1.
What is the relationship between life stage and an organism’s radiosensitivity?
-
Q2.
Please indicate which is the most radiosensitive parameter: mortality or reproduction?
-
Q3.
Please indicate the most important non-stochastic effects induced by organisms exposure to ionizing radiation at a population level.
-
Q4.
Which kind of exposure is more effective in causing organisms mortality?
-
Q5.
Regarding radioactive contamination, what information can be retrieved from the omics approaches? What can be the contribution of those studies for future remediation of radiologically contaminated sites?
-
Q6.
What are the main traits conferring radioresistance to plants compared to animals?
-
Q7.
What does “hormesis” in plants mean?
9.6 Exercise Solutions
-
SQ1.
The younger the organisms, the more sensitive they are to the deleterious effects of radiation exposure.
-
SQ2.
Reproduction and reproductive capacity is a more sensitive parameter to the effects of radiation exposure both for terrestrial and aquatic invertebrates and vertebrates, than mortality.
-
SQ3.
The non-stochastic effects that are most important at a population level are mortality, fertility, and fecundity.
-
SQ4.
Acute exposures to high doses of ionizing radiation are more effective in inducing higher injury than chronic exposures to low doses of ionizing radiation. The higher the dose the lower the ability of cells to divide and regenerate the damaged tissue which translates into a higher probability for organisms mortality.
-
SQ5.
The application of multiomics approaches, namely genomics, proteomics, metabolomics, and transcriptomics, has gained relevance in many different fields. These high-throughput techniques allow an analysis of the total set of molecules (DNA, proteins, and other metabolites) in a biological sample. Therefore, the integrated data have revolutionized biology and have contributed to advancing our understanding of different biological processes.
Genome sequencing, comparative genomics, and proteomics have allowed the identification of microbial essential genes (key players) that encode biomolecules, mainly proteins, involved in biological processes, including those involved in detoxification of radionuclides and metals. Furthermore, metagenomics approaches directed to the microbial communities of these contaminated environments allow for the identification, and characterization, of microorganisms with relevant functions in the bioremediation/decontamination processes. It is therefore expected that these broader approaches will contribute even more to the identification of microorganisms and to the elucidation of the metabolic pathways and key genes involved in those processes that may be further applied in the bioremediation/decontamination of these sites.
-
SQ6.
The elevated radioresistance of plants compared to animals relies on differences in cell structure and metabolism. Plant cells present some traits such as thickened cell walls, cuticles, hairs (pubescence), phenolic compounds, and often polyploidy.
-
SQ7.
Low doses of ionizing radiation induce positive outcomes in plants such as increasing growth and production of secondary metabolites engaged in the antioxidant defenses.
Notes
- 1.
Homologous recombination (HR) repair: while in eukaryotes the process occurs during meiosis and requires homologous DNA sequences, in bacteria HR is a major DNA repair mechanism that facilitates the incorporation of exogenous DNA.
- 2.
Non-homologous end joining (NHEJ) repair: in eukaryotic cells, DSB are repaired predominantly by this pathway. Broken double-stranded ends are repaired by direct ligation without the need for a homologous template.
- 3.
Extended Synthesis-Dependent Strand Annealing (ESDSA): a type of homologous recombination where the sequence around a DNA double-strand break (DSB) is replaced by a copy of a homologous DNA template, while the original configuration of the flanking regions is maintained.
- 4.
Chaperones—are proteins that assist other proteins folding.
- 5.
Protein carbonylation—Reaction of hydroxyl radicals with side chains of certain aminoacids causing irreversible oxidation of proteins.
- 6.
DNA methylation—DNA methylation of eukaryotic cells is an epigenetic signaling mechanism characterized by the transfer of a methyl group onto the C5 position of the cytosine to form 5-methylcytosine, by DNA methyltransferase enzymes. DNA methylation regulates gene expression by recruiting proteins involved in gene repression or by inhibiting the binding of transcription factor(s) to DNA.
- 7.
EDR10—effective dose rate inducing an effect of 10%.
- 8.
ED50—effective dose causing a 50% effect.
- 9.
- 10.
HDR5—the hazardous dose rate for 5% of the species.
- 11.
EC50 is the concentration of a substance in water causing death to 50% of the tested population.
- 12.
LOEC is the lowest concentration where an effect has been observed in chronic or acute ecotoxicity studies. NOEC is the highest concentration at which there is no statistically significant difference from the control condition in an acute or chronic ecotoxicity study.
References
Song Y, Xie L, Lee YK, Brede DA, Lyne F, Kassaye Y, et al. Integrative assessment of low-dose gamma radiation effects on Daphnia magna reproduction: toxicity pathway assembly and AOP development. Sci Total Environ. 2020;705:135912.
National Research Council (US). Committee on evaluation of EPA guidelines for exposure to naturally occurring radioactive materials. Evaluation of guidelines for exposures to technologically enhanced naturally occurring radioactive materials. Washington, DC; 1999.
IAEA. Protection of the environment from ionising radiation: the development and application of a system of radiation protection for the environment. Iaea-Csp-17. 2003;66(1–2).
Grauby A. Compartimentation de la radioactivite dans Ie milieu terrestre. Evacuation des dechets radioactifs. In: Proceedings, Reunion d’Information de l’AIEN. Paris: Organisation pour la Cooperation et Ie Developpement Economique (OCDE); 1972.
Parsons J, Droge S. Environmental compartments. In: van Gestel Cornelis AM, Van Belleghem FGAJ, van den Brink NW, Droge STJ, Hamers T, Hermens JLM, et al., editors. Environmental toxicology. Amsterdam: LibreTexts Libraries; 2019. p. 104–33. Available from: https://chem.libretexts.org/@go/page/294544.
Little KW. Environmental fate and transport analysis with compartment modeling. Boca Raton, FL: CRC Press; 2012.
Vandenhove H, Hurtgen C, Payne TE. Uranium: radionuclides encyclopedia of inorganic chemistry. 2010. (Major reference works). https://doi.org/10.1002/0470862106.ia739.
Langmuir D. Aqueous environmental geochemistry. Upper Saddle River, NJ: Prentice Hall; 1997.
Desmet GM, Van Loon LR, Howard BJ. Chemical speciation and bioavailability of elements in the environment and their relevance to radioecology. Sci Total Environ. 1991;100(C).
Whicker F, Schultz V. Radioecology nuclear energy in the environment. CRC Press; 1982. Available from: https://books.google.be/books?id=lmLwAAAAMAAJ.
Langmuir D, Herman JS. The mobility of thorium in natural waters at low temperatures. Geochim Cosmochim Acta. 1980;44(11):1753.
Bourg ACM. Speciation of heavy metals in soils and groundwater and implications for their natural and provoked mobility. In: Heavy metals. Springer; 1995.
Sparks DL. Environmental soil chemistry. 2nd ed. Amsterdam: Academic Press; 2003.
Mahmood Z, Mohamed C. Thorium. In: Radionuclides in the environment. John Wiley & Sons; 2010. p. 247–53.
Premuzic E, Francis A, Lin M, Schubert J. Chelation of thorium and uranium by Pseudomonas aeruginosa. Arch Environ Contam Toxicol. 1985;14:759–68.
Rogiers T, Claesen J, Van Gompel A, Vanhoudt N, Mysara M, Williamson A, et al. Soil microbial community structure and functionality changes in response to long-term metal and radionuclide pollution. Environ Microbiol. 2021;23(3):1670.
Atwood DA. Radionuclides in the Environment. John Wiley & Sons; 2010.
IAEA. Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater. Technical Reports Series 472. 2010;(472).
Kirchmann R, Van der Stricht E. Radioecology: radioactivity and ecosystems. Liège: Fortemps; 2001. Available from: http://lib.ugent.be/catalog/rug01:000853032.
Zhu YG, Smolders E. Plant uptake of radiocaesium: a review of mechanisms, regulation and application. J Exp Bot. 2000;51(351):1635–45 [cited 2017 Oct 6]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11053452.
Nyjfeler UP, Santschi PH, Li Y-H. The relevance of scavenging kinetics to modeling of sediment-water interactions in natural waters. Limnol Oceanogr. 1986;31(2):277.
Absalom J, Young S, Crout N. Radio-caesium fixation dynamics: measurement in six Cumbrian soils. Eur J Soil Sci. 1995;46(3):461.
Krouglov SV, Filipas AS, Alexakhin RM, Arkhipov NP. Long-term study on the transfer of 137Cs and 90Sr from Chernobyl-contaminated soils to grain crops. J Environ Radioact. 1997;34(3):267–86. Available from: https://www.sciencedirect.com/science/article/pii/0265931X96000434.
Sheppard SC, Sheppard MI, Gallerand MO, Sanipelli B. Derivation of ecotoxicity thresholds for uranium. J Environ Radioact. 2005;79(1):55–83. Available from: http://www.sciencedirect.com/science/article/B6VB2-4D3B1W1-3/2/681b494598ffb6a9c93d87d7ae43672a.
Vandenhove H, Van Hees M, Wannijn J, Wouters K, Wang L. Can we predict uranium bioavailability based on soil parameters? Part 2: soil solution uranium concentration is not a good bioavailability index. PG-577-86. Environ Pollut. 2007;145(2):577–86.
Semple KT, Doick KJ, Jones KC, Burauel P, Craven A, Harms H. Peer reviewed: defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ Sci Technol. 2004;38(12):228A–31A.
Hu Q-H, Weng J-Q, Wang J-S. Sources of anthropogenic radionuclides in the environment: a review. J Environ Radioact. 2010;101(6):426–37. Available from: http://www.sciencedirect.com/science/article/pii/S0265931X08001392.
Beresford NA, Horemans N, Copplestone D, Raines KE, Orizaola G, Wood MD, Laanen P, Whitehead HC, Burrows JE, Tinsley MC, Smith JT, Bonzom J-M, Gagnaire B, Adam-Guillermin C, Gashchak S, Jha AN, de Menezes A, Willey N, Spurgeon D. Towards solving a scientific controversy—the effects of ionising radiation on the environment. J Environ Radioact. 2020;211:106033.
Howard BJ, Larsson C.-M. The ERICA Integrated Approach and its contribution to protection of the environment from ionising radiation. J Environ Radioact. 2008;99(9):1361–63.
IAEA. Handbook of parameter values for the prediction of radionuclide transfer to wildlife., Technical Reports Series, vol. No. 479. Vienna: IAEA; 2014.
ICRP. Dose coefficients for nonhuman biota environmentally exposed to radiation. ICRP publication 136. Ann ICRP. 2017;46(2)
Andersson P, Garnier-Laplace J, Beresford NA, Copplestone D, Howard BJ, Howe P, Oughton DH, Whitehouse P. Protection of the environment from ionising radiation in a regulatory context (PROTECT): proposed numerical benchmark values. J Environ Radioact. 2009;100:1100–8.
ICRP. Annex D. Radiation effects in reference animals and plants. Ann ICRP. 2008;38(4–6):179.
Eckerman K, Endo A. ICRP publication 107. Nuclear decay data for dosimetric calculations. Ann ICRP. 2008;38:9–10.
Brown JE, Alfonso B, Avila R, Beresford NA, Copplestone D, Hosseini A. A new version of the ERICA tool to facilitate impact assessments of radioactivity on wild plants and animals. J Environ Radioact. 2016;153:141.
Vives i Batlle J, Ulanovsky A, Copplestone D. A method for assessing exposure of terrestrial wildlife to environmental radon (222Rn) and thoron (220Rn). Sci Total Environ. 2017;605–606:569.
Boyer P, Wells C, Howard B. Extended Kd distributions for freshwater environment. J Environ Radioact. 2018;192:128.
Beresford NA, Wright SM, Barnett CL, Wood MD, Gaschak S, Arkhipov A, et al. Predicting radionuclide transfer to wild animals: an application of a proposed environmental impact assessment framework to the Chernobyl exclusion zone. Radiat Environ Biophys. 2005;44(3):161.
Beresford NA, Gaschak S, Barnett CL, Howard BJ, Chizhevsky I, Strømman G, et al. Estimating the exposure of small mammals at three sites within the Chernobyl exclusion zone—a test application of the ERICA tool. J Environ Radioact. 2008;99(9):1496–502.
Dragović S, Mandić LJ. Transfer of radionuclides to ants, mosses and lichens in semi-natural ecosystems. Radiat Environ Biophys. 2010;49(4):625.
Beaugelin-Seiller K, Jasserand F, Garnier-Laplace J, Gariel JC. Modeling radiological dose in non-human species: principles, computerization, and application. Health Phys. 2006;90(5):485–93. https://doi.org/10.1097/01.HP.0000182192.91169.ed.
Thomas P, Liber K. An estimation of radiation doses to benthic invertebrates from sediments collected near a Canadian uranium mine. Environ Int. 2001;27(4):341.
Wood MD, Leah RT, Jones SR, Copplestone D. Radionuclide transfer to invertebrates and small mammals in a coastal sand dune ecosystem. Sci Total Environ. 2009;407(13):4062–74.
Mothersill CE, Oughton DH, Schofield PN, Abend M, Adam-Guillermin C, Ariyoshi K, Beresford NS, Bonisoli-Alquati A, Cohen J, Dubrova Y, Geras’kin SA, Hevrøy TH, Higley KA, Horemans N, Jha AN, Kapustka LA, Kiang JG, Madas BG, Powathil G, Sarapultseva EI, Seymour CB, Nguyen TK, Wood MD. From tangled banks to toxic bunnies; a reflection on the issues involved in developing an ecosystem approach for environmental radiation protection. Int J Radiat Biol. 2020; https://doi.org/10.1080/09553002.2020.1793022.
UNSCEAR. Sources and effects of ionizing radiation, United Nations Scientific Committee on the Effects of Atomic Radiation, Report to the general assembly with scientific annexes, vol. II, Scientific annexes C, D and E. New York: United Nations; 2008.
UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 1996 report to the general assembly, with scientific annexes. New York; 1996.
Copplestone D, Bielby S, Jones SR, Patton D, Daniel P, Gize I. Impact assessment of ionising radiation on wildlife. Environment Agency; 2001. Available from: https://aquadocs.org/bitstream/1834/27217/1/25_Impact_Assesment_of_ionising_Radiation_on_Wildlife.pdf.
IAEA. Effects of ionising radiation on plants and animals at levels implied by current radiation protection standards. Technical Report Series No. 332. Vienna; 1992. 74 pp. ISBN: 920 100992 5.
Upton AC. Environmental standards for ionizing radiation: theoretical basis for dose-response curves. Environ Health Perspect. 1983;52:31.
Beresford NA, Copplestone D. Effects of ionizing radiation on wildlife: what knowledge have we gained between the Chernobyl and Fukushima accidents? Integr Environ Assess Manag. 2011;7(3):371.
Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res. 2008;49(3):203–10.
Dartnell LR. Ionizing radiation and life. Astrobiology. 2011;11:551.
Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, et al. Mechanism of radiation-induced bystander effects: a unifying model. J Pharm Pharmacol. 2008;60(8):943–50.
UNSCEAR. Effects of ionizing radiation. Report to the general assembly, with scientific annexes. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2006.
Gomes T, Song Y, Brede DA, Xie L, Gutzkow KB, Salbu B, et al. Gamma radiation induces dose-dependent oxidative stress and transcriptional alterations in the freshwater crustacean Daphnia magna. Sci Total Environ. 2018;628–629:206.
Merino N, Aronson HS, Bojanova DP, Feyhl-Buska J, Wong ML, Zhang S, et al. Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol. 2019;10 https://doi.org/10.3389/fmicb.2019.00780.
Singh OV, Gabani P. Extremophiles: radiation resistance microbial reserves and therapeutic implications. J Appl Microbiol. 2011;110:851.
Becker J, Wittmann C. Microbial production of extremolytes—high-value active ingredients for nutrition, health care, and well-being. Curr Opin Biotechnol. 2020;65:118.
Coker JA. Extremophiles and biotechnology: current uses and prospects. F1000Research. 2016;5 https://doi.org/10.12688/f1000research.7432.1.
Shuryak I, Matrosova VY, Gaidamakova EK, Tkavc R, Grichenko O, Klimenkova P, et al. Microbial cells can cooperate to resist high-level chronic ionizing radiation. PLoS One. 2017;12(12):e0189261.
Bruckbauer ST, Martin J, Minkoff BB, Veling MT, Lancaster I, Liu J, et al. Physiology of highly radioresistant Escherichia coli after experimental evolution for 100 cycles of selection. Front Microbiol. 2020;11:582590.
Shuryak I. Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions. J Environ Radioact. 2019;196:50.
Ghirga G. Cancer in children residing near nuclear power plants: an open question. Ital J Pediatr. 2010;36(1):60. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-77958117954&partnerID=40&md5=bdf4aa7fe5e060d001f5dd26fc4ad520.
Webb KM, Yu J, Robinson CK, Noboru T, Lee YC, DiRuggiero J. Effects of intracellular Mn on the radiation resistance of the halophilic archaeon Halobacterium salinarum. Extremophiles. 2013;17(3):485.
de la Tour CB, Mathieu M, Servant P, Coste G, Norais C, Confalonieri F. Characterization of the DdrD protein from the extremely radioresistant bacterium Deinococcus radiodurans. Extremophiles. 2021;25(4):343.
Jung KW, Lim S, Bahn YS. Microbial radiation-resistance mechanisms. J Microbiol. 2017;55:499.
Bentchikou E, Servant P, Coste G, Sommer S. A major role of the RecFOR pathway in DNA double-strand-break repair through ESDSA in Deinococcus radiodurans. PLoS Genet. 2010;6(1):e1000774.
Gallois N, Alpha-Bazin B, Ortet P, Barakat M, Piette L, Long J, et al. Proteogenomic insights into uranium tolerance of a Chernobyl’s microbacterium bacterial isolate. J Proteome. 2018;177:148.
Lopez-Fernandez M, Jroundi F, Ruiz-Fresneda MA, Merroun ML. Microbial interaction with and tolerance of radionuclides: underlying mechanisms and biotechnological applications. Microb Biotechnol. 2021;14:810.
Nayak T, Sengupta I, Dhal PK. A new era of radiation resistance bacteria in bioremediation and production of bioactive compounds with therapeutic potential and other aspects: an in-perspective review. J Environ Radioact. 2021;237:106696.
Hoyos-Hernandez C, Courbert C, Simonucci C, David S, Vogel TM, Larose C. Community structure and functional genes in radionuclide contaminated soils in Chernobyl and Fukushima. FEMS Microbiol Lett. 2019;366(21):fnz180.
Theodorakopoulos N, Février L, Barakat M, Ortet P, Christen R, Piette L, et al. Soil prokaryotic communities in Chernobyl waste disposal trench T22 are modulated by organic matter and radionuclide contamination. FEMS Microbiol Ecol. 2017;93(8) https://doi.org/10.1093/femsec/fix079.
Arena C, De Micco V, De Maio A. Growth alteration and leaf biochemical responses in Phaseolus vulgaris exposed to different doses of ionising radiation. Plant Biol. 2014;16(Suppl. 1):194.
De Micco V, Arena C, Pignalosa D, Durante M. Effects of sparsely and densely ionizing radiation on plants. Radiat Environ Biophys. 2011;50(1):1.
Maity JP, Mishra D, Chakraborty A, Saha A, Santra SC, Chanda S. Modulation of some quantitative and qualitative characteristics in rice (Oryza sativa L.) and mung (Phaseolus mungo L.) by ionizing radiation. Radiat Phys Chem. 2005;74(5):391.
Zaka R, Vandecasteele CM, Misset MT. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J Exp Bot. 2002;53(376):1979.
Li F, Shimizu A, Nishio T, Tsutsumi N, Kato H. Comparison and characterization of mutations induced by gamma-ray and carbon-ion irradiation in rice (Oryza sativa L.) using whole-genome resequencing. G3 Genes, Genomes, Genet. 2019;9(11):3743.
Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6:836.
De Micco V, Arena C, Aronne G. Anatomical alterations of Phaseolus vulgaris L. mature leaves irradiated with X-rays. Plant Biol. 2014;16(Suppl. 1):187.
Mousseau TA, Møller AP. Plants in the light of ionizing radiation: what have we learned from Chernobyl, Fukushima, and other “hot” places? Front Plant Sci. 2020;11:552.
Møller AP, Mousseau TA. Are organisms adapting to ionizing radiation at Chernobyl? Trends Ecol Evol. 2016;31:281.
Yemets AI, Blume RY, Sorochinsky BV. Adaptation of the gymnosperms to the conditions of irradiation in the Chernobyl zone: from morphological abnormalities to the molecular genetic consequences. Cytol Genet. 2016;50:415.
Watanabe Y, Ichikawa S, Kubota M, Hoshino J, Kubota Y, Maruyama K, et al. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci Rep. 2015;5:13232.
Yoschenko V, Nanba K, Yoshida S, Watanabe Y, Takase T, Sato N, et al. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-Ichi Nuclear Power Plant. J Environ Radioact. 2016;165:60.
Koranda JJ, Robison WL. Accumulation of radionuclides by plants as a monitor system. Environ Health Perspect. 1978;27:165.
Ludovici GM, Oliveira de Souza S, Chierici A, Cascone MG, d’Errico F, Malizia A. Adaptation to ionizing radiation of higher plants: from environmental radioactivity to Chernobyl disaster. J Environ Radioact. 2020;222:106375.
Arena C, De Micco V, Macaeva E, Quintens R. Space radiation effects on plant and mammalian cells. Acta Astronaut. 2014;104(1):419.
De Micco V, Paradiso R, Aronne G, De Pascale S, Quarto M, Arena C. Leaf anatomy and photochemical behaviour of solanum lycopersicum L. Plants from seeds irradiated with low-let ionising radiation. Sci World J. 2014;2014:428141.
Nagata T, Todoriki S, Hayashi T, Shibata Y, Mori M, Kanegae H, et al. γ-Radiation induces leaf trichome formation in arabidopsis. Plant Physiol. 1999;120(1):113.
Ahuja S, Kumar M, Kumar P, Gupta VK, Singhal RK, Yadav A, et al. Metabolic and biochemical changes caused by gamma irradiation in plants. J Radioanal Nucl Chem. 2014;300:199.
Kurimoto T, Constable JVH, Huda A. Effects of ionizing radiation exposure on arabidopsis thaliana. Health Phys. 2010;99(1):49.
Gerlach SU, Herranz H. Genomic instability and cancer: lessons from Drosophila. Open Biol. 2020;10(6):200060.
González-Huici V, Wang B, Gartner A. A role for the nonsense-mediated mRNA decay pathway in maintaining genome stability in caenorhabditis elegans. Genetics. 2017;206(4):1853.
Lopez JV, Bracken-Grissom H, Collins AG, Collins T, Crandall K, Distel D, et al. The global invertebrate genomics alliance (GIGA): developing community resources to study diverse invertebrate genomes. J Hered. 2014;105:1.
Wilson-Sanders SE. Invertebrate models for biomedical research, testing, and education. ILAR J. 2011;52:126.
Clatworthy AL, Noel F, Grose E, Cui M, Tofilon PJ. Ionizing radiation-induced alterations in the electrophysiological properties of Aplysia sensory neurons. Neurosci Lett. 1999;268(1):45.
Vaiserman A, Cuttler JM, Socol Y. Low-dose ionizing radiation as a hormetin: experimental observations and therapeutic perspective for age-related disorders. Biogerontology. 2021;22(2):145–64. https://doi.org/10.1007/s10522-020-09908-5.
Geras’kin SA. Ecological effects of exposure to enhanced levels of ionizing radiation. J Environ Radioact. 2016;162–163:347.
Driver CJ. Ecotoxicity literature review of selected Hanford site contaminants. Richland, WA: Pacific Northwest Lab; 1994.
Cassidy CL, Lemon JA, Boreham DR. Impacts of low-dose gamma-radiation on genotoxic risk in aquatic ecosystems. Dose-Response. 2007;5(4):323.
Johnson TE, Hartman PS. Radiation effects on life span in Caenorhabditis elegans. J Gerontol. 1988;43(5):B137–41.
Allen RG. Relationship between γ-irradiation, life span, metabolic rate and accumulation of fluorescent age pigment in the adult male housefly, Musca domestica. Arch Gerontol Geriatr. 1985;4(2):169.
Vaiserman A, Litoshenko AI, Kvitnitskaia-Ryzhova TI, Koshel N, Mozzhukhina T, Mikhal’skiĭ S, et al. Molecular and cellular aspects of radiation hormesis in Drosophila melanogaster. Tsitol Genet. 2003;37(3):41–8.
Won EJ, Lee JS. Gamma radiation induces growth retardation, impaired egg production, and oxidative stress in the marine copepod Paracyclopina nana. Aquat Toxicol. 2014;150:17.
Han J, Won EJ, Kim IC, Yim JH, Lee SJ, Lee JS. Sublethal gamma irradiation affects reproductive impairment and elevates antioxidant enzyme and DNA repair activities in the monogonont rotifer Brachionus koreanus. Aquat Toxicol. 2014;155:101.
Han J, Won EJ, Lee BY, Hwang UK, Kim IC, Yim JH, et al. Gamma rays induce DNA damage and oxidative stress associated with impaired growth and reproduction in the copepod Tigriopus japonicus. Aquat Toxicol. 2014;152:264.
Fuller N, Ford AT, Nagorskaya LL, Gudkov DI, Smith JT. Reproduction in the freshwater crustacean Asellus aquaticus along a gradient of radionuclide contamination at Chernobyl. Sci Total Environ. 2018;628–629:11.
Kuzmic M, Galas S, Lecomte-Pradines C, Dubois C, Dubourg N, Frelon S. Interplay between ionizing radiation effects and aging in C. elegans. Free Radic Biol Med. 2019;134:657.
Krisko A, Leroy M, Radman M, Meselson M. Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers. Proc Natl Acad Sci U S A. 2012;109(7):2354.
Mothersill C, Lyng F, Mulford A, Seymour C, Cottell D, Lyons M, et al. Effect of low doses of ionizing radiation on cells cultured from the hematopoietic tissue of the Dublin Bay prawn, Nephrops norvegicus. Radiat Res. 2001;156(3):241.
Alonzo F, Hertel-Aas T, Gilek M, Gilbin R, Oughton DH, Garnier-Laplace J. Modelling the propagation of effects of chronic exposure to ionising radiation from individuals to populations. J Environ Radioact. 2008;99(9):1464–73.
Depledge MH. The ecotoxicological significance of genotoxicity in marine invertebrates. Mutat Res Fundam Mol Mech Mutagen. 1998;399(1):109.
Fuller N, Lerebours A, Smith JT, Ford AT. The biological effects of ionising radiation on crustaceans: a review. Aquat Toxicol. 2015;167:55–67.
Alonzo F, Hertel-Aas T, Real A, Lance E, Garcia-Sanchez L, Bradshaw C, Battle JVI, Oughton DH, Garnier-Laplace J. Population modelling to compare chronic external radiotoxicity between individual and population endpoints in four taxonomic groups. J Environ Radioact. 2016;152:46–59.
Harrison FL, Anderson SL. Effects of acute irradiation on reproductive success of the polychaete worm, Neanthes arenaceodentata. Radiat Res. 1994;137(1):59.
Jönsson KI, Harms-Ringdahl M, Torudd J. Radiation tolerance in the eutardigrade Richtersius coronifer. Int J Radiat Biol. 2005;81(9):649.
Nakamori T, Yoshida S, Kubota Y, Ban-nai T, Kaneko N, Hasegawa M, et al. Effects of acute gamma irradiation on Folsomia candida (Collembola) in a standard test. Ecotoxicol Environ Saf. 2008;71(2):590.
Styron CE. Effects of beta and gamma radiation on a population of springtails, Sinella curviseta (Collembola). Radiat Res. 1971;48(1):53.
Suzuki J, Egami N. Mortality of the earthworms, Eisenia foetida, after γ-irradiation at different stages of their life history. J Radiat Res. 1983;24(3):209.
Watanabe M, Sakashita T, Fujita A, Kikawada T, Horikawa DD, Nakahara Y, et al. Biological effects of anhydrobiosis in an African chironomid, Polypedilum vanderplanki on radiation tolerance. Int J Radiat Biol. 2006;82(8):587.
Gladyshev E, Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci U S A. 2008;105(13):5139.
Harrison FL, Rice DW, Moore DH, Varela M. Effects of radiation on frequency of chromosomal aberrations and sister chromatid exchange in the benthic worm, Neanthes arenaceodentata. In: Capuzzo JM, Kester DR, editors. Oceanic processes of marine pollution, vol. 1. Malabar, FL: R. E. Krieger Publishing Company; 1986. p. 145–56.
Parisot F, Bourdineaud JP, Plaire D, Adam-Guillermin C, Alonzo F. DNA alterations and effects on growth and reproduction in Daphnia magna during chronic exposure to gamma radiation over three successive generations. Aquat Toxicol. 2015;163:27–36.
Zadereev E, Lopatina T, Oskina N, Zotina T, Petrichenkov M, Dementyev D. Gamma irradiation of resting eggs of Moina macrocopa affects individual and population performance of hatchlings. J Environ Radioact. 2017;175–176:126.
Murphy JF, Nagorskaya LL, Smith JT. Abundance and diversity of aquatic macroinvertebrate communities in lakes exposed to Chernobyl-derived ionising radiation. J Environ Radioact. 2011;102(7):688.
Fuciarelli TM, Rollo CD. Ionizing radiation alters male Acheta domesticus courtship songs that are critical for mating success. Anim Behav. 2021;178:209.
Krivolutskiy DA, Tikhomirov FA, Fedorov EA, Pokargevsky AD, Taskaev AI. Effect of ionizing radiation on biogeocenosis. M Geo. 1988.
Møller AP, Barnier F, Mousseau TA. Ecosystems effects 25 years after Chernobyl: pollinators, fruit set and recruitment. Oecologia. 2012;170(4):1155.
Dallas LJ, Bean TP, Turner A, Lyons BP, Jha AN. Exposure to tritiated water at an elevated temperature: genotoxic and transcriptomic effects in marine mussels (M. galloprovincialis). J Environ Radioact. 2016;164:325.
Mousseau T, Milinevsky G, Kenney-Hunt J, Møller A. Highly reduced mass loss rates and increased litter layer in radioactively contaminated areas. Oecologia. 2014;175(1):429–37. https://doi.org/10.1007/s00442-014-2908-8.
Bezrukov V, Møller AP, Milinevsky G, Rushkovsky S, Sobol M, Mousseau TA. Heterogeneous relationships between abundance of soil surface invertebrates and radiation from Chernobyl. Ecol Indic. 2015;52:128–33.
Møller AP, Mousseau TA. Reduced colonization by soil invertebrates to irradiated decomposing wood in Chernobyl. Sci Total Environ. 2018;645:773.
Jackson D, Copplestone D, Stone DM, Smith GM, Jackson D, Copplestone D, et al. Terrestrial invertebrate population studies in the Chernobyl exclusion zone, Ukraine. Radioprotection. 2005;40(S1):S857–63.
Garnier-Laplace J, Della-Vedova C, Gilbin R, Copplestone D, Hingston J, Ciffroy P. First derivation of predicted-no-effect values for freshwater and terrestrial ecosystems exposed to radioactive substances. Environ Sci Technol. 2006;40(20):6498.
Garnier-Laplace J, Geras’kin S, Della-Vedova C, Beaugelin-Seiller K, Hinton TG, Real A, et al. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J Environ Radioact. 2013;121:12–21. Available from: http://www.sciencedirect.com/science/article/pii/S0265931X12000240.
Real A, Sundell-Bergman S, Knowles JF, Woodhead DS, Zinger I. Effects of ionising radiation exposure on plants, fish and mammals: relevant data for environmental radiation protection. J Radiol Prot. 2004;24:A123.
Hinton TG, Alexakhin R, Balonov M, Gentner N, Hendry J, Prister B, et al. Radiation-induced effects on plants and animals: findings of the United Nations Chernobyl forum. Health Phys. 2007;93:427.
Paunesku T, Stevanović A, Popović J, Woloschak GE. Effects of low dose and low dose rate low linear energy transfer radiation on animals–review of recent studies relevant for carcinogenesis. Int J Radiat Biol. 2021;97:757.
ICRP. Environmental protection—the concept and use of reference animals and plants. ICRP publication 108. Ann ICRP. 2008;38(4–6).
Garnier-Laplace J, Beaugelin-Seiller K, Della-Vedova C, Métivier J-M, Ritz C, Mousseau TA, et al. Radiological dose reconstruction for birds reconciles outcomes of Fukushima with knowledge of dose-effect relationships. Sci Rep. 2015;5:16594. Available from: https://pubmed.ncbi.nlm.nih.gov/26567770.
Bonisoli-Alquati A, Møller AP, Rudolfsen G, Mousseau TA. Birds as bioindicators of radioactive contamination and its effects. NATO Sci Peace Secur Ser A Chem Biol. 2022; https://doi.org/10.1007/978-94-024-2101-9_11.
Ulsh BA, Mühlmann-Díaz MC, Whicker FW, Hinton TG, Congdon JD, Bedford JS. Chromosome translocations in turtles: a biomarker in a sentinel animal for ecological dosimetry. Radiat Res. 2000;153(6):752.
Lourenço J, Mendo S, Pereira R. Radioactively contaminated areas: bioindicator species and biomarkers of effect in an early warning scheme for a preliminary risk assessment. J Hazard Mater. 2016;317:503–42. Available from: http://www.sciencedirect.com/science/article/pii/S0304389416305751
Pouget JP, Mather SJ. General aspects of the cellular response to low- and high-LET radiation. Eur J Nucl Med. 2001;28:541.
Antunes S, Pereira R, Gonçalves F. Acute and chronic toxicity of effluent water from an abandoned uranium mine. Arch Environ Contam Toxicol. 2007;53:207–13. Available from: http://www.ingentaconnect.com/content/klu/244/2007/00000053/00000002/00000011.
Semaan M, Holdway DA, Van Dam RA. Comparative sensitivity of three populations of the cladoceran Moinodaphnia macleayi to acute and chronic uranium exposure. Environ Toxicol. 2001;16(5):365.
Ribeiro R, Lopes I. Contaminant driven genetic erosion and associated hypotheses on alleles loss, reduced population growth rate and increased susceptibility to future stressors: an essay. Ecotoxicology. 2013;22(5):889.
Reis P, Lourenço J, Carvalho FP, Oliveira J, Malta M, Mendo S, et al. RIBE at an inter-organismic level: a study on genotoxic effects in Daphnia magna exposed to waterborne uranium and a uranium mine effluent. Aquat Toxicol. 2018;198:206.
Reis P, Pereira R, Carvalho FP, Oliveira J, Malta M, Mendo S, et al. Life history traits and genotoxic effects on Daphnia magna exposed to waterborne uranium and to a uranium mine effluent—a transgenerational study. Aquat Toxicol. 2018;202:16–25 [cited 2019 Feb 12]. Available from: https://www.sciencedirect.com/science/article/pii/S0166445X18304363?via%3Dihub.
Antunes S, Castro B, Nunes B, Pereira R, Gonçalves F. In situ bioassay with Eisenia andrei to assess soil toxicity in an abandoned uranium mine. Ecotoxicol Environ Saf. 2008;71(3):620–31.
Fernandes S, Nogueira V, Lourenço J, Mendo S, Pereira R. Inter-species bystander effect: Eisenia fetida and Enchytraeus albidus exposed to uranium and cadmium. J Hazard Mater. 2020;399:122972.
Lourenço J, Pereira R, Gonçalves F, Mendo S. SSH gene expression profile of Eisenia andrei exposed in situ to a naturally contaminated soil from an abandoned uranium mine. Ecotoxicol Environ Saf. 2013;88:16–25. Available from: http://www.sciencedirect.com/science/article/pii/S0147651312003636.
Lourenço J, Pereira R, Silva A, Carvalho F, Oliveira J, Malta M, et al. Evaluation of the sensitivity of genotoxicity and cytotoxicity endpoints in earthworms exposed in situ to uranium mining wastes. Ecotoxicol Environ Saf. 2012;75(1):46.
Lourenço J, Pereira RO, Silva AC, Morgado JM, Carvalho FP, Oliveira JM, et al. Genotoxic endpoints in the earthworms sub-lethal assay to evaluate natural soils contaminated by metals and radionuclides. J Hazard Mater. 2011;186:788–95. Available from: http://www.sciencedirect.com/science/article/B6TGF-51JF898-4/2/3357f762ece1cad9d6c524082f058883.
Lourenço J, Silva A, Carvalho F, Oliveira J, Malta M, Mendo S, et al. Histopathological changes in the earthworm Eisenia andrei associated with the exposure to metals and radionuclides. Chem Int. 2011;85(10):1630–4. Available from: http://www.sciencedirect.com/science/article/pii/S004565351100988X.
Gagnaire B, Bado-Nilles A, Betoulle S, Amara R, Camilleri V, Cavalié I, et al. Former uranium mine-induced effects in caged roach: a multiparametric approach for the evaluation of in situ metal toxicity. Ecotoxicology. 2014;24(1):215–31. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84922004530&partnerID=40&md5=87693d63b4821bc695c0733fe67c12dd.
Geng F, Hu N, Zheng J-F, Wang C-L, Chen X, Yu J, et al. Evaluation of the toxic effect on zebrafish (Danio rerio) exposed to uranium mill tailings leaching solution. J Radioanal Nucl Chem. 2012;292(1):453–63. https://doi.org/10.1007/s10967-011-1451-x.
Driessnack MK, Dubé MG, Rozon-Ramilo LD, Jones PD, Wiramanaden CIE, Pickering IJ. The use of field-based mesocosm systems to assess the effects of uranium milling effluent on fathead minnow (Pimephales promelas) reproduction. Ecotoxicology. 2011;20(6):1209–24. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-79960306220&partnerID=40&md5=34a4d22e6c656218ebcf18f10daffd51.
Pyle GG, Swanson SM, Lehmkuhl DM. Toxicity of uranium mine-receiving waters to caged fathead minnows, Pimephales promelas. Ecotoxicol Environ Saf. 2001;48(2):202–14.
Lourenço J, Marques S, Carvalho FP, Oliveira J, Malta M, Santos M, et al. Uranium mining wastes: the use of the fish embryo acute toxicity test (FET) test to evaluate toxicity and risk of environmental discharge. Sci Total Environ. 2017;605–606:391.
Marques S, Antunes S, Nunes B, Gonçalves F, Pereira R. Antioxidant response and metal accumulation in tissues of Iberian green frogs (Pelophylax perezi) inhabiting a deactivated uranium mine. Ecotoxicology. 2011;20:1–13. https://doi.org/10.1007/s10646-011-0688-z.
Marques SM, Antunes SC, Pissarra H, Pereira ML, Gonçalves F, Pereira R. Histopathological changes and erythrocytic nuclear abnormalities in Iberian green frogs (Rana perezi Seoane) from a uranium mine pond. Aquat Toxicol. 2009;91(2):187–95. Available from: http://www.sciencedirect.com/science/article/B6T4G-4SD29J0-1/2/e98e5c48284d016d7b01e874627d9048.
Marques SM, Chaves S, Gonçalves F, Pereira R. Differential gene expression in Iberian green frogs (Pelophylax perezi) inhabiting a deactivated uranium mine. Ecotoxicol Environ Saf. 2013;87:115–9. Available from: http://www.sciencedirect.com/science/article/pii/S0147651312003624.
Marques SM, Chaves S, Gonçalves F, Pereira R. Evaluation of growth, biochemical and bioaccumulation parameters in Pelophylax perezi tadpoles, following an in-situ acute exposure to three different effluent ponds from a uranium mine. Sci Total Environ. 2013;445–446:321–8. Available from: http://www.sciencedirect.com/science/article/pii/S0048969712016373.
Marques SM, Gonçalves F, Pereira R. Effects of a uranium mine effluent in the early-life stages of Rana perezi Seoane. Sci Total Environ. 2008;402(1):29–35. Available from: http://www.sciencedirect.com/science/article/B6V78-4SP49T5-2/2/f725d35f227e9c86e7ed9f4b27544219.
Cleveland D, Hinck JE, Lankton JS. Assessment of chronic low-dose elemental and radiological exposures of biota at the Kanab North uranium mine site in the Grand Canyon watershed. Integr Environ Assess Manag. 2019;15(1):112.
Lourenço J, Pereira R, Gonçalves F, Mendo S. Metal bioaccumulation, genotoxicity and gene expression in the European wood mouse (Apodemus sylvaticus) inhabiting an abandoned uranium mining area. Sci Total Environ. 2013;443:673–80. Available from: http://www.sciencedirect.com/science/article/pii/S0048969712014179.
Abreu MM, Lopes J, Santos ES, Magalhães MCF. Ecotoxicity evaluation of an amended soil contaminated with uranium and radium using sensitive plants. J Geochemical Explor. 2014;142:112–21. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84901694794&partnerID=40&md5=1278d81e4282258a06d8953bb93d8ed2.
Černe M, Smodiš B, Štrok M, Benedik L. Radiation impact assessment on wildlife from an uranium mine area. Nucl Eng Des. 2012;246:203–9. Available from: http://www.sciencedirect.com/science/article/pii/S0029549311005498.
Evseeva T, Majstrenko T, Geras’kin S, Brown JE, Belykh E. Estimation of ionizing radiation impact on natural Vicia cracca populations inhabiting areas contaminated with uranium mill tailings and radium production wastes. Sci Total Environ. 2009;407(20):5335–43. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-68149137727&partnerID=40&md5=62d27dfe96db81a24fa7d4404afefd42.
Evseeva TI, Geras’kin SA, Shuktomova II. Genotoxicity and toxicity assay of water sampled from a radium production industry storage cell territory by means of allium-test. J Environ Radioact. 2003;68(3):235–48. Available from: http://www.sciencedirect.com/science/article/B6VB2-488NWY5-1/2/f2d891ce12b59c2d0779c65806b2620a.
Favas P, Pratas J. Uranium in soils, waters and plants of the abandoned uranium mine (central Portugal). In: 12th International Multidisciplinary Scientific GeoConference and EXPO—Modern Management of Mine Producing, Geology and Environmental Protection, SGEM 2012. 2012. p. 1023–8. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84890629266&partnerID=40&md5=756bdfe151a3708aadedc7c171cd295c.
Favas PJC, Pratas J, Varun M, D’Souza R, Paul MS. Accumulation of uranium by aquatic plants in field conditions: prospects for phytoremediation. Sci Total Environ. 2014;470–471:993–1002. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84887589392&partnerID=40&md5=ba64c1dcc67c875b03c4c0f5cd8ed2d1.
Gramss G, Voigt K-D. Forage and rangeland plants from uranium mine soils: Long-term hazard to herbivores and livestock? Environ Geochem Health. 2014;36(3):441–52. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84899137530&partnerID=40&md5=c7e6f1194420a00e2f7b3b08cb18a8cf.
Jha VN, Tripathi RM, Sethy NK, Sahoo SK, Shukla AK, Puranik VD. Bioaccumulation of 226Ra by plants growing in fresh water ecosystem around the uranium industry at Jaduguda, India. J Environ Radioact. 2010;101(9):717–22. Available from: http://www.sciencedirect.com/science/article/pii/S0265931X10001037.
Joner EJ, Munier-Lamy C, Gouget B. Bioavailability and microbial adaptation to elevated levels of uranium in an acid, organic topsoil forming on an old mine spoil. Environ Toxicol Chem. 2007;26(8):1644–8. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-34547959229&partnerID=40&md5=3a8d955c604d169b608a071ff5ee997c.
Lottermoser BG, Schnug E, Haneklaus S. Cola soft drinks for evaluating the bioaccessibility of uranium in contaminated mine soils. Sci Total Environ. 2011;409(18):3512–9. Available from: http://www.sciencedirect.com/science/article/pii/S0048969711005675
Neves MO, Figueiredo VR, Abreu MM, Transfer of U. Al and Mn in the water-soil-plant (Solanum tuberosum L.) system near a former uranium mining area (Cunha Baixa, Portugal) and implications to human health. Sci Total Environ. 2012;416:156–63. Available from: http://www.sciencedirect.com/science/article/pii/S0048969711013817.
Pereira R, Marques CR, Ferreira MJS, Neves MFJV, Caetano AL, Antunes SC, et al. Phytotoxicity and genotoxicity of soils from an abandoned uranium mine area. Appl Soil Ecol. 2009;42(3):209–20. Available from: http://www.sciencedirect.com/science/article/B6T4B-4WB378H-1/2/51f679adb63af1388ae7c40e787ba34d.
Stojanović M, Stevanović D, Iles D, Grubišić M, Milojković J. The effect of the uranium content in the tailings on some cultivated plants. Water Air Soil Pollut. 2009;200(1–4):101–8. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-67349201727&partnerID=40&md5=4dea9ddea2ffd866cf80f485b5a2cc56.
André A, Antunes SC, Gonçalves F, Pereira R. Bait-lamina assay as a tool to assess the effects of metal contamination in the feeding activity of soil invertebrates within a uranium mine area. Environmental Pollution. 2009;157(8–9):2368–77. https://doi.org/10.1016/j.envpol.2009.03.023.
Kratz W. The bait-lamina test: General aspects, applications and perspectives. Environmental Science and Pollution Research International. 1998;5(2):94–96. https://doi.org/10.1007/bf02986394.
Further Reading
Cinelli G, Tollefsen T, Bossew P, Gruber V, Bogucarskis K, De Felice L, et al. Digital version of the European Atlas of natural radiation. J Environ Radioact. 2019;196:240. https://doi.org/10.1016/j.jenvrad.2018.02.008.
Dartnell LR. Ionizing radiation and life. Astrobiology. 2011;11:551. https://doi.org/10.1089/ast.2010.0528.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Lourenço, J. et al. (2023). Environmental Radiobiology. In: Baatout, S. (eds) Radiobiology Textbook. Springer, Cham. https://doi.org/10.1007/978-3-031-18810-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-18810-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18809-1
Online ISBN: 978-3-031-18810-7
eBook Packages: MedicineMedicine (R0)