Abstract
This chapter gives an overview of molecules and mechanisms able to intervene with the biological effects of ionizing radiation (IR), either related to their clinical use in radiotherapy or in the field of radiation protection in case of an accidental exposure to radiation and/or nuclear emergencies. According to the National Cancer Institute, “radiomodifiers” can be classified into (a) radioprotectors (protect molecules and tissues from direct and indirect damage induced by IR) or (b) radiomitigators (reduce and help to repair damage), depending on whether they are administered pre- or post-IR exposure, respectively. Most of them are free radical scavengers and antioxidants (or enhancers of the antioxidant defenses), increase DNA repair mechanisms, have anti-inflammatory properties, and/or prevent cell death. On the other hand, (c) radiosensitizers directly or indirectly enhance DNA damage and ROS production, increasing IR toxicity on tumor cells, thus they are used to increase radiotherapy efficacy in cancer patients. The section “Radionuclides and methods to treat contaminated individuals” describes the medical consequences and treatment modalities of internal contamination by radionuclides. Overall, the chapter discusses the effects of most currently known radiomodifiers, their specific properties, and their mechanisms of action, by emphasizing results obtained in recent preclinical and clinical trials.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Antioxidants
- Radionuclide
- Radioprotector
- Radiomitigator
- Radiosensitizer
- Radiotherapy
- Decorporation
- Contamination
-
To understand how radioprotectors, radiomitigators, and radiosensitizers work in increasing the effect of radiotherapy (RT) through enhanced apoptosis of cancer cells while simultaneously reducing or diminishing the effect on normal cells.
-
To review the characteristics of an ideal radioprotector and to understand mechanisms by which natural or synthetic compounds can prevent or avoid the damage associated with low or high doses of ionizing radiation (IR).
-
To learn how radiomitigators can reduce the damage caused by IR and contribute to the repair/regeneration of damaged tissues even when they are administered after exposure.
-
To understand the mechanisms underlying cancer cell radioresistance and how radiosensitizers (natural or synthetic) are able to sensitize cancer cells.
-
To learn about the radiosensitization phenomenon and the associated molecular mechanisms. The combined action of these molecules with radiation offers a new strategy for enhanced IR cytotoxicity in cancer cells together with reducing normal tissue toxicity.
By the end of this chapter, readers are expected to understand the importance of applying current knowledge in the development of new synthetic or natural radioprotectors and radiosensitizers and develop an understanding of their cellular and molecular mechanisms of action.
11.1 Introduction
Radiation protection aims to reduce unnecessary radiation exposure with the intention to minimize the harmful effects of radiation on human health. With increasing use of radiation technologies and radioisotopes in medicine and industry, the risk of radiological and nuclear accidents escalates, affecting human health. Nuclear power plants and industrial accidents pose a serious threat to public health. Emergency preparedness in an event of nuclear terrorism and nuclear warfare requires the use of existing radiomodifiers and public health measures such as sheltering in place and the use of personal protective equipment (PPE). New approaches are urgently needed for protecting the persons working in a radiation field, first responders, and general population in the form of safe, effective, and easily accessible radioprotective agents.
Cellular exposure to IR induces genomic instability or mutations predisposing to carcinogenesis and/or cell death. Upon exposure, radiation induces DNA damage, lipid peroxidation, oxidation of thiol groups located in the plasma membrane and membranes of the cellular organelles, DNA strand breaks, and base alterations in cells, tissues, and organs. These changes may trigger a series of cellular responses, including activation of DNA damage repair pathways, signal transduction responses, gene transcription, and immune and proinflammatory responses. Triggering these pathways helps to recover damaged cells or eliminate the dysfunctional cells. However, they may also result in the development of tissue toxicities. The radiation research program of the National Cancer Institute (NCI) has proposed the following pharmacological classification of agents with IR response modification properties according to the timing of administration (Fig. 11.1):
A radioprotective agent/drug prevents harmful effects of radiation exposure while a radiosensitizing agent makes tumor cells more susceptible to radiation, in order to maximize the effect of radiotherapy while having less effect on normal tissues. Radiomitigators can attenuate IR damages even when they are delivered at the same time or after radiation exposition. The use of radiation-effect modulators (radioprotectors, radiomitigators, and/or radiosensitizers) can mitigate side effects and increase the efficacy of RT in cancer patients (Fig. 11.2).
11.1.1 Radioprotectors
The extent of radiation damage to living cells and organisms depends on the type of radiation (alpha (α) particles, beta (β) particles, positrons, X-rays, gamma rays (γ-rays), UV, etc.). Attempts to protect against the damaging effects of radiation were made as early as 1949. Efforts are actively being continued to search for radioprotectors suitable to be used in specific scenarios of radiation exposure. Possible applications of radioprotectors are outlined in Fig. 11.3.
Over the last few decades, many natural and synthetic compounds have been investigated for their potential as radioprotectors. Natural or synthetic radioprotectors are able to (i) reduce direct or indirect radiation damage, (ii) repair direct and indirect damage once they have occurred, and (iii) facilitate the repair of damaged cells or recover depleted cell populations [1].
It should be stressed that the majority of the compounds discussed below are currently not used in routine clinical practice and are still under preclinical or clinical evaluation.
Early development of synthetic radioprotectors focused on thiol compounds (e.g., amifostine) and their derivatives, which have been used in cancer patients, to prevent complications of RT. In addition, they have been thought to be useful in accidental radiation exposure scenarios [2]. However, the practical applicability of the majority of these synthetic compounds remained limited owing to their limited administration routes, narrow administration window for efficacy, high toxicity at high doses or at recurrent usage, and cost factors as well. Besides thiol compounds, various compounds with different chemical structures are being investigated to develop an ideal radioprotector; there is still an urgent need to identify and develop novel, nontoxic, effective, and biocompatible compounds which can adequately protect normal tissues with no sparing of the tumor cells.
An interest has been emerging in developing potential new candidate drugs from natural plants and phytochemicals. Plant products could bridge the gaps in the search for an ideal radioprotector due to its abundance, typically low toxicity, and relatively low cost.
Characteristics of an Ideal Radioprotector
An ideal radioprotective agent should (a) be efficient in providing multifaceted protection, (b) prevent direct and indirect acute or chronic effects on normal tissue, (c) be easily and comfortably administered without toxicity, (d) cause no or minimal adverse effects on the test organism, (e) have a sufficiently long time window of effectiveness after administration and also have a sufficiently long shelf life, (f) have an acceptable stability profile (both of bulk active product and formulated compound), (g) be compatible with a wide range of other drugs, (h) not protect tumors from IR, and (i) be easily accessible and economical and should not require special handling and transportation temperatures (Box 11.1).
Box 11.1: Radioprotectors
-
Radioprotectors (synthetic compounds, natural plant extracts, and phytochemical derivatives) are designed to lessen the effects of radiation-induced damage in healthy tissues.
-
Radioprotective drugs are effective when administered prior to or during radiation exposure to reduce the radiation-induced injuries/toxicities.
-
Safe, novel, nontoxic, and easily accessible radioprotective agents are needed to be developed for human health.
Underlying Mechanisms of Radioprotectors
Radioprotectors are diverse and elicit their action by various mechanisms (Fig. 11.4) such as:
-
Scavenging free radicals (either by suppressing the formation or by detoxifying radiation-induced free radical species).
-
Inducing hypoxia in cells in order to avoid synthesis of reactive oxygen species (ROS).
-
Increasing levels of antioxidant defenses such as GSH (reduced glutathione) and/or antioxidant enzymes (superoxide dismutase (SOD), glutathione peroxidase (GPx), thioreductase, catalase (CAT), etc.).
-
Triggering one or more cellular DNA damage repair pathways.
-
Impeding cell division or inhibiting apoptotic cell death.
-
Modulating redox-sensitive genes.
-
Modulating growth factors and cytokine production.
-
Controlling inflammatory response.
-
Chelating or decorporating radionuclides.
-
Promoting tissue regeneration (intestinal or hematopoietic and immunostimulant compounds), gene therapy, and/or stem cell therapy. In most cases, these molecules are administered after exposure to radiation, which is why they should be also considered radiomitigators.
The most common mechanisms of radioprotection are the scavenging of free radicals, repair of DNA damages, inhibition of apoptosis or inflammation, increase antioxidant defenses, and modulation of growth factors, cytokines, and redox genes. Thus, the management of radiation exposure may require a holistic multimechanistic approach to achieve optimal radiation protection during RT of cancer patients and in cases of nuclear accidents or emergencies [3] (Box 11.2).
Box 11.2: Possible Mechanisms of Radioprotectors
-
Radioprotectors can be screened for their effective emerging strategies, such as modulation of growth factors, cytokines, redox genes, and tissue renewal.
-
The radioprotective agents are often antioxidants, which may suppress or scavenge the radiation-induced free radicals from the cell.
-
These compounds are cofactors or can induce/stimulate antioxidants enzymes (like SOD, GPx, and) activity, which would likely lead to both prevent DNA damage and decrease in lipid peroxidation.
-
They may have the ability to enhance DNA repair, reduce the postradiation inflammatory response, or even delay cellular division allowing more time for cells to repair the DNA damage or undergo cell death.
Therapeutic Principles to Develop Radioprotectors (Portrayed in Fig. 11.5)
Antioxidant Activity
General therapeutic approaches to develop novel radioprotective agents. IR, directly or indirectly, causes damage to macromolecules such as DNA, lipids, and proteins. As a result, oxidative stress is generated, which either triggers DNA damage repair or induces p53-mediated cell disorders, such as cell cycle arrest and cell apoptosis. When the damage exceeds the cell’s ability to repair itself, the cell appears to follow the death program. The protective activities of potential radioprotectors should target such phases/mechanisms (described in blue dotted box) with the aim to shield the normal cells from harmful insults of irradiation. Inspired from/based on “General principles of developing novel radioprotective agents for nuclear emergency” from Radiation Medicine and Protection (Volume 1, Issue 3, Pages 120–126), by Du et al. 2020, Copyright Elsevier (2022)
Radioprotectors should prevent/suppress the formation of radiation-induced free radicals (most of them are produced during radiolysis with water), thereby inhibiting their reactions with biomolecules, reducing the incidence of DNA strand breaks, and preventing the occurrence of cellular malfunction (more detail in Chap. 2). Since free radicals are short-lived (approximately 10−10 s) and interact rapidly with biomolecules, it is necessary that radioprotectors are present in sufficient concentration in the cellular milieu, at the time of radiation exposure.
Molecules or compounds which increase the activity or expression of antioxidant enzymes are also considered radioprotectors. Many antioxidants have the potential to act as radioprotectors; however, not all antioxidants offer radioprotection, and this paradox may be explained by the relative activity of a compound when reacting with radiation-induced reactive species compared with those generated under H2O2 induced oxidative stress. Conventional antioxidants may not be able to scavenge this less reactive secondary species because either they do not accumulate in proximity to the secondary radicals or they may not have enough kinetic reactivity to scavenge them effectively. Thiols (e.g., amifostine), hydrophilic antioxidants (e.g., GSH), and newly developed cyclic nitroxides have adequate reactivity to effectively scavenge •OH and secondary radicals as well.
Molecule-Based Radioprotection or Molecular Radioprotection
Molecules or events that play a role late in signaling and IR-induced apoptotic pathways may act as potential targets for post-irradiation interventions.
-
ATM/ATR is activated by DNA damage and DNA replication stress; however, they often work together to signal DNA damage and trigger apoptotic cell death by upregulating proapoptotic proteins such as apoptotic protease-activating factor-1 (Apaf-1), phorbol-12-myristate-13-acetate-induced protein 1 (Noxa), and Bcl2-associated X (Bax) after IR.
-
Pifithrin (PFT)-μ (2-phenylethynesulfonamide) directly inhibits p53 binding to mitochondria as well as inactivates the antiapoptotic proteins Bcl-xL and Bcl-2 on the mitochondrial surface, thereby suppressing subsequent release of cytochrome c and apoptosis, whereas PFT-μ reversibly inhibits transcriptionally mediated p53-dependent apoptosis.
-
Signal transducer and activator of transcription 3 (STAT3) can be activated by various growth factors and protects against IR damage. The protection mediated by STAT3 is attributed to its genomic actions as a transcription factor (such as upregulating genes that are antioxidative, antiapoptotic, and proangiogenic, but suppressing anti-inflammatory and antifibrotic genes) and other nongenomic roles targeting mitochondrial function and autophagy.
-
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a well-characterized ubiquitous master transcription factor, whose activity is tightly controlled by cytoplasmic association along with its redox-sensitive transcriptional inhibitor Kelch-like ECH-associated protein 1 (Keap1). A well-known mechanism of activation of Nrf2 signaling protects cells against radiation-induced oxidative stress and also maintains cellular reduction-oxidation homeostasis. Upon oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus to activate a series of antioxidant response elements, such as GPx, SOD, CAT, and heme oxygenase-1 (HO-1), increasing total cellular antioxidant capacity (TAC), accompanied by suppressed expression of inflammatory-related genes, avoiding oxidative stress and excessive inflammatory response, which is particularly important in radioprotection.
-
Heat-shock proteins (HSPs), molecular chaperones, are induced in cells during stress conditions. Importantly, HSPs are cytoprotective and can mediate cell and tissue repair after IR-induced deleterious effects. Higher cytosolic levels of HSPs have been shown to induce radioprotective effects by interfering with apoptotic pathways.
-
Peroxisome proliferator-activated receptor-γ (PPAR-γ), ligand-activated transcription factors, is a part of the nuclear hormone receptor family. It suppresses IR-induced survival signals and DNA damage responses and enhances IR-induced apoptosis signaling in human cells.
11.1.1.1 Thiol-Containing Molecules
In the search for an effective radioprotective agent, the Walter Reed Army Research Institute (USA) screened approximately 4500 compounds from the late 1950s. Cysteine was the first agent to confer radiation protection in mice after total body irradiation (TBI) in 1949. Later, various synthetic compounds with the aminothiol group were developed and proved to be highly effective in preclinical models [4]. Among them, the most effective was WR-2721 or amifostine, a prodrug activated by alkaline phosphatase to an active sulfhydryl compound WR-1065, and at this moment, it is the only cytoprotective agent specifically approved by the FDA as a radioprotector (Fig. 11.6). The efficacy of amifostine is attributed to the free radical scavenging, along with DNA protection and repair, all of which are coupled with the initial induction of cellular hypoxia. At the cellular level, amifostine has significant effects on cell cycle progression and has antimutagenic and anticarcinogenic properties [5]. In fact, amifostine indirectly induces the expression of proteins involved in DNA repair and triggers antiapoptotic pathways [6] and expression of antioxidant enzymes. Some authors have also proposed that it may enhance protective effects by increasing nuclear accumulation and inducing transcription factors related to p53 expression [7].
Moreover, WR-1065 accumulates more rapidly in normal tissues than in malignant cells, because the concentration of membrane-bound alkaline phosphatase tends to be higher on normal cells. Moreover, the lower vascular supply and the acidic environment of many tumors reduce the rate of dephosphorylation of WR-2721 and its uptake. It thus seems to be a really unique molecule that might potentiate radiotherapy (RT) efficacy in two opposite ways at the same time [8]. The US FDA has approved the use of amifostine in preventing/reducing xerostomia (dry mouth) in head and neck cancer patients undergoing RT [5]. It has also been assayed in clinical trials to reduce mucositis, dysphagia, dermatitis, and pneumonitis during radiotherapy of head and neck cancers [9].
However, like other radioprotective aminothiols, the safety profile of amifostine has considerable limitations. Although the side effects such as nausea, vomiting, and hypotension are not life threatening, they can further aggravate the gastrointestinal syndrome. As it will be exposed latter, amifostine has been assessed in combination with other FDA-approved drugs (growth factors, cytokines, vitamin E, metformin, etc) looking for additive or synergistic radioprotective effects to prevent Acute Radiation Syndrome (ARS). Nevertheless, in most of cases none of these novel strategies completely counteracts amifostine’s toxic side effects at the doses needed to be efficacious as radioprotector [5].
Dimethyl sulfoxide (DMSO) has been shown to prevent the loss of proliferative lingual epithelial stem and progenitor cells upon irradiation by facilitating DNA DSB repair, thereby protecting against radiation-induced mucositis without tumor protection. Given its high efficacy and low toxicity, DMSO appears to be a potential treatment option to prevent radiation-induced oral mucositis [10].
GSH (L-γ-glutamyl-L-cysteinyl-glycine) plays a crucial role in the detoxification of reactive oxygen species, H2O2, lipid peroxyl radicals, peroxynitrites through enzymatic reactions, such as those catalyzed by GPxs, glutathione-S-transferases (GSTs), formaldehyde dehydrogenase, maleylacetoacetate isomerase, and glyoxalase I [11]. GSH not only protects DNA and other biomolecules against oxidative stress and radioinduced damages, it is also essential to activate DNA repairment mechanisms, to activate proliferation and to avoid radio-induced cell death [12]. In fact, the selective depletion of GSH in cancer cells has been shown to have potent radiosensitizing effects on tumor cells [13].
N-acetylcysteine (NAC) has a powerful antioxidant capacity, preserves GSH cellular levels, and prevents oxidative stress-induced apoptosis. NAC treatment (300 mg/kg, subcutaneous), starting either 4 h prior to or 2 h after radiation exposure reduced early deaths in abdominally irradiated (X-rays, 20 Gy) C57BL/6 mice, attenuating gastrointestinal syndrome [14]. More recently, preclinical studies have evidenced that NAC can prevent/reduce cardiac, ovarian, renal, and testicular radiation-induced toxicity in rats. Nevertheless, NAC and GSH cannot be used as a radioprotector in cancer patients because they also enhance antioxidant defenses in cancer cells and may increase their metastatic potential [12].
Treatment with erdosteine (a homocysteine derivative) before γ-radiation exposure ameliorated nephrotoxicity and altered kidney function in rats. It is a potent scavenger of free radicals, increases GPx and CAT activity, and reduces oxidized glutathione levels displaying almost normal concentrations with respect to the irradiated group. Moreover, IL-1, IL-6, and TNF-α circulating levels were also significantly improved thus erdosteine provide substantial protection against radiation-induced inflammatory damage as evidenced in the biochemical and histopathological samples [15].
Phosphorothioates and other aminothiols are usually administered shortly before irradiation. They have been hypothesized to act as radioprotectors by one or a combination of the following effects: scavenging radiation-induced free radicals before their reaction with biomolecules; inducing hypoxia; scavenging metals; repairing DNA damage through hydrogen donation to carbon-centered radicals; and stabilizing genome. Moreover, high doses of phosphorothioates administered to mice before radiation have demonstrated anticarcinogenic effects [4]. However, as it happens with other more powerful thiolic radioprotectors (such as amifotine), its use is limited due to undesirable side effects.
11.1.2 Cyclic Nitroxides (NRs)
NRs, like Tempol, JP4–039, XJB-5-131, TK649.030, or JRS527.084, are stable free radicals containing a nitroxyl group (-NO.) with an unpaired electron. The action of nitroxides to metabolize ROS is ascribed primarily to cyclic one- or two-electron transfer among three oxidation states: the oxoammonium cation, the nitroxide, and the hydroxylamine. Nitroxides undergo a very rapid, one-electron reaction to the corresponding hydroxylamine, which has antioxidant activity. In addition to their ability to neutralize free radicals, NR can easily diffuse through the cell membranes (and have SOD-like activity) (Fig. 11.7), prevent Fenton and Haber-Weiss reactions by oxidation of transition metal ions to a higher oxidation state, confer catalase-like activity on heme proteins, and inhibit lipid peroxidation. NRs are able to mitigate TBI-induced hematopoietic syndrome, when are administered before or as late as 72 h after radiation exposition [16].
Radioprotective properties of cyclic nitroxides include scavenger free radical capacity and SOD-like activity. Adapted from “Nitroxides as Antioxidants and Anticancer Drugs,” by Lewandowski M. and Gwozdzinski K. 2017, Licensed under CC BY 4.0
Gramicidin S-derived nitroxide (JP4–039) is an effective TBI mitigator when is delivered intravenously up to 72 h after exposure. JP4–039 treatment ameliorated head and neck radiation-induced mucositis and distant marrow suppression in mice [17]. In a comparative study with other four nitroxides, JP4-039 demonstrated the best median survival after radiation exposition [18]. The potential of this type of molecules as radioprotectors and/or mitigators has raised the interest of researchers, and nitroxidic structures has evidenced radioprotective activity. That is the case of nitronyl- nitroxide radical spin-labeled resveratrol [19].
11.1.3 Antimicrobials
Primary experiments performed in the 1960s reported that antibiotic treatment and a single transfusion of allogeneic platelets significantly reduced mortality among monkeys exposed to TBI X-irradiation. Oral administration of streptomycin, kanamycin, neomycin, or gentamicin with drinking water (4 mg/mL) for 2 weeks before supralethal TBI (28.4 Gy) prolonged mean survival in mice (8.2–8.9 days vs. 6.9 for controls) [20]. The efficacy of antibiotics and other antimicrobials (antifungal and antiviral agents) is best explained as a countermeasure for radiation-induced neutropenia and immunosuppression.
Tetracycline and ciprofloxacin protected human lymphoblastoid cells, reducing radiation-induced DNA double-strand breaks (DSB) by 33% and 21%, respectively. Their radioprotective efficacy was attributed to the activation of the Tip60 histone acetyltransferase and altered chromatin structure [21]. Tetracycline hydrochloride is a free radical scavenger, protects DNA, and increases survival of C57BL/6 mice by 20% upon a lethal radiation dose of 9 Gy [22].
Mucositis is the most common side effect of RT for head and neck cancers. Preventive measures used in clinical medicine include good oral hygiene, dental and periodontal treatment, avoidance of tobacco products and alcohol, and frequent oral rinsing with a bland mouthwash such as povidone-iodine. Nonabsorbable antibiotic lozenges and/or antifungal topical agents (i.e., bicarbonates and amphotericin B) are also recommended [23].
Minocycline prevented radiation-induced apoptosis and promoted radiation-induced autophagy in primary neurons in vitro. Minocycline also increases the counts of splenic macrophages, granulocytes, natural killer cells, and lymphocytes, and accelerates neutrophil recovery in C57BL/6 mice exposed to 1-3 Gy 60Co γ-rays. The mechanisms involved in this radioprotective effect were the suppression of cytokines that could prevent hematopoiesis (e.g. macrophage inflammatory protein-1α, TNF-α and INF-γ) and the increased production of IL-1α and β, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) [24].
Furazolidone (FZD) is an antimicrobial agent effective on both Gram+ and Gram− bacteria by interfering with bacterial oxidoreductase activity. In vitro, FZD treatment reduced unstable chromosomal aberrations (CAs) (such as acentric and dicentric chromosomes (DC)), chromosome breaks, and radiosensitivity of intestinal epithelial cells. Ma et al. [25] showed that FZD treatment significantly improved the survival of lethal dose-irradiated mice, decreased the number of micronuclei (MN), increased the number of leukocytes and immune organ indices, and reversed the apoptosis and autophagy in the small intestine, thus restoring intestinal integrity. Their experiments showed that irradiation resulted in villous shortening and crypt dilation accompanied by epithelial atrophy or slough, and even marked edema and inflammatory cell infiltration, and how FZD significantly induced damage recovery. FZD is a clinically used antibiotic with few side effects and has been proposed as an efficacious medical countermeasure (MCM). However, detailed radiation protection activity and clinical applications need to be further studied, because radioprotective efficacy of antibiotics has not yet been tested in clinical trials.
11.1.4 Phytochemicals
11.1.4.1 Plant Extracts
Considerable information from in vivo, ex vivo, and/or in vitro studies suggests that crude extracts, fractionated extracts, isolated phytoconstituents, and plant polysaccharides from various plants such as Alstonia scholaris, Centella asiatica, Hippophae rhamnoides, Ginkgo biloba, Ocimum sanctum, Panax ginseng, Podophyllum hexandrum, Amaranthus paniculatus, Emblica officinalis, Phyllanthus amarus, Piper longum, Tinospora cordifolia, Mentha arvensis, Mentha piperita, Syzygium cumini, Zingiber officinale, Ageratum conyzoides, Aegle marmelos, and Aphanamixis polystachya protect against radiation-induced lethality, lipid peroxidation, and DNA damage [26]. From these extracts, polyphenolic and nonpolyphenolic active principles and a range of secondary metabolites (e.g., carotenoids, alkaloids, sulfur compounds), already known for their anticancer properties, have also demonstrated radioprotective potential. Although many have been tested for brevity, this chapter focuses on those with the most promising results in vivo.
11.1.4.2 Polyphenolic Phytochemicals
Over the last decades, plant-derived polyphenols have been screened for their potential ability to confer radioprotection. The free radical scavenger potential and antioxidant activity of polyphenols depends, in part, on their ability to delocalize electron distribution, resulting in a more stable phenoxy group. Moreover, intercalation in DNA double helices induces stabilization and condensation of DNA structures making them less susceptible to free radicals’ attack, reducing genotoxic damage induced by IR [27]. They are capable of trapping and neutralizing lipoperoxide radicals and can chelate metal ions (i.e., iron and copper), which play an important role in the initiation of oxidative stress reactions [28, 29]. Polyphenols radioprotective efficacy is mainly attributed to its (Fig. 11.8) antioxidant and antiinflammatory properties, to their capacity to detoxify free radicals, eliciting DNA repair pathways, stimulating the recovery of hematopoietic and immune functions [28, 29].
In addition to the biochemical scavenger theory, there is also evidence of another potential mechanism by which polyphenols activate Nrf2, exhibiting cellular protection against excessive ROS production, oxidative stress, and inflammation as well. Since the chemical features of these natural organic compounds are analogous to phenolic substances, their antioxidant and antiradical/scavenging radical (such as H2O2, 2,2-diphenyl-1-picrylhydrazyl) properties may be correlated positively with the number of hydroxyl groups bonded to the aromatic ring. They can exert their protection against environmental stimuli with the aid of remarkable antioxidant power by balancing the organic oxidoreductase enzyme system, regulating antioxidant-responsive signaling pathways, and restoring mitochondrial function.
Although topically administered polyphenols may provide strong antioxidant protection, various challenges still exist and are onerous as well: (1) improving the bioavailability of polyphenols more effectively in order to promote their effectiveness is challenging; (2) if the polyphenols are extracted as the medicine or as health supplements, attention should be paid to the activity loss and degradation of polyphenols during the extraction process; (3) the effects cannot be generalized for all kinds of polyphenols, because each polyphenol has its own unique features; and (4) polyphenols have limited water solubility, and so it is important for polyphenols to be involved in rapid metabolism and also prove its chemical stability and solubility under in vivo conditions. To overcome this limitation, Obrador et al. [30] suggested a few feasible options: structural modifications of natural molecules (e.g., in the form of salts) to increase their hydro-solubility for intravenous administration or oral formulations to increase their bioavailability (e.g., cocrystals, nanoparticles, nanozymes). The promising phytochemical, pharmacodynamic, and toxicological research into the properties of polyphenols may serve as potential candidates for radioprotection in the near future.
Apigenin exhibits anticancer properties associated with its prooxidant activity, inhibiting tumor growth and inducing cell cycle arrest and apoptosis. Apigenin pretreatment displayed efficacy for radioprotection in TBI Swiss albino mice by reducing cytogenetic alterations and biochemical and hematological changes [31]. Further, when apigenin was administered intraperitoneally at a dose level equal to 15 mg/kg body, it was found to ameliorate radiation-induced gastrointestinal (GI) damages and restore intestinal crypt-villus architecture [32]. These attributes could be due to its ability to activate the endogenous antioxidants, suppress lipid peroxidation, and modulate inflammatory (NF-κB) and apoptotic signaling mediator/marker (p53, p21, Bax, caspase-3, caspase-9) expression. The in vivo efficacy of apigenin was also evidenced when it was intraperitoneally administered to mice 3 h after receiving γ-rays [33]. A significant reductions in the level of 8-hydroxy-2-deoxyguanosine (8-OH-dG), suppressed expression of NF-κB and NF-κB-regulated proinflammatory cytokines were observed, thus showing the radioprotective potential of apigenin.
Curcumin, a yellow pigment of turmeric, is naturally found in the rhizome of Curcuma longa and other Curcuma spp. It is an active immunomodulatory agent which has many scientifically proven health benefits, such as the potential to improve symptoms of anxiety, depression, arthritis, and heart health and prevent Alzheimer’s, cancer, and oxidative and inflammatory conditions. Administration of curcumin in patients undergoing RT has demonstrated a dual action: radioprotection to normal cells through its ability to reduce oxidative stress, scavenge free radicals, inhibit transcription of genes related to oxidative stress, and suppress inflammatory response, as well as radiosensitization in tumor cells [34]. Curcumin, administered before or after a single 50 Gy radiation dose, showed protective effect on radiation-induced cutaneous damage in mice by significantly decreasing mRNA expression of early-responding cytokines (IL-1, IL-6, IL-18, TNF-α, and lymphotoxin-beta) and fibrogenic cytokines [35]. Oral administration of curcumin in mouse before irradiation resulted in a significant rise in activities of GPx and SOD enzymes while declining lipid peroxidation significantly, which indicates increased antioxidant status in mouse exposed to different doses of fractionated γ-radiation [36]. These protective qualities of curcumin may be due to free radical scavenging and upregulation of Nrf2 expression.
Ellagic acid (EA), a strong natural antioxidant, has a major protecting role against different diseases associated with oxidative stress and inflammation. It also exerts antiangiogenesis effects via down regulation of vascular endothelial growth factor-2 (VEGF-2) signaling pathways in cancer. The amount and duration of EA used play a significant role in suppressing in vivo and in vitro oxidative stresses. In vitro studies [37] displayed high DPPH radical scavenging and lipid peroxidation inhibition activities of EA. It triggered the actions of antioxidant enzymes such as SOD, CAT, and GPx in V79–4 cells; reduced cell proliferation; and induced apoptosis in human osteogenic sarcoma cells as evidenced by chromosomal DNA degradation and apoptotic body appearance. When the human breast cancer cells (MCF-7) were treated with EA (10μM) and exposed with γ-radiation, the rate of apoptotic cell death in sub-G1 phase of cell cycle was high due to decreased mitochondrial membrane potential, upregulated proapoptotic Bax, and downregulated Bcl2, suggesting EA’s role in tumor toxicity to improve cancer radiotherapy [38].
Epicatechin (EC) is a common flavanol found in tea, cocoa, dark chocolates, and red wine. It has the ability to cross the blood-brain barrier and activate brain-derived neurotrophic factor pathways, suggesting its neuroprotective effects. In addition to general antioxidant activities, it aids with the modulation of metabolism of nitric oxide (NO) and other reactive nitrogen species (RNS). To evaluate the radioprotective effects of EC, Swiss albino mice were administered with EC for three consecutive days before exposing them to 5 Gy 60Co γ-irradiation [39]. EC pretreatment ameliorated γ-radiation-mediated alterations in mice, protected the liver and testis from radiation-induced oxidative stress, prevented systemic and cellular stress, and developed inflammation. It may possibly be due to the influence on the endogenous antioxidant defenses system after TBI in mice [40]. Another study [41] intended to investigate the effectiveness of EC in scavenging mitochondrial ROS and mitigating mitochondrial damage as radiation countermeasure agents by using human and mouse cells. It was observed that preradiation and postradiation treatments with EC mitigated ROS-mediated mitochondrial damage and IR-induced oxidative stress responses in mice. Also, oral administration of EC significantly enhanced the recovery of mouse hematopoietic cells from radiation injury in vivo, suggesting EC as a potentially viable countermeasure agent which is immediately effective against accidental IR exposure.
Epigallocatechin-3-gallate (EGCG) is a natural polyphenolic antioxidant found in a number of plants, predominantly in green tea and black tea and also in small amounts in fruits and nuts. It gets a lot of attention for its potential positive impact on health. It aids weight loss, reduces inflammation, and helps prevent certain chronic conditions, including heart disease, diabetes, and cancers. Pretreatment with EGCG significantly enhanced the viability of human skin cells which were irradiated with X-rays and decreased radiation-induced apoptosis [42]. It was found that EGCG suppressed IR-induced damage to mitochondria via upregulation of SOD2 and induced expression of cytoprotective molecule HO-1 in a dose-dependent manner via transcriptional activation. The therapeutic effects and mechanism of EGCG on radiation-induced intestinal injury (RIII) have not yet been determined; however, Xie et al. recently [43] investigated it both in vitro and in vivo and revealed that treatment with EGCG not only prolonged the survival time of lethally irradiated mice, but also mitigated RIII. Besides, it significantly augmented proliferation and survival of intestinal stem cells and their progeny cells in irradiated mice. Their findings demonstrated that EGCG protected against RIII by reducing the level of IR-induced ROS and DNA damage, inhibiting apoptosis and ferroptosis through activating transcription factor Nrf2-mediated signaling pathway and its downstream targets comprising antioxidant proteins Slc7A11, HO-1, and GPx4, suggesting that EGCG could be a promising medical countermeasure for the alleviation of RIII.
Genistein (GEN), an isoflavonoid compound, is commonly found in soybeans and its products. Mechanistic insights reveal its potential beneficial effects on human diseases such as cancer, by inducing apoptosis and cell cycle arrest. GEN has antiangiogenic, antimetastatic, and anti-inflammatory effects. Besides, various studies of GEN have revealed its radioprotective properties by protecting against radiation-induced DNA damage, scavenging free radicals, and altering cell cycle effects. Davis et al. [44] revealed GEN-induced radioprotection against hematopoietic- acute radiation syndrome (H-ARS) by altering the cell cycle of hematopoietic stem and progenitor cells in a murine model. The extracted GEN displayed protection against IR-induced GI injury and bone marrow toxicity by upregulating the Rassf1a and Ercc1 genes to effectively attenuate DNA damage in a TBI mouse model [45]. Moreover, Song et al. [46] showed that low concentration of GEN (1.5μM) lessened radiation-induced injuries by way of inhibiting apoptosis, alleviating chromosomal and DNA damage, downregulating GRP78, and upregulating HERP, HUS1, and hHR23A. In contrast, high concentration of GEN (20μM) demonstrated radiosensitizing characteristics in cancer cells. The role of genistein as a radiosensitizer will be further discussed in Sect. 11.4.
Naringin, a predominant flavone glycoside, is present in citrus fruits. Manna et al. [47] demonstrated that pretreatment with naringin significantly prevented γ-radiation (6Gy)-induced intracellular ROS-mediated oxidative DNA damage; inhibited radiation-induced G1/S-phase cell cycle arrest by modulating p53-dependent p21/WAF1, cyclin E, and cyclin dependent kinase 2 (CDK2) activation; and reversed the inflammation through downregulating nuclear factor kappa B (NF-kB) signaling pathways and balancing the expression of C-reactive protein, monocyte chemoattractant protein-1 (MCP-1), and iNOS2 at sites of inflammation in murine splenocytes. Besides, naringin pretreatment could effectively deter UVB-mediated DNA damage, alter apoptotic marker expression (Bax, BCl-2, caspase-9, and caspase-3), and potentially modulate NER gene (XPC, TFIIH, XPE, ERCC1, and GAPDH) expression, thereby augmenting DNA repair [48].
Naringenin is present in peppermint and citrus fruits such as oranges, grapefruit, and tangerines. It is endowed with biological effects on human health, which includes a great ability to modulate signaling pathways; efficient impairing of plasma lipid and lipoprotein accumulation; and antiatherogenic and anti-inflammatory effects. To evaluate radioprotective effects of naringenin in vivo, Swiss albino mice were orally administered 50 mg/kg body weight of naringenin prior to radiation exposure [49], and it protected mice against radiation-induced DNA, chromosomal, and membrane damage. Naringenin pretreatment increased antioxidant status and survival chances, inhibited NF-kB pathway, and downregulated radiation-induced apoptotic proteins (p53, Bax, and Bcl-2) in normal cells resulting in radioprotection at the cellular, tissue, and organism levels.
Resveratrol (RV), a natural polyphenol, is produced in several plants in response to stress, injury, and UV radiation. It is present in fruits such as grapes, strawberries, and red wine. It is known for its analgesic, antiviral, cardioprotective, neuroprotective, and antiaging actions. Different doses of RV were administered intraperitoneally to mice prior to total-body γ-irradiation (2 Gy), and it was observed that RV significantly reduced lymphocyte damage in mice caused by γ-radiation due to its ability to scavenge free radicals, restore the levels of intracellular antioxidants (GPx, SOD, CAT activity), and cause cell cycle arrest [50]. RV is also known to have a significant effect in stabilizing p53 and altering proapoptotic and antiapoptotic protein concentration [51]. Zhang et al. [52] treated with RV IR-exposed C57BL/6N mice. RV reduced radioinduced-intestinal injury (upregulating Sirt1 and acetylating p53 expression), improved intestinal morphology, decreased apoptosis of crypt cells, maintained cell regeneration, and ameliorated SOD2 expression, evidencing its radioprotective potential. The role of RV together with pterostilbene as a radiosensitizer will be further discussed in Sect. 11.4.
Pterostilbene (PT), is another stilbenoid compound, structurally similar to RV, present in blueberries, grapes, and other similar fruits. It is an active phytonutrient with many biomedical applications in cancer treatment, insulin sensitivity, cardiovascular diseases, aging, and cognition. Moreover, it has a greater bioavailability, efficacy and lower toxicity than RV [53]. Sirerol et al. [54] evidenced that pterostilbene reduced chronic UVB irradiation-induced skin damage and carcinogenesis in hairless mice through maintaining antioxidant defenses, including GSH, CAT, SOD, and GPx. Recently, a combination of natural polyphenols (PT and silibinin) with a NAD+ precursor and a TLR2/6 ligand was shown to protect mice against lethal γ-radiation, increasing long term survival up to 90% of the treated mice [55].
11.1.4.3 Nonpolyphenolic Phytochemicals
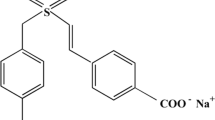
Caffeic acid/caffeic acid phenethyl ester (CAPE) is found in coffee, tea, chocolate, and colas. It has numerous pharmacological and physiological effects, including cardiovascular, respiratory, renal, and smooth muscle effects, as well as effects on mood, memory, alertness, and physical and cognitive performance. It is essentially regarded as a radiosensitizer by virtue of its inhibition of DNA repair after irradiation. The radioprotective properties of CAPE have also been shown in the bone marrow chromosomes of mice exposed to TBI (1.5 Gy 60Co γ-rays), regardless of its time of administration [56]. Caffeic acid, a known dietary antioxidant, could be used as a supplemental drug which has a dual effect: ameliorating hematopoietic stem cell (HSC) senescence-accompanied long-term BM injury in single (sublethal dose of 5 Gy) TBI and stimulating apoptotic cell death of colon cancer cells in mice [57]. Khayyo et al. [58] intraperitoneally administered CAPE prior to total-head γ-irradiation and observed that the oxidant stress parameters (total oxidant status, oxidative stress index, and lipid hydroperoxide) were significantly reduced, whereas antioxidant parameters (activity of paraoxonase, arylesterase, total GSH levels) were increased in the rat brain tissue, signifying the protective role of CAPE as an important antioxidant against ROS accumulation induced by total-head irradiation. The role of CAPE as a radiosensitizer will be further discussed in Sect. 11.4.1.7.
Sesamol is found in sesame seeds and oil. It has many biological activities and health-promoting benefits such as inducing growth arrest and apoptosis in cancer and cardiovascular cells and enhancing vascular fibrinolytic capacity, antioxidant activity, chemoprevention, antimutagenic, and antihepatotoxic activities. Naturally occurring or synthetic substances of sesamol counteract the damaging effects of oxidation by inhibiting or retarding oxidation reactions. Also, it has the potential to scavenge free radicals and therefore reduces the radiation-induced cytogenetic damage in cells. Kumar et al. [59] investigated its radioprotective potential against radiation-induced genotoxicity in hematopoietic bone marrow of whole-body 𝛾-irradiated (2Gy) mice. Preadministration of 20 mg/kg body weight sesamol reduced the frequency of radiation-induced MN, CAs, and comets (% damaged DNA streak in tail), suggesting its major role in direct scavenging of free radicals to protect bone marrow, spleen, and lymphocytes from radiation-induced cytogenetic damages and genotoxicity. Besides, intraperitoneal pretreatment of sesamol offered protection to hematopoietic and GI systems against γ-radiation-induced injury in C57BL/6 male mice by inhibiting lipid peroxidation; translocating gut bacteria to spleen, liver, and kidney; enhancing regeneration of crypt cells in GI; reducing the expression of p53 and Bax apoptotic proteins in the bone marrow, spleen, and GI; and alleviating the total antioxidant capacity in spleen and GI tissue [60]. Recently, Majdaeen et al. [61] concluded that regular oral consumption of sesamol extract is more effective than consuming it once before irradiation.
3,3′-Diindolylmethane (DIM), a small-molecule compound and a major bioactive metabolite, is formed by acid hydrolysis of indole-3-carbinol (one of the best characterized components in Cruciferae). It can inhibit invasion, angiogenesis, and proliferation and induce apoptosis in tumor cells by modulating signaling pathways involving AKT, NF-kB, and FOXO3 [62]. Chen et al. [63] investigated the radioprotective effects of DIM in normal tissues using a mouse model approach. It was indicated that treatment with DIM increased the expression of some stress-responsive genes without causing DNA damage, delayed radiation-induced cell cycle arrest, and apoptosis. Fan et al. [64] reported that administration of DIM in a multidose schedule protected rodents against lethal doses of TBI up to 13 Gy. Transcriptomic profiling showed that DIM’s mechanism of radioprotection involved regulation of responses to DNA damage and oxidative stress by inducing ataxia-telangiectasia mutated (ATM)-driven DDR-like response, enhancing radiation-induced ATM signaling and NF-κB activation, suggesting its potential role as a MCM in protecting or mitigating adverse effects of RT.
11.1.5 Vitamins
With the understanding that free radicals perpetuate a significant amount of the damage caused by IR, vitamins with antioxidant potential (A, C, and E and its derivatives) have been assayed as radioprotectors (Fig. 11.9). Vitamin A and β-carotenes (lutein, lycopene, phytofluene, phytoene, and others) reduced mortality and morbidity in mice exposed to partial or TBI. Dietary vitamin A offered protection in mice subjected to localized radiation exposure focused on the intestine (13 Gy, TBI) and the esophagus (29 Gy) [30]. A single dose of vitamin A injected intraperitoneally 2 h before 2 Gy of γ-radiation exposition, significantly reduced the number of MN in the bone marrow and the genetic damages, due to its capacity to trap free radicals [65]. Carotenoids such as crocin and crocetin (isolated from the dietary herb saffron) have antioxidant, anti-inflammatory, and antiapoptotic effects. In mice bearing pancreatic tumors, crocin significantly reduced tumor burden, radiation-induced toxicity, and hepatic damage and preserved liver morphology [66] while crocetin also reduced radiation injury in intestinal epithelial cells [67].
Lutein is a pigment classified as a carotenoid, found in plants such as green leafy vegetables (spinach, kale), fruits, corn, egg yolk, and animal fats. While this pigment plays an important role in eye health, lutein supplements also help to prevent colon and breast cancer, diabetes, and heart disease due to its powerful antioxidant potential. In vitro and in vivo lutein was found to scavenge free radicals and inhibit lipid peroxidation by increasing the activity of CAT, SOD, and glutathione reductase [68]. Lutein showed maximum survival in mice treated with 250 mg/kg body weight against a lethal dose of 10 Gy γ-radiation. Pretreatment of lutein maintained near-normal levels of hematological parameters indicating resistance/recovery from the radiation-induced damages [69]. Furthermore, lycopene has the highest antioxidant activity among carotenoids, and it reduces proinflammatory cytokine expression such as IL-8, IL-6, and NF-κB. Many preclinical studies evidence its radioprotective efficacy, in particular, if it is administered before or as soon as possible after radiation exposure [70].
Vitamin C is the reduced form of ascorbic acid (AA) and a water-soluble vitamin. The intake of vitamin C decreases the risk of getting cataracts after radiation exposition. AA has low toxicity and cost and is easily available, making it an attractive radioprotective agent. Administration of AA before γ-irradiation prevents chromosomal damage in bone marrow cells, mainly due to its scavenging activity of ROS, protecting lipid membranes and proteins from oxidative damage. It has also been reported that AA can prevent the adverse effects of TBI by increasing the antioxidant defense systems in the liver and kidney of irradiated animals [71]. Sato et al. [72] demonstrated the significant radioprotective effect of AA on the ARS in special GI syndrome, especially if it is administered before or not later than 24 h after radiation exposition.
Vitamin E is an essential fat-soluble nutrient with antioxidant, neuroprotective, and anti-inflammatory properties. Vitamin E family includes eight vitamers, four saturated (α, β, γ, and δ) called tocopherols, and four unsaturated analogs (α, β, γ, and δ) referred to as tocotrienols, which are collectively called tocols, with α-tocopherol being the most abundant in human tissues. Tocols administered subcutaneously 1 h prior to or during 15 min postirradiation improved the 30-day survival in mice, and other tocopherol derivatives, such as α-tocopherol-succinate and α-tocopherol-mono-glucoside, have also shown radioprotective effects in vivo. Moreover, subcutaneous injection of γ-tocotrienol (100–200 mg/kg) 24 h prior to 60Co γ-irradiation showed a significant protective effect in mice facing radiation doses as high as 11.5 Gy and increased mice survival rate [73].
Preclinical studies have provided evidence that tocotrienols exert radioprotection at least in part via induction of G-CSF, reducing inflammatory response suppressing the expression of TNFα, inducible NO synthase (iNOS), and IL-6 and 8, as well as inhibiting NF-κB signaling [74]. Endothelial cells activated through IR downregulate the expression of thrombomodulin (TM) and increase endothelial surface expression of adhesion molecules, which allow the attachment of immune cells, and thereby contribute to inflammation and activation of the coagulation cascade. The greater efficacy of tocotrienols is attributed to their higher antioxidant potential and its ability to inhibit HMG-CoA reductase activity (decreasing serum cholesterol levels) and increase TM expression in endothelial cells, which result in antipermeability, anti-inflammatory, and antithrombotic response in order to decrease radiation-induced vascular damages.
Nevertheless, low bioavailability of tocotrienols is an important limiting factor for their use as radioprotectants, and thus a novel water-soluble liposomal formulation of γ-tocotrienol (GT3) has been developed. GT3 has shown to increase the delivery of γ-tocotrienol in the spleen and bone marrow and offered significant radioprotection in vivo [75]. Despite these promising results, the use of vitamin E derivatives as radioprotectants must be evaluated with caution for their potential toxic effects. More recently, several laboratories have assayed the potential synergistic effect of tocols with other radioprotectants, such as pentoxifylline (PTX) (an antioxidant and anti-inflammatory xanthine derivative, approved by the FDA) which increased survival of mice subjected to 12 Gy 60Co γ-irradiation. Efficacy of PTX and α-tocopherol against radiation-induced fibrosis has been observed in animal models and clinical studies, even though the treatment started after radiation-induced fibrosis manifested clinically. Three clinical trials have evaluated if PTX enhances the radiation-protective properties of γ-tocotrienol, but the results of these studies have not yet been published [74]. At least, two randomized controlled trials provided evidence that dietary supplementation of α-tocopherol and β-carotene during radiation therapy could reduce the severity of treatment adverse effects, but these trials also evidenced that the use of high doses of antioxidants might compromise radiation treatment efficacy. Other combinations like α-tocopherol, acetate and AA showed radioprotective effects and enhanced apoptosis in irradiated cancer cells [76].
Cholecalciferol (D3) and ergocalciferol (D2) are the two forms of vitamin D provided by the food. Exposure to UV radiation of the skin also induces the endogenous synthesis of D3, and for that reason, it is also called the “sunshine vitamin.” D3 and D2 have to undergo a double hydroxylation (in the liver and in the kidney) to form the biologically active derivative, that is, calcitriol (1,25-(OH)2-vitamin D), an essential hormone in the regulation of phosphocalcic metabolism. In vitro and in vivo studies evidenced the radioprotective efficacy of calcitriol enhancing the expression of genes coding for antioxidant enzymes (such as SODs and GPxs) and metallothioneins which are ROS scavengers [77]. Jain and Micinski [78] showed a positive link between vitamin D and GSH concentrations, as well as reduction in levels of ROS and proinflammatory cytokines, which is undoubtedly beneficial in protecting against IR. Populations of radiologically contaminated areas close to the Chernobyl accident had lower vitamin D blood levels compared to those in the uncontaminated Ukrainian regions [79]. Therefore, oral supplementation with vitamin D during RT or in medical professionals chronically exposed to low IR doses should be taken into consideration also because radiation toxicity can reduce mineral bone density. Recent studies evidence that calcitriol also radiosensitizes cancer cells by activating the NADPH/ROS pathway, which can makes it a promising adjuvant in RT [80].
11.1.6 Oligoelements
Many antioxidant/defense enzymes, like SOD and metalloproteins, require trace elements as cofactors. The main oligoelements showing protective effects against radiation-induced DNA damage are zinc (Zn), manganese (Mn), and selenium (Se) [81] (Fig. 11.10). Se is an essential component of selenoenzymes such as GPx, thioredoxin reductase-1 (TR1), and ribonucleotide reductase (RNR). Se compounds and their metabolites possess a wide range of biological functions including anticancer and cytoprotection effects and modulation of hormetic genes and antioxidant enzyme activities. Exposure to radiation has been associated with a decrease in Se blood levels, and thus administration of seleno-compounds has emerged as a radioprotective strategy to reduce radiation toxicity. Mechanisms underlying the radioprotection effects include Nrf2 transcription factor activation and the consequent upregulation of the antioxidant-adaptive response in bone marrow stem cells and hematopoietic precursors [82]. 3,3-Diselenopropionic acid (at an IP dose of 2 mg/kg for 5 days prior to γ-TBI) showed radioprotection in mice by decreasing DNA damage and apoptosis [83]. Another recent formulations, poly-vinylpyrrolidone and selenocysteine-modified Bi2Se3 nanoparticles, improved the RT efficacy against tumors while exerting radioprotection in normal tissues [84]. Cancer patients, treated orally with Selenium Selenite, experienced a a lower incidence of diarrhoea compared to the placebo group [85]. Selenomethionine also reduces mucositis in patients with advanced head and neck cancer who are receiving cisplatin and radiation therapy (NCT01682031, www.clinicaltrials.gov).
Radiation-induced lung pneumonitis is a major dose-limiting side effect of thoracic RT, and the therapeutic options for its prevention are limited. 3,3′-Diselenodipropionic acid (DSePA), a synthetic organoselenium compound, shows moderate GPx-like activity and is an excellent scavenger of ROS. DSePA reduced the radiation-mediated infiltration of polymorphonuclear neutrophils (PMN) and suppressed NF-kB/IL-17/G-CSF/neutrophil axis as well as elevation in levels of proinflammatory cytokines such as IL1-β, ICAM-1 (intercellular adhesion molecule-1), E-selectin, IL-17, and TGF-β in the bronchoalveolar fluid of irradiated mice, thus ameliorating inflammatory responses. Administration of DSePA has shown a survival advantage against TBI and a significant protection to lung tissue against thoracic irradiation [86]. Wang et al. [87, 88] developed a highly efficient radioprotection strategy using a selenium-containing polymeric drug, with low toxicity and long-term bioavailability, The radioprotection activity of (VSe) and N-(2-hydroxyethyl) acrylamide shows more remarkable effects both in cell culture and mice models compared to the commercially available ebselen (organoselenium compound) and also exhibits a much longer retention time in blood (half-life ∼10 h).
Crescenti et al. [89] evaluated in vivo the tolerance induced by the combination of Se, Zn, and Mn (4 microg/mL each) plus Lachesis muta venom (O-LM) (4 ng/mL) to high doses of TBI (10 Gy, 137Cs source) IR in mice. Mice who received daily O-LM subcutaneous injections, starting 30 days before irradiation, showed a higher number of crypts, enhanced villous conservation, and lack of edema or vascular damage compared to the untreated and irradiated group. O-LM treatment also decreased vascular damage and grade of aplasia of mice bone marrow. O-LM treatment safety and efficacy were tested in a phase I clinical trial, and results indicated that it is an attractive candidate as a radioprotective agent for patients undergoing RT. Other clinical evidence indicates that Zn supplementation may act as an effective radioprotector in patients during RT. In a randomized clinical study, patients treated with Zn sulfate suffered a lower degree of mucositis compared to the placebo group [90]. Orally administered Zn-carnosine reduced oral mucositis and xerostomia in head and neck cancer patients [91].
11.1.7 Superoxide Dismutase (SOD) Mimetics and Nanoparticles
SODs are a group of metalloenzymes that catalyze the dismutation of superoxide radicals (O2˙-) to H2O2 and O2, thus are first line of defense to prevent IR damages. In the event of a radio-nuclear attack or nuclear accident, the skin damage used to be severe. A synthetic SOD/CAT mimetic (EUK-207) administered 48 h after irradiation significatively mitigated radiation dermatitis, suppressed indicators of tissue oxidative stress, and enhanced wound healing [92].
Clinical applications of SODs mimetics are limited by their structural instability deficient availability and high cost. Compared with natural enzymes, nanozymes (nanomaterials with enzyme-like activity) are more stable, are economically affordable, and can be easily modified. Due to these characteristics, nanozymes are expected to become effective substitutes for natural enzymes for medical applications. Nanozymes with SOD-like activity have been developed and proved to have a mitigating effect on diseases involving oxidative stress [93]. As shown in Fig. 11.11, after administration, they are internalized by the cells and imitate SOD2 activity in order to inhibit ROS-induced cell damage.
Patients treated with RT for cancers of the head, neck, or lung suffer damage to the mucosa of the upper aerodigestive tract. Most of them develop ulcerative forms of mucositis, and severe forms lead to inability to eat solid foods, and in some cases, they cannot drink liquids. Results of clinical trials (now in phase III, e.g., NCT03689712) demonstrated the efficacy of the SOD mimetic, avasopasem manganese (GC4419) [94].
Mn porphyrin-based SOD mimics (MnPs) are reactive with superoxide and with other reactive oxygen, nitrogen, and sulfur species (Fig. 11.12). MnPs have CAT and GPx-like activities and peroxynitrite-reducing activity [93]. MnPs administered before and continued after radiation exposure protect from γ-ray, X-ray, and proton beam irradiation damages in different animal models, and a few studies indicate that beginning treatment with MnPs after radiation exposure is also effective. In normal tissues, MnPs treatment reduces oxidative stress, NF-κB, and TGF-β signaling pathways and activates Nrf2-dependent pathways. On the contrary, MnPs administration in combination with cancer therapy results in more oxidative stress in cancer cells, which leads to the reduction of NF-κB and HIF-1α and their downstream signaling pathways (Fig. 11.12). These changes are associated with increasing apoptosis and reducing overall cancer growth [95].
BMX-001 is a porphyrin mimetic of the human mitochondrial manganese SOD, with the capacity to cross the blood-brain barrier and protect the brain against IR while acting as a tumor radiosensitizer [96]. It has been assayed as a radioprotector in different clinical trials, e.g., NCT03386500 (patients with recently diagnosed anal cancer), NCT03608020 (cancer patients with multiple brain metastases), NCT02990468 (head and neck cancer), and NCT02655601 (high-grade glioma treated with radiation therapy and temozolomide) [30].
All previous SOD mimetics suppress oxidative stress-mediated injuries, supporting the survival of the normal tissue, while promoting apoptotic processes in tumor tissues. The results from the clinical trials will provide us invaluable information on their real clinical utility as radioprotectors.
11.1.8 Hormonal and Hormonal Mimetic Radioprotectors
11.1.8.1 Catecholamine Agonist
Radiation dermatitis is a common side effect of irradiation that limits cancer RT courses. It has already been described how the induction of hypoxia limits the damage associated with radiation, and consequently the option of using vasoconstrictor substances as radioprotectors has been proposed. Topical application of adrenergic vasoconstrictors (epinephrine or norepinephrine) to rat skin before radiation exposition (17.2 Gy) confers 100% protection against radiation dermatitis [97], and similar results were obtained when phenylephrine was topically administered to prevent radiation mucositis.
Indralin is an α1-adrenoceptor agonist with vasoconstrictor effects similar to those of epinephrine. Indralin (120 mg/kg)-treated rhesus monkeys survived better (five of six) after being exposed to a lethal TBI 60Co ɣ-irradiation of 6.8 Gy, than nontreated ones (all died). Moreover, less pronounced manifestations of hemorrhagic syndrome, leukopenia, and anemia were also noted [98]. Norepinephrine and α1-adrenoceptor agonists accelerate differentiation of hematopoietic stem cells by blocking their proliferation, thus avoiding, at least, earlier manifestation of radiation injury. A common feature of the radioprotective action of biogenic amines like indralin and aminothiols is the induction of hypoxia, although their mechanisms of action differ significatively. Norepinephrine and indralin exert their effect through the neurohormonal α1-adrenoceptors, but sulfur-containing radioprotectors act directly on tissues. Nevertheless, the use of α-catecholaminergic agonists entails a high risk of increased blood pressure or pressure decompensation in hypertensive patients, which would compromise their widespread use in an accidental emergency radiation exposure.
11.1.8.2 Somatostatin Analogs
GI radiation vulnerability to a certain extent can be caused by release of potent pancreatic enzymes into the intestinal lumen after radiation exposure. Therefore, reducing intraluminal proteolytic activity may help attenuate intestinal radiation toxicity.
Somatostatin and its analogs (octreotide and pasireotide) inhibit exocrine pancreatic secretions. Octreotide reduces both acute and delayed intestinal radiation injury and diarrhea [99], as it has also been evidenced in a randomized controlled trial in patients who were undergoing radiation therapy to the pelvis (NCT00033605, www.clinicaltrials.gov). Nevertheless, some common side effects such as allergy, nausea, rash, and light-headedness may limit the routine use of octreotide. Moreover, it could also induce hypoglycemia [99] and reduce secretion of GH and IGF1, which could be highly counterproductive for the recovery of damaged tissues.
SOM230 (pasireotide) is another somatostatin analog under preclinical evaluation as a radioprotector. SOM230 reduced intestinal mucosa injury and increased mouse survival after TBI by inhibiting exocrine pancreatic secretion. Moreover, SOM230 has a 40-fold improved affinity to somatostatin receptor 5 than other somatostatin analogs, and it proved to be beneficial when administered prior to radiation exposure, and also when the treatment started up to 48 h following the exposure [100].
11.1.8.3 Melatonin
Several hormones are known to exhibit radioprotective characteristics, and melatonin, N-acetyl-5-methoxytryptamine, is one of them. It is the main secretory product of the pineal gland. Its radioprotective properties are outlined in Figs. 11.13 and 11.14.
Melatonin has the ability to neutralize both ROS and NO directly leading to the production of less/nontoxic agents or indirectly increasing the activity of antioxidant enzymes such as SODs, GPx, GR, and CAT at the same time suppressing prooxidant enzymes like xanthine oxidase (XO) [101]. In addition, melatonin induces DNA repair mechanisms, which reduce mutagenic damage and also induction of DNA DSBs, which are lethal events for the cell. Melatonin administration before irradiation with a lethal dose of 60Co γ-rays reversed the upregulation of Bax and p53 proapoptotic genes and elevated Bcl-2, which led to 100% survival and preservation of hematopoietic and GI systems in mice [102]. Inflammation and fibrosis are two degenerative phenomena that are typical pathophysiological processes following RT. Melatonin via inhibition of NF-kB, COX-2, and iNOS enzymes has the ability to reduce the release of inflammatory cytokines and chemokines. Attenuation of these enzymes’ activities is associated with reduced level of oxidative stress, infiltration of macrophages and lymphocytes, as well as suppression of fibrosis, which prevents radio-induced pneumonitis and lung fibrosis [103], and also heart [104] and brain [105] damage associated with radiation exposition.
The physiological concentrations of melatonin in the human blood are approximately much lower during the day than during the night. Therefore, it seems that radiation therapy with supplementary melatonin leads to more beneficial effects during the nighttime. Melatonin exhibits multiple neutralizing actions to reduce radio-induced damage. Together with its low toxicity and its ability to cross biological barriers, these are all significant properties to consider it for clinical RT applications as well as for mitigation of radiation injury in a possible radiation accident scenario; however, its short half-life in vivo (<1 h) and the need of high doses to achieve radioprotective effects could limit its use in practice.
At this moment, just a few clinical trials have studied the therapeutic usefulness of melatonin as a radiosensitizer. Many preclinical studies evidence that it increases ROS production, inhibits telomerase activity and DNA repair mechanisms in cancer cells, reduces tumor angiogenesis and inflammatory response associated with high doses of radiation exposure, and enhances anticancer immunity. All these oncostatic properties make melatonin an interesting molecule to increase the efficacy of RT on cancer cells [106].
11.1.9 Metformin (MTF)
Apart from being a common antidiabetic drug, MTF has demonstrated potential antioxidant, radioprotective, and anticarcinogenic properties [107]. It is a hydrogen-rich agent able to neutralize free radicals, increase GSH, and upregulate the activity of SOD and CAT enzymes [108], which all favors the antioxidant defense of normal cells. MTF has been reported to reduce the generation of ROS at the complex 1 and to prevent mitochondrial mediated apoptosis [109]. It also decreases production of the inflammatory cytokine IL-1β in response to lipopolysaccharide (LPS) in macrophages [110] and inhibits NADPH oxidase, COX-2, and inducible NO synthase, thereby limiting macrophage recruitment and inflammatory responses. MTF stimulates the DNA repair pathways of nonhomologous end joining (NHEJ) or homologous recombination (HR), and nucleotide excision repair (NER) pathways [111].
In contrast to other radioprotectors, MTF has shown modulatory effects through induction of several redox-related genes, such as the PRKAA2 gene (which encodes the AMPK), thereby suppressing redox reactions, protecting cells from accumulation of unrepaired DNA, and attenuating initiation of inflammation as well as fibrotic pathways [108] involved in lung fibrosis [104]. Cardiovascular disease is one of the most pivotal disorders after RT. The administration of MTF to γ-irradiated (5 Gy) rats significantly ameliorated the changes in cardiac disease biomarkers (LDH and CK-MB) and in NF-κB, IL-6, and TNF-α levels compared to the control group. MTF also reduced E-selectin as well as ICAM and VCAM-1. These results demonstrate that concomitant administration of MTF during RT can act as an efficient heart protector from oxidative stress, inflammatory mediators, and endothelial dysfunction-induced damages [112,113,114]. MTF does not have significant adverse effects at the normal clinical level, but it may cause severe lactic acidosis and increase the risk of hypoglycemia. In animal models, MTF has demonstrated synergistic effects with melatonin mitigating the radiation-associated damages, and both of them radiosensitize cancer cells increasing RT efficacy (this will be later exposed in Sect. 11.4).
11.2 Radiomitigators
Radiomitigators are those agents/compounds which can be administered during or shortly after radiation therapy or IR exposure to reduce the effects of radiation on normal tissues before the onset of symptoms. These compounds are capable of minimizing the toxicity even after radiation has been delivered, which differentiates them from radioprotectors (reducing direct damage caused by radiation in normal tissues). At this moment, all FDA-approved radiation countermeasures (filgrastim, a recombinant DNA form of the naturally occurring G-CSF; pegfilgrastim, a PEGylated form of the recombinant human G-CSF; sargramostim, a recombinant GM-CSF) are classified as radiomitigators [30].
In some cases, these agents have protective properties that are similar to the action of “classic” radioprotectors, even if they are administered after radiation exposure. However, these agents are most effective not only at administration shortly after irradiation, but also during the irradiation. For radiologic terrorism and space research, much of the focus of radiomitigators has been in the field of developing chemopreventives strategies in order to reduce carcinogenesis of TBI.
Characteristics of an Ideal Radiomitigator
An ideal radiomitigator should (a) offer the possibility of easy administration, (b) protect normal sensitive tissues which are associated with dose-limiting toxicity and significant reduction in quality of life, (c) be stable and easily available, and (d) have no relevant toxicity.
Mechanism of Action
Postradiation changes in normal tissue such as constant mitotic cell death and perpetually active cytokine cascades can sooner or later lead to vascular damage, tissue hypoxia, and excessive extracellular matrix deposition [115]. Radiation mitigators should aim to interrupt these cascades prior to the manifestation of toxicity or intervene to prevent the prolongation of molecular and cellular damage, and therefore reduce the expression of radiation-induced tissue toxicity or prevent the acute toxicity.
Potential radiation mitigators are described in this chapter; their possible mechanism of action is represented in Fig. 11.15.
Radiomitigators can modulate the radiation-induced molecular, cellular, and tissue toxicity/injuries even when they are administered after radiation exposure. Gene and stem cell therapies as therapeutic radiation countermeasures are being developed and may be applied in the near future to minimize the side effects of radiation exposure through tissue regeneration.
DNA Repair and Cell Recovery Process
Several studies have suggested that cellular recovery and repair processes can be enhanced by radiomitigators. Double-strand breaks (DSBs) are the most common form of DNA damage associated with IR. After DSBs are generated, a cascade of enzymatic processes, such as, HR and NHEJ (mediated by BRCA 1 and BRCA 2 enzymes), activation of p53, and induction of cell cycle arrest triggered to allow DNA repair or to induce apoptosis. The pharmacological improvement of these mechanism can contribute to mitigate IR damages. However, this must be done with care, because failure of these processes can lead to carcinogenic transformation [116]. Future studies should focus on compounds that have the potential to enhance the process of DNA repair after radiation exposure. In that sense, higher cellular pools of DNA precursors can create a radioprotective cellular environment, and drugs and chemicals that stimulate the activity of precursor-synthesizing enzymes can function as radiomitigators [117].
Anti-inflammatory Activity

IR is indirectly toxic by activating immune response, and patients undergoing radiation therapy may occasionally suffer from widespread inflammation. Various proinflammatory cytokines and chemokines are generated after radiation exposure, which particularly mediate inflammation, fibrosis, and other serious injuries in tissues and organs. Some natural products and their bioactive components can reduce the expression of these small cell signaling protein molecules and relieve the inflammation-associated side effects through their healing properties. Some phytochemicals, nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoid, and other molecules reduce inflammatory response, reducing long-term side effects like fibrosis.

Hematopoietic and Immunostimulating Activity (Regeneration)
Hematopoietic stem cell injury is the primary cause of death after accidental or intentional exposure to a moderate or high dose of IR. Hence, compounds which can stimulate the regeneration of hematopoietic cells and immune system by mechanisms such as increasing spleen colony-forming units in synergy with interleukins may have good ability to protect cells and tissues against radiation exposure. A range of endogenous compounds like IL-1, TNFα, G-CSF, stem cell factor (SCF), erythropoietin (EPO), and GM-CSF stimulate stem cell progenitors and promote hematopoietic bone marrow repopulation and thus have been further investigated as potential radiomitigators. So, agents that upregulate endogenous radioprotective factors can also act as radioprotectors.
A variety of agents (such as vitamins, TLR ligands, and β-glucan) and many natural antioxidants are classified as immunomodulators as they regulate different cytokines (cell growth factors, colony-stimulating factors, etc.) in order to facilitate patient recovery from IR-induced injuries. These regulators inhibit cell apoptosis, promote differentiation and development of gastrointestinal or hematopoietic stem cells, and have radiomitigator effects (Box 11.3).
Box 11.3: Radiomitigators
-
Radiomitigators (cytokines, growth factors, hormones, synthetic analogs, immunological adjuvants, immune regulatory peptides, etc.) accelerate the postradiation restoration of radiosensitive tissues.
-
At times, these agents can exert protective effects in a similar way to the action of “classic” radioprotectors; therefore, radiomitigators reduce oxidative stress and inflammatory damages, activate enzymes involved in repair mechanisms, and/or stimulate the replenishment of damaged tissues.
-
Compared to radioprotectors, they have the advantage of being effective despite being administered after exposure to IR. They are usually administered during the early period of postradiation and prior to the development of acute radiation syndrome (ARS).
11.2.1 Growth Factors and Cytokines
Four radiomitigators have been approved by the US Food and Drug Administration (US FDA) for the management of hematopoietic acute radiation syndrome (H-ARS). They include human recombinant G-CSF (filgrastim/Neupogen®), long-acting PEGylated form of G-CSF (filgrastim/Neulasta®), GM-CSF (sargramostim/Leukine®), and romiplostim (Nplate®), a fusion protein containing a peptide region that binds to the thrombopoietin receptor (c-Mpl) and an Fc carrier domain that increases its circulating half-life. Romiplostim was approved for use in radiation injury by the FDA based on the Animal Rule (United States Food and Drug Administration, 2021, Highlights of prescribing information. Nplate® (romiplostim) for injection, for subcutaneous use). Except for romiplostim, they have all been used to treat radiation accident victims with beneficial results [118,119,120,121].
G-CSF and PEGylated G-CSF promote the differentiation and proliferation of myeloid progenitor cells and their progeny. These effects promote neutrophil recovery after radiation-induced neutropenia. In addition, they enhance the function of neutrophils and improve survival. A World Health Organization Consultancy recommended that Neupogen and Neulasta should be administered subcutaneously, as soon as possible to individuals who have been exposed to radiation doses of >2 Gy [118]. Neulasta has the advantage that it is administered weekly, compared to daily administrations that is required for Neupogen treatment. GM-CSF increases the differentiation and proliferation of macrophage and granulocyte progenitor cells. When administered as late as 48 h after radiation exposure, GM-CSF reduced the recovery time for neutropenia and thrombocytopenia and decreased the rate of infection [5]. In addition, GM-CSF appears to exhibit an antifibrotic effect in the setting of radiation-induced lung injury (RILI) in experimental animals and humans [122, 123].
Keratinocyte growth factor (KGF), a factor that is produced by mesenchymal cells, protects and repairs epithelial tissues. Early studies suggested that KGF promotes the recovery of the oral mucosa after radiation-induced injury, improves gastrointestinal barrier function, and limits bacterial translocation and subsequent sepsis after irradiation. In clinical studies, Palifermin®, a human recombinant KGF product, reduced the incidence, duration, and severity of oral mucositis and esophagitis in patients treated with chemoradiotherapy and stimulated immune reconstitution following hematopoietic stem cell transplantation [124].
Many cell types release epidermal growth factor (EGF), which promotes the regeneration of hematopoietic stem cells in vivo. EGF was reported to have an additive effect on overall survival with G-CSF (survival of 20% for controls, 67% for EGF, 86% for EGF plus G-CSF) [125]. Fibroblast growth factor (FGF) is found in many tissues throughout the body, and its levels decrease after irradiation. FGF-P is a human recombinant derivative that is capable of activating FGF receptor-1, resulting in protection of the crypts located in the duodenum and improved survival in a GI-ARS mouse model. In addition, platelet counts were found to be higher in FGF-P-treated animals, resulting in decreased hemorrhages and cutaneous ulcerations postirradiation. It has been suggested that FGF-P has the potential to treat radiation-induced skin ulcerations and thermal burns and that it holds potential promise in the management of ischemic wounds and the promotion of tissue engineering and stem cell regeneration [125].
Interleukin-12 (IL-12) has pleiotropic effects on the innate and adaptive immune cells, including stimulation of hematopoiesis. Treatment with HemaMax® (human recombinant IL-12) restored all cell types in bone marrow when administered at 24 and 48 h post-TBI in non-human primates (HNPs) and mice, respectively. Compared to Neupogen, Neulasta, and Leukine, the single administration of HemaMax® is another advantage in the event of a mass casualty incident [126]. A novel, PEGylated IL-11 (Neumega®) is approved to treat thrombocytopenia in cancer patients, but must be injected daily, making its use inconvenient as a radiomitigator. To circumvent this problem, another mono-PEGylated IL-11 analog (BBT-059) was designed and demonstrated higher bioavailability and potency in vivo. In mouse model exposed to high TBI doses, BBT-059 leads to bone marrow cell reconstitution, leading to an accelerated recovery of platelets, erythrocytes, and neutrophils and an increase of survival higher than that obtained with treatment with the PEGylated derivatives of G-CSF and GM-CSF [125].
Erythropoietin is prescribed for the treatment of severe anemia arising from intense chemo- and/or radiation therapies. Erythropoietin and thrombopoietin (TPO) have been used for the victims of radiation exposure in the Tokaimura accident. Romiplostim (Nplate) is a synthetic TPO receptor agonist that preferentially increases platelet generation in bone marrow; contributes to mitigation of radiation-induced thrombocytopenia, anemia, and leukopenia; gives protection; and enhances regeneration of vascular endothelium. Romiplostim has recently received FDA approval to treat acutely irradiated and severely myelosuppressed adult and pediatric patients. More recently, ALXN4100TPO (a TPO receptor agonist) has been shown to stimulate megakaryopoiesis, reduce bone marrow atrophy and radiation-induced mortality in acutely irradiated mice, with the advantage of being less immunogenic than Nplate.
Combinations of hematopoietic growth factors and cytokines (G-CSF, GM-CSF, EPO, SCF, and IL-3) have already been used in the treatment of radiological accident victims, but the relative efficacy of this combined treatment is difficult to evaluate due to differing radiation sources, exposure doses, and other circumstances [127].
As explained in detail in Chap. 2, irradiation directly causes ROS overproduction, apoptosis, and/or necrosis, which activate the inflammatory response. In the short term, proinflammatory cytokines, such as IL-1, IL-6, IL-8, IL-33, TNF-α, and TGF-β, help to activate the immune response and bone marrow cellular recovery, but if it is excessive or is maintained for a long time, it can contribute to bystander/nontargeted effect (damages in tissues that have not been directly exposed), in special autoimmune diseases, fibrosis, and/or cancer initiation and progression. Therefore, the use of cytokines or growth factors capable of increasing the inflammatory response should be carefully evaluated. Moreover, the use of substances that inhibit its release or antagonize its proinflammatory effects has been shown to have mitigating effects on the damage caused by IR.
Fibrogenic cytokines like TGF-β, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) are involved in radiation-induced fibrosis. TGF-β is able to stimulate ROS and NO production by the immune system, involved in the initiation and progression of chronic oxidative damage after exposure to a high dose of radiation. It is therefore not surprising that combined inhibition of TGF-β and PDGF signaling attenuates radiation-induced pulmonary fibrosis associated with decreased pneumonitis and leading to prolonged survival. Inhibition of TGF-β also reduced radiation-induced endothelial vascular damages [113, 114]. Moreover, different phase I/II clinical trials have shown more successful RT response with the combined use of TGF-β inhibitor in metastatic breast cancer patients (LY2157299, NCT02538471). This is of special interest, because the reduction in plasma levels of TGF-β is associated with greater efficacy of RT on different types of cancer and some studies have proposed that attenuation of cytokines by genistein or quercetin ameliorates late effects of IR such as pneumonitis and fibrosis [128].
The necrosis of central nervous system (CNS) tissue is one of brain irradiation’s main risk factors. The same is true for radiation-induced increase of capillary permeability resulting from cytokine release, causing extracellular edema. A recombinant human monoclonal antibody (bevacizumab), which prevents the VEGF from binding to its receptors, reduced brain necrosis in a patient subjected to cranial irradiation and further experiments evidenced its efficacy for the management of edema associated with radiation necrosis [129].
Toll-like receptors (TLRs) play critical roles in basal resistance to IR in animals and multiple radiosensitive tissues. Several TLR ligands had been proved to exert protective roles against IR both in vitro and in vivo, downstream effectors including NF-κB (controller of inflammation, and immune response), interferon regulatory factors, and stress-activated protein kinase (Jnk), which in turn results in inhibition of apoptosis, promotion of cell proliferation, regulation of cell cycle, and secretion of cytokines. In cultured cells, TLR2, TLR5, or TLR9 agonists inhibit radiation-induced apoptosis and increase cell survival. CBLB502 (a TLR5 agonist) was reported to alleviate bone marrow and intestinal injuries in mice and rhesus monkeys. Activation of TLR4 by its agonist LPS can protect bone marrow damage and lower mice mortality after irradiation. Moreover, some kinds of TLR agonists, such as TLR2/6 coagonist CBLB613, were reported to be more effective in radiomitigation than single-TLR agonists. In conclusion, TLRs and their ligands provide novel strategies for radiation protection in nuclear accidents [28, 29, 55].
11.2.2 Cell Therapy Replacement
IR is known to be especially damaging on highly proliferative tissues. Cellular sensitivities in approximate descending order from most to least sensitive are lymphocytes, germ cells, proliferating bone marrow and intestinal epithelial cells, and epithelial stem cells. Hematopoietic syndrome (HS) is the dominant manifestation after whole-body doses of about 1–6 Gy and consists of a generalized pancytopenia, due to bone marrow stem cell depletion, although, excepting lymphocytes, mature blood cells in circulation are largely unaffected. Patients remain asymptomatic during a latent period as the impediment to hematopoiesis progresses. Risk of infection and sepsis is increased as a result of neutropenia (most prominent at 2–4 weeks) and decreased antibody production. Petechiae and bleeding result from thrombocytopenia, which develops within 3–4 weeks and can persist for months. Anemia develops more slowly because circulating erythrocytes have a longer life span. Clinical management of the HS with risk of sepsis, hemorrhage, and/or acute anemia is related to the standard clinical protocols. Therapy would certainly encompass, but not limited to, the use of antibiotics, blood, and platelet transfusion, although the latter is limited by the recipient’s own immune response. Moreover, aseptic protocols must be rigidly employed. Allogeneic hematopoietic stem cell transplantation can restore bone marrow and immune functions. In the past, stem cells were harvested directly from donor bone marrow in the operating room, but at present, peripheral blood is most used as a source of stem cells for both autologous and allogeneic grafts [130]. Bone marrow stromal cell transplantation has also been shown to renew the irradiated intestinal stem and alleviate radiation-induced GIS [131]. To date, about 50 patients with acute radiation sickness have been treated with allogeneic hematopoietic stem cell transplants, but the median survival time has not yet exceeded 1 month. Despite these results, the efficacy of bone marrow transplantation in patients undergoing RT treatments highlights the need to have mechanisms in place to implement this procedure for patients exposed during a nuclear emergency [132].
Mesenchymal stem cells (MSCs) are nonhematopoietic adult stem cells with self-renewal and multilineage differentiation potential, low immunogenicity, and capacity to restore cell loss in damaged microenvironments. Moreover, MSCs secrete different interleukins, which help in the repair and recovery of cells. Although MSCs were traditionally isolated from bone marrow, cells with MSC-like characteristics are much easier to isolate from a variety of neonatal and adult tissues, including amniotic fluid, umbilical cord, peripheral blood, fat tissue, etc. Treatment with MSCs has shown efficacy in protecting the liver against radiation-induced injury; healing irradiated skin in mice; mitigating radiation-induced GIS, HS, brain injury, and neurological complications of RT; and increasing survival in irradiated mice [102]. Moreover, MSCs have successfully been assayed against radiation-induced pulmonary fibrosis (NCT02277145) and xerostomia (NCT03876197) (www.clinicaltrials.gov) [30]. Nevertheless, despite the extensive use of MSCs in preclinical and ongoing clinical trials, there is a lack of long-term safety in humans. During recent years, it has been demonstrated in animal models that MSC-derived extracellular (EVs). EVs can exert the same therapeutic effect of MSC; therefore, EVs can be used as an alternative MSC-based therapy [133]. To cite some examples, EVs inhibit DNA damage and cell death and preserve intestinal function [134] and bone marrow activity providing long-term survival in mice exposed to TBI [135].
11.2.3 Nonsteroidal (NSAIDs) and Steroidal Anti-inflammatory Radiomitigators
Radiation initiates many enzymes such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) to produce ROS or NO, involved in the activation of inflammatory response. Most NSAIDs, such as aspirin, ibuprofen, indomethacin, diclofenac, and flurbiprofen, are able to inhibit COX-1 and COX-2 enzymes. The protective action COX inhibitors (COXi) is ascertained to the inhibition of the prostaglandin synthesis and directly or indirectly linked with the ability of NSAIDs to arrest cells in the G0 or G1 phase where cells are less sensitive to radiation damage and/or stimulation of the hematopoietic recovery [136]. Both pre- and post-irradiation treatments with sodium diclofenac reduced radiation-induced formation of DC and MN formation in human peripheral blood lymphocytes [137]. Flurbiprofen also showed radioprotection in clinical studies, e.g., delaying the onset of mucositis and reducing its severity after RT in 12 head and neck cancer patients, although the overall severity or duration of mucositis was not improved [138]. A recent meta-analysis of randomized controlled trials indicates that aspirin reduces the overall risk of recurrence and mortality of colorectal cancer and/or colorectal adenomas, which increases the interest in its possible use as a radiomitigator [24, 25]. However, nonselective COXi are known to cause undesirable side effects including GI ulcers and bleeding when taken for continued periods of time.
Increase of COX-2 gene expression is associated with decreased survival in patients receiving RT [139]. COX-2 selective inhibitors (COXi, as celecoxib, meloxicam, indomethacin) lack the GI toxicity of classical NSAIDs, and therefore, the use of COX-2i like meloxicam has been extensively assayed. Meloxicam administered either before or repeatedly after irradiation exposure has enhanced the recovery of hematopoietic progenitor cells committed to granulocyte-macrophage and erythroid development in sublethally irradiated mice [140], but the increase in survival was only observed when meloxicam was applied before lethal TBI. Sequential administration of PGE2 and meloxicam was shown to increase hematopoiesis and survival in irradiated mice [141], and meloxicam combined with ibuprofen treatment reduced bone loss after radiation exposure [142]. Radiation pneumonitis is a severe and dose-limiting side effect in lung cancer treatment. In this regard, celecoxib was tested in rats after single-dose X-ray irradiation of the right hemithorax and mediastinal region with 20 Gy revealing a dose-dependent protective effect on lipid peroxidation (MDA levels) and histopathological parameters. Celecoxib treatment induced a decrease in severe skin reactions after a high single dose of 50 Gy [136]. Moreover, celecoxib was also found to alleviate radiation-induced brain injury by maintaining the integrity of the BBB (blood-brain barrier) and reducing the inflammation in the rat brain tissues by inhibition of apoptosis in vascular endothelial cells [143]. RIVAD018 is another selective COX-2i which adds to its anti-inflammatory effects the ability to exert antioxidant activity, preventing oxidation of low-density lipoproteins, showing protection on both cellular and vascular models [144].
Several studies have also described that overexpression of COX-2 in cancer cells results in increased tumor angiogenesis, growth, and metastasis; thus, several COX-2 inhibitors have been described as radiosensitizers [136]. Celecoxib restricts neoangiogenesis, leading to a reduction in the survival of hepatocarcinoma and lung and skin cancer cells. In glioblastoma cells, the combined effect of radiation and celecoxib increased tumor cell necrosis, showing a significant reduction in tumor microvascular density compared to irradiation alone [139].
Radiation exposure of skin with high doses (>20 Gy) results in erythema, blistering, and necrosis in sequence. The necrosis generally occurs 10–30 days after exposure, although it may appear earlier in the most severe cases. The earliest administration of systemic and topical anti-inflammatory agents reduces the need for surgical excision of the affected tissue. Current therapy might make use of transplanted autologous keratinocytes combined with allogeneic stem cells. Advances in the knowledge of the radiomitigating properties of these compounds may prove to be very useful, particularly for the relatively low cost and toxicity, and specially for their analgesic effects [139].
Steroidal anti-inflammatory drugs such as dexamethasone can be administered after radiation exposure to attenuate fever and inflammatory or pain symptoms or to treat acute pathologies such as pneumonitis. Some authors reported that dexamethasone administration prior or immediately after radiation exposure reduced the risk of cardiac and other tissue fibrosis. Moreover, dexamethasone is often used to manage the inflammatory response in the brain during RT treatment of glioblastoma and other intracranial tumors. The effects of dexamethasone on patient survival however remain controversial because several clinical studies suggest that dexamethasone could potentially restrict effective RT [145].
11.2.4 Probiotics, Prebiotics, and Fecal Microbiota Transplantation (FMT)
Pathologically, acute intestinal epithelium damage is described as dilatation or destruction of crypt cells, decrease in villous height and number, ulceration, severe mucosal and submucosal inflammation, and sepsis associated with a pathogen bacterial translocation. Because of the rapid turnover of intestinal mucosa, the acute-phase symptoms (nausea, vomiting, diarrhea, abdominal pain, and acute mucositis) persist for hours to several months, while other intestinal complications such as obliterative vasculitis, mucosal ulceration, bowel wall thickening or progressive interstitial fibrosis, bowel obstruction, and fistulae formation, with or without fecal incontinence, are late events, often associated with chronic radiation exposition [146]. The reported incidence of severe late chronic radiation enteritis varies between 5 and 15% of patients treated with pelvic RT.
Probiotics, prebiotics, and FMT target intestinal microbiota by inhibiting colonization of pathogenic bacteria and restoring microbiome normobiosis. They increase production of mucin in the intestinal epithelial cells and expression of tight junction protein and occludin, thereby enhancing mucus layer function and improving survival of intestinal crypts (Fig. 11.16).
A diverse and healthy commensal intestinal microbiota plays an essential role in GI homeostasis. It has been found that postirradiation enteropathy is associated with low mucosal microbiota diversity, in particular, a decrease of Lactobacillus and Bifidobacterium spp. and an increase in the relative abundance of opportunistic pathogens. Gut microbiota dysbiosis aggravates radiation enteritis, reduces the absorbing surface of intestinal epithelial cells, weakens intestinal epithelial barrier function, promotes intestinal inflammation, and contributes to the development of mucositis, leading to a persistent diarrhea and bacteremia [147]. Correction of the microbiome by application of probiotics, prebiotics, FMT, and/or antibiotics helps to prevent and treat radiation-induced enteritis [148].
Probiotics are live microorganisms, added to aliments, that have a beneficial role in reducing pathogenic bacteria multiplying without competitors, promoting intestinal immune barrier function, and preventing translocation of harmful bacteria. Preparations containing Bifidobacterium, Lactobacillus, and Streptococcus ameliorated radiation-induced gut toxicity, reducing the incidence of diarrhea, and delaying the necessity for rescue treatment with loperamide [147]. Randomized controlled trial evidenced that live Lactobacillus acidophilus plus Bifidobacterium bifidum treatment reduced the incidence of radiation-induced diarrhea and the need for antidiarrheal medication and had a significant benefit on stool consistency [149]. The anti-inflammatory effect of probiotics has been shown in other pathologies such as ulcerative colitis and Crohn’s disease. The administration of Lactobacillus spp. decreased levels of different colonic inflammatory cytokines such as IL-6, TNF-α, or NF-κB p65 and recruitment of leukocytes to the colonic mucosa. In mice model, administration of Lactobacillus rhamnosus increased the crypts survival in radiation-induced enteritis by approximately twofold and reduced epithelial cell apoptosis, which depends on intact TLR2 and COX-2 inhibition in mesenchymal stem cells of crypt [150]. Genetically engineered species of Lactobacillus plantarum and Lactococcus lactis release SOD inducing anti-inflammatory effects and attenuation of enteritis symptoms [151]. Increased production of short-chain fatty acids is one of the most important probiotic protective effects implicated in GI and hematopoietic tissue protection and increased survival of irradiated mice [152]. Several clinical trials seem to indicate that probiotics reduce the incidence of radiotherapy-induced mucositis [148], even though results are difficult to evaluate, as they vary in the type of cancer patients recruited, radiotherapy modalities used, and type of bacteria used as probiotic [146]. In this regard, choosing the right probiotic can be crucial, and a recently published systematic review concludes that a combination of Bifidobacterium longum, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium infantis, and Saccharomyces boulardii could be a good combination of probiotics to reduce incident rates of mucositis or ameliorate its symptoms in chemo- or radiotherapy-treated patients [153].
Prebiotics offer a source of enrichment to the microbiome, and dietary interventions have demonstrated to reduce the severity of inflammatory intestinal pathologies and thus can potentially serve as a radiomitigative strategy. In fact, a clinical trial (NCT01549782) evidenced that increased consumption of certain prebiotics (fiber and plant sugars) was associated with a reduction in days of diarrhea and improved quality of life for irradiated patients [154].
FMT increased the survival rate, elevated peripheral white blood cell counts, and alleviated GI toxicities and intestinal epithelial integrity in irradiated mice [155]. Radiation-induced intestinal edema was strikingly alleviated after 8 weeks of FMT of gut microbes from healthy donors, enhancing beneficial bacteria such as Alistipes, Phascolarctobacterium, Streptococcus, and Bacteroides recovery, whereas the abundance of Faecalibacterium decreased. FMT can reduce the intestinal leakage and enhance the intestinal functions and epithelial integrity in patients with chronic radiation enteritis [156].
Researchers have long known that administering antibiotics to irradiated animals can enhance survival by avoiding opportunistic infections. As previously have been exposed, antibiotics such as fluoroquinolones and ciprofloxacin also have the advantage of reducing radiation damage to hematopoietic progenitor cells. Antibiotic cocktail and metronidazole pretreatment are beneficial to the reconstruction of gut microbes in irradiated mice. Abx pretreatment regulates macrophage polarization in the ileum and downregulates the expression of TGF-β1, thereby preventing intestinal fibrosis and ultimately improving the survival of mice with radiation-induced intestinal injury [157].
11.2.5 Angiotensin Axis-Modifying Agents
Radiation nephropathy has emerged as a significant complication in RT and is a potential sequela of radiological terrorism and radiation accidents. The use of a high-salt diet in the immediate post-irradiation period significantly decreases renal injury but is deleterious for the treatment of established disease. FDA-approved drugs that modify the renin-angiotensin system are habitually used for the treatment of hypertension and cardiac and/or renal insufficiency. ACEIs constrain angiotensin-converting enzyme (ACEs) and reduce the formation of angiotensin II (AII). Angiotensin receptor blockers (ARBs) impede the function of the angiotensin AT1 or AT2 receptors and decrease the actions of AII.
The efficacy of ACEIs and ARBs has also been long studied for their effects in radiation protection, modulation, or mitigation (Fig. 11.17). Clinical trials have evidenced the potential of ACE inhibitors to reduce radiation-induced pneumonitis and fibrosis (enalapril, NCT01754909, www.clinicaltrials.gov).
Results of a recent meta-analysis review evidenced that the use of ACEIs, but not ARBs, effectively reduced the incidence of radiation pneumonitis in most lung cancer patients. That has important clinical implications because lung cancer patients receiving thoracic radiation could take an appropriate dose of ACEIs to prevent radiation-induced pneumonitis, during or after the period of RT, which would greatly improve the quality of life and therapeutic effect. By contrast, even the most expensive ARBs were ineffective [158].
Five different ACEIs (captopril, lisinopril, enalapril, ramipril, and fosinopril), at clinically relevant doses, have been examined for efficacy as mitigators of radiation-induced nephropathy. Overall, survival in rats is higher after an 11–12 Gy TBI when treated with any of the ACEIs captopril, enalapril, or fosinopril starting 1 week postirradiation [159]. All, except fosinopril, effectively abrogated radiation nephropathy, with captopril being the most effective [160].
Captopril treatment increased survival from thoracic irradiation to 75% compared with 0% survival in vehicle-treated animals, and suppression of inflammation and senescence markers, combined with an increase of anti-inflammatory factors, was part of the mechanism involved in its therapeutic effects [161]. Captopril reduced radiation-induced cytokines EPO, G-CSF, and SAA (Non-invasive serum amyloid A) in the plasma, mitigated brain microhemorrhage at 21 days postirradiation, and increased EPO levels postirradiation if started prior to radiation exposure. These data suggest that captopril may be an ideal countermeasure to mitigate H-ARS following accidental radiation exposure [162]. A trial of captopril in patients receiving TBI demonstrated not only safety, but also efficacy against renal and pulmonary injury [163]. Moreover, prophylactic administration of captopril reduced radiation-induced hypertension and renal failure and mitigated pulmonary endothelial dysfunction and radiation-induced pneumonitis and fibrosis. The isoflavone genistein appears to work synergistically with captopril, improving the 30-day survival in mice receiving both drugs from 0 to 95% after 8.25 Gy TBI. The combination therapy reduced anemia and increased the number of circulating hematopoietic cells [164].
In murine models, administration of AT1 receptor antagonist before, during, and after fractionated whole-brain irradiation prevented or reduced cognitive impairment. It is also hypothesized that ARBs may attenuate radiation-induced brain injury by increasing the generation of anti-inflammatory peptide, angiotensin (1–7). ACEI or AT1 antagonist treatment in hypertensive patients increases blood levels of angiotensin (1–7); prevents oxidative stress, inflammatory cytokine release, and fibrotic events; and also has anticarcinogenic effects, thus having radiomitigating potential as it has been evidenced recently [165].
While other types of antihypertensive drugs are ineffective, ACEIs and AII receptor antagonist type I are effective in the mitigation of radiation damages. Moreover, some of them also exhibit antitumor effects; thus, there is a strong case for the clinical use of these agents in the treatment of radiation-induced late effects.
11.2.6 Statins
The incidence of cardiovascular disease was observed in the atomic bomb survivors, and cardiovascular disease is a known side effect of radiation therapy [166]. Statins (simvastatin, lovastatin, pravastatin, and others) are inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase, which is a rate-limiting enzyme for the synthesis of cholesterol and serves to upregulate low-density lipoprotein (LDL) synthesis. Therefore, statins are clinically used to reduce LDL levels in the blood and, consequently, to treat atherosclerosis and hypercholesterolemia. Statins also strongly induce thrombomodulin (TM) expression, which in turn forms a complex with thrombin. Thrombin-TM complexes activate protein C, which has anti-inflammatory, anticoagulant, and antioxidant properties. All these beneficial effects may help to attenuating radiation injuries [167].
Radiation exposure (5 Gy X-rays) increased cholesterol levels, and those were reduced by simvastatin treatment [168]. Simvastatin treatment (20 mg/kg/d over 2 weeks) mitigates, to a limited extent, radiation-induced enteric injury (4–8 Gy), as evidenced by improved structural integrity of the mucosa, reduced neutrophil infiltration, decreased thickening of the intestinal wall, and reduced accumulation of collagen I in jejunum and bone marrow in male C57BL/6J mice [169]. Simvastatin also prevented radiation-induced marrow adipogenesis and provided radioprotection to the niche cells [170], and attenuated radiation-induced salivary gland dysfunction in mice [171]. Pathak et al. [167] demonstrated that a single subcutaneous dose of γ-tocotrienol (GT3) rescues mice from lethal radiation doses, and combined treatment (GT3 + simvastatin) provides substantial protection against radiation-induced lethality, hematopoietic injury, and bone marrow damage compared to the single treatment.
A combination of statin and ACEI agents has shown efficacy in reducing GI toxicity in patients receiving pelvic RT [172]. Lovastatin treatment of irradiated mice (15 Gy whole-lung irradiation), starting immediately after irradiation or 8 weeks post-irradiation (three times a week), demonstrated a reduction in lung tissue lymphocytes and macrophages, decreased collagen content, prevented lung fibrosis, and improved rates of survival [173].
Pravastatin (30 mg/kg body weight given 4 h before irradiation) protected the normal intestine and lung tissues from radiation. The radiomitigating effect of pravastatin was associated with a reduction in the level of radiation-induced DNA DSB. The pravastatin-treated group showed a significantly lower apoptotic index of the lung and intestinal epithelial cells and reduced the intestinal expression of ataxia-telangiectasia mutated and γ-H2A histone family member X (H2AX) after irradiation [174]. Statins are generally well tolerated, and their effect was pronounced for delayed radiation injury and for that reason shows potential as radiomitigators.
11.2.7 Growth Hormone (GH) and Somatomedin C (IGF1) Analogs
Long et al. [175] demonstrated that chimeric protein dTMP-GH, a tandem dimer formed by thrombopoietin mimetic peptide and GH treatment, increased survival in mice exposed to 60Co γ-ray photons (6 Gy). Meanwhile, dTMP-GH treatment accelerated the recovery of bone marrow hematopoiesis, promoted skin wound closure, and mitigated ileum injury. Zinc sulfate and GH administration prevented radiation-induced dermatitis in rats [176], and increased GH/IGF1 levels also reduced radio-induced intestinal epithelial cell apoptosis preserving, in the short term, the efficacy of RT on tumors [177]. GH significantly restored follicular development and preserved fertility in female rats exposed to a single TBI of 3.2 Gy [178]. However, in oncology, GH and IGF1 reduce the effectiveness of RT and may frequently cause metastasis and cancer recurrence. Therefore, even if GH/IGF-derived radiomitigative effects are confirmed, further studies of these hormonal treatments would be necessary before translating the results to human clinical trials.
11.2.8 Molecular Hydrogen (H2)
Hydrogen can mitigate IR damages through various mechanisms [122, 123]: (a) directly neutralizes hydroxyl radicals and peroxynitrite [179]; (b) indirectly reduces oxidative stress, by upregulating the expression of different endogenous antioxidant enzymes, i.e., SOD, CAT, and GPx; and (c) shows antiapoptotic and anti-inflammatory properties [180]. H2 reduces 8-hydroxy-2′-deoxyguanosine and malondialdehyde levels and increases SOD activity and GSH levels.These findings suggest that the radioprotective effect of H2 is largely due to the inhibition of oxidative stress. In that sense, H2 has demonstrated in vitro radioprotective effects in cells especially sensitive to IR, such as intestinal epithelial cells, hematopoietic precursors, and spermatogonia [180] these protective effect of H2 are not significant when it is administered after radiation [181].
Shin et al. [182] observed that application of H (H2O) to human skin prevented UV-induced erythema and DNA damage, administered even after exposure to RI. Although a lot of in vitro and in vivo research has been done to investigate the potential use of H2 as a radiomitigator, there are scarce clinical data. Kang et al. [183] performed a placebo-controlled, randomized study to evaluate the validity of ingesting hydrogen-rich water in 49 patients with malignant liver tumors, while they were receiving RT at the same time. Patients drinking H2-rich water had considerably higher quality of life (QOL) scores, notably less appetite loss, and much fewer tasting disorders than patients drinking placebo water, and most importantly, no differences were found in tumor response to RT comparing both groups of patients [183]. In cancer patients, H2 has also shown protective effects against brain, lung, and myocardial injury associated with RT, furthermore preventing side effects like anorexia, taste disorders, or bone marrow damage without compromising the antitumor effects of the treatment [180].
The use of H2 is feasible in the clinical practice because it is stable at normal temperatures; it can be easily administered through various routes such as inhalation, drinking, injection, etc. (Fig. 11.18); it can even cross the blood-brain barrier; has a very favorable tolerability profile; and it shows great efficacy as a potential radioprotective agent [122, 123]. Although the human body does not have the enzymes necessary to produce H2, the colonic microbiota can produce about 12 L of H2 per day under physiological conditions. Many results support the idea that upregulation of H2 gas produced by intestinal bacteria could be used as a valid treatment strategy for various diseases. Since there are several methods to supply external H2, it can be easily administered with little or no adverse effects.
11.2.9 Vitamins
1-Methyl nicotinamide (MNA), a derivative of vitamin B3, significantly prolonged survival of mice irradiated at LD30/30 (6.5 Gy), LD50/30 (7.0 Gy), or LD80/30 (7.5 Gy) of γ-rays when the MNA administration started as late as 7 days post-irradiation. Another vitamin B3 derivative, 1-methyl-3-acetylpyridine, was slightly less efficient when it was administered after 7.5 Gy γ-ray exposition. These prosurvival effects might be related to the anti-inflammatory and/or antithrombotic properties of the vitamin B3 derivatives and do not seem to be mediated by stimulation of hematopoiesis. These results show that MNA may represent a prototype of a radiomitigator because it reduces the severity and/or progression of radiation-induced injuries when applied several hours or days after exposure to high doses of IR [184].
11.3 Internal Contamination by Radionuclides and Treatment
After various radiological and nuclear incidents, radioactive materials (radionuclides) may be released in the atmosphere where they could be either inhaled as gas, ingested as particulates, or absorbed through intact skin or subcutaneous tissue [185].
The medical consequences of internal contamination are determined primarily by radiation dose and radiation quality. Deleterious effects include dose-dependent deterministic (i.e., predictable) effects; stochastic (i.e., random) effects such as cancer in tissues where radionuclides are retained for prolonged times, and at a sufficiently high quantity of contamination; multiorgan failure; and death. The radiation quality or specific radionuclide(s) has (have) a characteristic emitted energy (alpha, beta, or gamma/X-ray), solubility, radioactive half-life, and biological half-life, which is determined by the time required for a compartment, defined by a body organ or tissue or part of an organ or tissue (see Fig. 11.19) to eliminate half of its radionuclide content. The particle size and chemical composition of the radioactive material impact the site of deposition within the body and route of elimination. Finally, comorbidities such as renal insufficiency, hepatic failure, and pulmonary disorders may impair pathways needed for radionuclide elimination from the body, thereby prolonging exposure [186].
Biological compartments for radionuclide intake and distribution. Reproduced from Dainiak N and Albanese J, Assessment and clinical management of internal contamination, JRP, 2022, in press, and modified from ICRP, 2015, Occupational Intakes of Radionuclides: Part 1. ICRP Publication 130. Ann. ICRP 44(2)
The internal contamination with radionuclides involves four metabolic phases:
-
1.
Intake (incorporation)
-
2.
Uptake (absorption into the circulatory system)
-
3.
Retention (deposition)
-
4.
Excretion (decorporation)
The excretion of these radionuclides by natural processes can be accelerated using decorporation therapies. This consists of enhancing the action of biological processes through chemical or biological agents, thereby facilitating radionuclide elimination. In the event that radionuclides have been incorporated internally, the objective of the therapy is to reduce the internal dose and thus the risk of biological effects on health. This can be achieved by preventing the incorporation, reducing the absorption and internal deposit of radionuclides, and also promoting their excretion. The decorporation process may have adverse side effects. Therefore, these therapies must be based on risk criteria and applied as soon as possible.
The general procedures are intended to reduce or inhibit the absorption of radionuclides from the GI system, the respiratory tract, or the skin and wounds (Fig. 11.19). Some examples of general procedures are the use of emetics, gastric lavage, laxatives, gastric alkalinization, and irrigation if there are wounds, especially in an emergency scenario. The use of specific drugs to impede the deposition of radionuclides (decorporation agents) in organs or tissues could avoid accumulation and retention of radionuclides and, obviously, is more effective if treatment is started immediately after internal contamination. Decorporation agents can reduce radionuclide absorption, entry, and deposit in organs and tissues and/or accelerate its excretion, finally minimizing the absorbed dose.
11.3.1 Blockers (Metabolic Blocking)
Blocking agents work by reducing the absorption of the radionuclide in the body, since they saturate tissues, organs, and metabolic processes using a stable isotope (identical to the nonradioactive element). Among these agents, the best known is potassium iodide (KI), used to prevent the deposit in the thyroid gland of radioactive iodine delivered to the atmosphere as a result of uncontrolled nuclear accident, which can lead to an increased risk of developing thyroid cancers, particularly in infants and young children [187]. KI prevents binding of radioiodine by three mechanisms: a) it will dilute the radioiodine circulating inside the body and available for thyroid uptake; b) it will saturate the active transport mechanism of iodine mediated by the sodium iodine symporter (NIS); and c) it will inhibit the organification of iodine, also called Wolff-Chaikoff phenomenon, a mechanism that could lead to a decrease in the synthesis of thyroid hormones and a possible hypothyroidism; but this effect is usually of short duration. This measure only protects the thyroid from radioactive iodine, not other parts of the body.
Pharmacologic thyroid blockade by oral KI (50–100 mg in adults) can substantially reduce radioiodine thyroid uptake and was one of the first and urgent protective actions recommended by the World Health Organization (WHO) (1960–1970s).
The recommendations adopted for iodine prophylaxis, in particular those regarding the administration timing, the iodine quantity to be given, and the possible side effects occurring as a result of this measure, are included in the Guide [188]. Although stable iodine is usually considered as the standard for thyroid protection against radioiodine [189], perchlorate can be considered as an alternative, provided that it is administered at equi-effective dosages (1000 mg perchlorate is as effective as 100 mg stable iodine in the aftermath of an acute radioiodine exposure). Perchlorate also protects the thyroid by competition with radioiodine at the NI-symporter site. Considering its simpler protective mechanism and potential advantages in particularly vulnerable subpopulations and its acceptable adverse effects, it seems promising for future studies to focus more closely on perchlorate as an alternative to stable iodine for thyroid protection against radioiodine [187].
11.3.2 Reduced Absorption
Absorption is defined as a movement of material that reaches the blood regardless of the mechanism. This generally applies to the entrance in the bloodstream of soluble substances and material dissociated from particles (NCRP 161).
Prussian blue, a nonabsorbable resin (approved by the FDA), acts as a laxative agent that promotes the fecal elimination of ingested radiocesium and thallium. The most effective form of this compound is its colloidal soluble form. This compound was used in the Goiânia accident extensively and successfully for the decorporation of 137Cs. Different silica-based materials have also been tested to capture various radionuclides of plutonium, americium, uranium, and thorium [190].
Natural products have also been used to reduce the absorption of radionuclides. An example is that orally administered Chlorella algae inhibited the absorption of strontium (90Sr) into the blood and enhanced its fecal elimination [191].
11.3.3 Dilution (Isotopic Dilution)
Increasing the intake of liquids, such as water, milk, and tea, or intravenous administration of isotonic saline solution, is a rapid method to increase the excretion of soluble radionuclides. This would be the case of tritium, where ingestion of sufficient liquids reduces the time of permanence in the body [192].
11.3.4 Displacement
Displacement shares the same principle as dilution and blocking therapies. However, in this specific case, an element is used that has a different atomic number. Thereby, that element will compete for internal scavenging sites, displacing the radioisotope from a receptor/target. Calcium gluconate, for example, competes with radiostrontium in bone deposition, or stable iodine, which displaces technetium-99m [193].
This method consists of increasing the natural renewal process of the release of radionuclides from organs and tissues, thus reducing deposition and improving the elimination rate by diuresis. As an example, ammonium chloride, which if administered orally, lowers the pH of the blood and increases the elimination of radiostrontium once internalized. Or the use of sodium bicarbonate increases the pH of the blood and favors the removal of uranium [194].
11.3.5 Chelators and Functional Sorbents
Chelating agents are classified as organic or inorganic agents capable of binding to metal ions and forming complex ring structures, known as “chelates.” These agents possess atoms of union or “ligands” that generally form covalent bonds and facilitate the excretion by the kidneys or other organs [186].
Some examples of this method are the one used to facilitate the elimination of plutonium complexes by the kidneys and the GI. DTPA (diethylenetriaminepentaacetic acid with calcium or zinc) is the chelator with the widest range of potential use [186]. Other chelators commonly used are dimercaptosuccinic acid, dimercaprol, and deferoxamine. Different silica-based materials (such as isomers of diphosphonic acid, hydroxypyridinone, acetamide phosphonic acid, DTPA, and glycinyl-urea) have also been tested to capture various radionuclides of plutonium, americium, uranium, and thorium [190]. Importantly, factors that can potentially affect the stability of any chelating agent must always be taken into account, i.e., (but not limited to) acidity and alkalinity, chemical properties of the agent, its selectivity, and concentration of competing metals.
Internal contamination with actinides, whether by inhalation, ingestion, or injuries, represents a serious risk to the health. Some guidelines to assist physicians or other professionals in treating workers or members of the public who may suffer internal contamination with compounds such as plutonium tributyl phosphate, plutonium nitrate, americium oxide, or nitrate can be found in [195].
The use of these types of agents is most effective when administered immediately after exposure to radiation because the radionuclides are still circulating in the body and may not yet have deposited in target organs or cells (liver and bone are examples of preferred targets).
11.3.6 Surgical Excision
This method is used for the elimination of a fixed radionuclide contaminant in the body. The surgery must be evaluated carefully, taking into account risks and benefits, and must be carried out with the support and collaboration of radiation protection staff [196].
Occasionally, debridement and excision of the wound may be necessary in order to remove the fixed contamination. It is important that a well-established evaluation is carried out by specialized personnel to support the medical decision, considering the benefits and risks of the surgical procedure. When surgical exploration is necessary, as well as the removal of tissue/foreign material, it should be performed with the help of a radiation protection professional, a radiophysicist who uses a specific probe for wounds. Once the surgical material has been removed, it should be saved for subsequent radioanalysis. There are no contraindications regarding the use of local anesthetics or systemic anesthetic agents.
11.3.7 Lung Lavage (Mechanical)
Lung lavage is an invasive medical procedure that involves the same risks as general anesthesia and is only indicated for a limited number of cases. The parameters that are taken into account are the patient’s age, clinical status, existence of comorbidities, radiotoxicity of the contaminant, and dose.
This technique will only be used after a meticulous medical and dosimetric evaluation, and in case inhaled and insoluble radioactive particles (plutonium for example) are deposited in the lungs. Other isotopes and focal accumulation are depicted in Fig. 11.20. A flexible bronchoscopy should be performed to enhance bronchoalveolar lavage [197]. This type of bronchoscopy should be performed only if the lung load is high and incorporates a large amount of insoluble inhaled particles, such as alpha particles (α).
The objective of this procedure is to avoid deterministic effects for pulmonary doses above 6 Gy-equivalents (Gy-Eq) and is stochastic when the committed doses are lower in the lung. All this within a period of 30 days and individualized for each case.
The Clinical Decision Guide (CDG) and the IAEA EPR 2018 Guide provide bases that can be used by healthcare providers to treat cases where radionuclides have been deposited internally as explained above. Both guides are useful for medical management of individuals contaminated with radionuclides as a consequence of a nuclear or radiological emergency, or due to an industrial scintigraphy accident, or in patients undergoing treatments with radionuclides.
See Annex 1: Contamination by radionuclides and MCMs table.
11.4 Radiosensitizers
Radiotherapy (RT) is a treatment that uses high doses of radiation to kill cancer cells and shrink tumors. Radiosensitizers are chemicals or pharmaceutical agents that increase the cytotoxic effect of IR on cancer cells by accelerating DNA damage and producing free radicals, suppressing the antioxidant mechanism of defenses, or inhibiting the repair of biomolecules, among others. In most cases, radiosensitizers have less effect on normal cells; however, some can also be administered after radiation exposure to treat or reduce the late side effects to healthy tissue. The effectiveness of potential radiosensitizers is measured in terms of the enhancement ratio (ER) (Box 11.4):
Box 11.4: Radiosensitizers
-
Radiosensitizers specifically target tumor cells and make them more susceptible to IR during RT.
-
These therapeutic compounds apparently enhance the radiation-induced damage to cancer cells at the molecular level and may also further limit the harmful effects of radiation on normal tissue.
-
Radiosensitizing agents promote fixation of free radicals by their electron affinity, rendering the molecules incapable of repair.
-
Their mechanism of action is comparable to the oxygen effect, as biochemical reactions of the damaged molecules preclude the repair of cellular damage.
Characteristics of an Ideal Radiosensitizer
For use as an adjunct in RT, an ideal radiosensitizer should not be harmful to healthy tissues and not interfere with other therapies, as well as should be highly efficient on tumor and hypoxic cells. It should also be economically affordable.
A radiosensitizer should be nontoxic and should produce an advantage in enlarging the therapeutic window, increasing tumor control probability, and limiting the normal tissue toxicity. This effective gain could result from a selective uptake or absorption rate or half-life of the radiosensitizing molecule in a tumor with respect to normal tissue.
Mechanism of Action
Radiosensitizers have been developed to modulate the response that occurs during or after the radiation exposure. At a molecular level, these molecules stimulate the fixing of free radicals generated by radiation. Similarly to the oxygen effect, the biochemical mechanism prevents the repair of damaged molecules. The electron affinity of the radiosensitizers captures independently existing free radicals, rendering the molecules incapable of repair [198]. Although each radiosensitizer has different rationales and limitations, they interact with specific biological targets, i.e., the signaling pathway/cascade (Table 11.1) at diverse levels (Fig. 11.21) from molecules to cells to tissues to organs to a whole organism. The core mechanisms for radiosensitization include:
-
Inhibiting repair of radiation-induced DNA damage, thereby increasing the degree of radiation-induced apoptosis and DNA damage
-
Improving cytotoxicity by disrupting the cell cycle and organelle function
-
Activating and regulating the expression of radiation-sensitive genes or silencing genes related to radioresistance
Classification
Based on the DNA damage and repair mechanisms, radiosensitizers are divided into five groups [199, 200]: (1) reduction of thiols or other intracellular radioprotective molecules; (2) radiolysis of the radiosensitizer, which results in the production of cytotoxic chemicals; (3) inhibitors of repair of biomolecules; (4) thymine analogs incorporated into DNA chain; and (5) oxygen mimetics with electrophilic properties.
With the continuous technological innovation, radiosensitizers can be classified into three categories: (1) molecular structures of small molecules (Table 11.2); (2) macromolecules with their mechanism of radiosensitivity (Table 11.3); and (3) nanomaterials (Table 11.4) with low cytotoxicity, good biocompatibility, usability, and functionality (Box 11.5).
Box 11.5: Radiation Sensitizers
-
Small molecules are classified based on radiation-induced free radicals, pseudo-substrates, and other mechanisms.
-
Macromolecules such as miRNAs, proteins, peptides, and oligonucleotides have been explored to develop radiosensitizers as they are capable of regulating radiosensitivity.
-
Promising nanotechnology methods used as radiosensitizers include well-developed nanomaterials with low toxicity, good biocompatibility, and functionalization ease.
-
Other technologies, such as molecular cloning technology, analysis of molecular structure, and bioinformatics, can speed up the development of new effective radiosensitizing drugs.
11.4.1 Nutraceutical Compounds
Several nutraceutical chemicals have attracted significant interest in recent decades due to their possible involvement in the prevention and treatment of various illnesses, as well as their favorable effects in boosting human and animal health. In particular, literature data often report their positive effect in combination with chemotherapy in cancer care. Even while intriguing results have been published on this issue at multiple cellular levels, less is known about their role as radiosensitizers. Presence of these compounds during radiation augments their effect by several mechanisms including the lethal reactions of free radicals.
Among compounds of various origins that showed radiosensitizer potential, numerous studies have revealed the important role of molecules of natural origin, when administered in combination to IR.
The use of nutraceuticals as sensitizers, in addition to being generally well tolerated, is also easily recovered and less expensive in comparison to synthesized drugs. Their administration reduces the collateral effects frequently associated with medication delivery, and in certain situations, they can help attenuate IR adverse effects through biological processes like those shown in Fig. 11.22. Indeed, in most cases, they show anti-inflammatory and antioxidant properties, which are precious arms to counter the RI side effects on healthy tissues.
Radiation therapy and nutraceutical substances may influence signaling pathways involved in migration, inflammatory response, autophagy, and formation of reactive oxygen species (ROS). Adapted from “Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy,” by [203], Licensed under CC BY 4.0
However, in most cases, they showed direct anticancer activity, as demonstrated by numerous scientific papers. The most studied natural compounds are exposed in Table 11.5 [203].
11.4.1.1 Curcumin
Curcumin, the main component in the Indian culinary spice turmeric (Curcuma longa), has been shown to have anticancer potential in several studies. The biological mechanism can be ascribed to cell signaling pathway effects, resulting in the inhibition of cell proliferation and induction of apoptosis.
Regarding its radiosensitizing properties evaluated by an in vitro approach, the inhibition of survival and proliferation has been observed on the MCF-7 breast cancer cell line. In addition, the effect of vehicolated curcumin, using solid nanoparticles, combined with X-ray radiation was tested by Minafra and coworkers [204] on the human nontumorigenic breast epithelial MCF10A cell line and the breast adenocarcinoma MCF7 and MDA-MB-231 cell lines. The vehicolated curcumin has been shown to be more effective than the free curcumin on MCF7 and MCF10A, whereas the free molecule resulted to be slightly more effective on MDA-MB-231. The dose-modifying factors (DMFs) were calculated to quantify the radiosensitizing effect, which resulted in 1.78 for MCF7 using vehicolated curcumin and 1.38 with free curcumin on MDA-MB-231 cells. Transcriptomic and metabolomics approach supported this study, revealing the double-positive effect of curcumin as an autophagy enhancer for tumor cells and antioxidant agents [204].
Antiapoptotic signals and block in G2/M cell cycle phase mediated by Bcl-2 were demonstrated in human immortalized prostate adenocarcinoma cells (PC-3) after 5 Gy irradiation combined with 2μM curcumin. Instead, an increased radiosensitivity was observed in HCT116 and HT29 human colorectal cancer cell lines treated with 25μM of curcumin and a single dose of X-ray radiation (10 Gy). Curcumin was also able to decrease COX-2 expression by the inhibition of EGFR phosphorylation both in vitro on the human head and neck squamous cell carcinoma (HNSCC) cell line and in two in vivo models of head and neck tumor.
On the human glioblastoma U87MG cell line, the viability was reduced in a dose-dependent manner by 3 Gy of X-ray combined with curcumin at a concentration range of 5–10μM, sustained by the arrest of cell cycle in phase G2/M (which is the most sensitive step to radiation) and the inhibition of two master regulators of tumor progression, the MAP kinases ERK and JNK [203].
However, curcumin is an unstable, nonbioavailable compound due to its poor absorption in the GI system. Hence, its therapeutic application is delimited by its pharmacokinetics. Despite promising preclinical studies, no double-blinded placebo-controlled clinical trial, using curcumin as a radiosensitizer, has been successful. The interaction of curcumin with RT on different cancer types has been reviewed by Verma [205]; however, there is still a lack of solid clinical evidence of radiosensitization. For instance, in vitro and in vivo studies together with clinical bioavailability data do not give evidence for a radiosensitizing effect of curcumin in the treatment of high-grade brain tumors (glioblastoma multiforme). On the other hand, there is limited data on curcumin’s radioprotective function, despite the fact that some clinical trials suggest that curcumin is beneficial for the management of radiation toxicities [205].
11.4.1.2 Resveratrol (RV) and Pterostilbene (PT)
The antineoplastic ability of RV encouraged its application also as a radiosensitizer to overcome radioresistance of many cancers.
A dose-dependent reduction in the surviving fraction of a non-small cell lung cancer (NSCLC) cell line after irradiation with 0–8 Gy of γ-rays in combination with 20μM of RV was observed along with accelerated senescence and cell death following enhanced DNA DSB induced by ROS [203].
However, an increased expression of LC3-II for autophagy response after X-ray and RV treatment (75μM) was demonstrated in SU-2 glioblastoma multiforme cell lines [206]. Also, in GBM, RV showed inhibition of the hypoxia-inducible factor HIF-1α, which is responsible for a well-known mechanism of radioresistance. Moreover, the interaction of RV with other agents as iododeoxyuridine (IUdR) was also tested and demonstrated the ability to decrease the formation of cancer colonies [203].
In the HNSCC cancer model, suppression of cell proliferation was obtained on a cell line, treated with 100μM of RV combined with 10 Gy of X-ray, also observing the inhibition of STAT3 phosphorylation, a well-known transcription factor driving inflammation and cancer progression. Even the peanut stem extract (PSE), which contains a high amount of RV, has been tested in combination with X-rays, which showed similar radiosensitization effects on radioresistant human prostate cancer cell lines. In this regard, the tumor growth of a prostate cancer xenograft mouse model was reduced with RV and/or PSE (total dose 12 Gy, 5 or 250 mg/kg, respectively) [203]. RV was also used as a pretreatment (25–150μM) to treat the human NPC CNE-1 cell line with X-ray irradiation (0–6 Gy), revealing the inhibition of the AKT phosphorylated form, a known proproliferative marker. These effects were also confirmed in NPC xenograft models, combining the RV treatment with 4 Gy for 3 days, resulting in a tumor volume reduction.
Nevertheless, a key problem is the short RV half-life and low bioavailability under in vivo conditions. In vivo, pterostilbene was proven to be beneficial in the treatment of melanoma and pancreatic cancer. This study demonstrated that PT can be helpful against melanoma by inhibiting the generation of adrenocorticotropic hormone in the brain of a mouse, which impairs the Nrf2-dependent antioxidant defenses of melanoma and pancreatic tumors. This produces tumor growth restraining and tumor sensitization to oxidative stress. In addition, PT has been shown to increase cancer cell death by the induction of lysosomal membrane permeabilization [53].
11.4.1.3 Withaferin A
Withaferin A (WA) was the first withanolide to be isolated and extracted from the plant Withania somnifera. WA-induced radiosensitization has been observed in human histiocytic lymphoma, renal carcinoma, and liver, breast, and several other types of cancer. Overall, these studies highlight the effect of combined treatment, mediated by the increase of apoptosis and production of ROS.
WA has been shown to suppress cancer cell growth by targeting the intermediate filament protein vimentin, a structural protein of the cell cytoskeleton. In light of its anticancer capability, WA was also tested in order to investigate its effect in inducing the radiosensitization of cancer cells.
WA’s effects were initially investigated in vitro and in combination with γ-irradiation on a lung fibroblast cell line, and WA was found to be well tolerated by cells and to mediate a synergistic impact with γ-rays in terms of cell death. Based on these encouraging results, WA was further tested in vivo to assess its effect as a radiosensitizing agent in several cancer models such as the spontaneous murine mammary adenocarcinoma (Ehrlich ascites carcinoma—EAC), a mouse model of fibrosarcoma, and a mouse model of melanoma. Overall, each of the studies demonstrated that the WA and γ-ray combined treatment inhibits tumor growth, increasing tumor-free survival and median survival time of animals [203].
11.4.1.4 Celastrol
Celastrol, also known as tripterine, is a triterpenoid derived from the root of the “thunder god wine” plant often found in China and utilized in traditional Chinese medicine for its anti-inflammatory qualities in a variety of conditions, including autoimmune diseases. Moreover, anticancer properties have been revealed, due to its proteasome inhibitory activity and antimetastatic ability.
A study has evaluated its radiosensitizer effect on PC-3 cells, both in vitro and in vivo. The in vitro pretreatment with celastrol before irradiation with X-rays resulted in a significant dose-dependent enhancement of IR-induced clonogenic cell killing. This effect was explained by (1) a longer gH2AX activation for a longer time in combined treated cells with respect to the only irradiated ones, thus revealing a DNA repair impairment action by celastrol, and (2) a major expression of apoptosis markers (cleaved PARP and caspase-3).
Thus, the same group tested the celastrol radiosensitizer effect on a PC-3 xenograft model. 1 mg/kg of celastrol (5 days/week for 3 weeks) was given to the mice 1 h before irradiation with a single dose of 2 Gy (5 days/week for 2 weeks). The histological analysis showed a significantly increased apoptosis and angiogenesis reduction in the combined treated tumors [203].
Similar effects have been found on the NCI-H460 human lung cancer cell line, combining celastrol with 0–4 Gy of X-rays. Indeed, the EGFR, ErbB2, and survivin irradiation markers were found to be reduced, whereas the celastrol-dependent inhibition of HSP90 was observed. Furthermore, celastrol induces a more pronounced ROS generation after irradiation, thanks to its quinone methide moiety [203].
Finally, the effect of celastrol as a radiosensitizer was evaluated on lung cancer with different approaches. Indeed, one research group has identified it as one of the most promising sensitizer candidates among 30 drugs, by an in silico study. Thus, they tested its effect in vitro on A549 and H460 cells, subjected to pretreatment with celastrol and 2–10 Gy of dose range. The encouraging results from this in vitro study were the premises for a preclinical study on a A549 xenograft mouse model. The combined treatment using 2 mg/kg/5 each day and 10 Gy of IR for 12 days produced larger intratumoral necrotic areas [203].
11.4.1.5 Ursolic Acid
Ursolic acid (UA) belongs to the family of the pentacyclic triterpenoids. It is generally obtainable from the peel of many fruits, e.g., apples, blueberries, and prunes, and also in many herbs, such as rosemary and thyme. Recently, the following therapeutic properties of UA have been described: anticancer, anti-inflammatory, and antimicrobial, and also its radiosensitization activity in models in in vitro and in vivo studies. For example, in human prostate and colon cancer cells, and in mouse melanoma cells, the UA is able to radiosensitize cells with a significant reduction in cell viability associated with an increase of typical signs of apoptosis cascade, such as cell volume reduction, nuclei fragmentation or condensation, caspase-3 activation (one of the key enzymes involved in the apoptotic pathway), increased levels of cleaved PARP (enzyme involved in DNA repair processes), DNA fragmentation, and also increased ROS generation. In melanoma mouse models, the treatment with UA and IR is able to inhibit tumor growth owing to a downregulation of Bcl-2 and survivin, two known key protein regulators of cell survival [203].
Moreover, UA can also exert a differential effect after exposure of normal or cancer cells to UV, acting as a photosensitizer for the latter and as a photoprotector for normal ones. This action was observed in human melanoma cells and in human retinal pigment epithelium control cells, where induced oxidative stress by ROS production, cell cycle arrest, and cell death induction were evaluated following UA and UV treatments. Furthermore, the UA has a significant radiosensitizing effect in human gastric adenocarcinoma cells, as evidenced by (i) a decrease in the cell survival fraction and otherwise an increase in the number of apoptotic cells (positive to the propidium iodide and annexin V apoptotic markers); (ii) the arrest of the cell cycle (in the G1 and G2/M phases); and (iii) the increase in ROS amount and a decrease of Ki-67-positive proliferating cells [207].
11.4.1.6 Zerumbone
Zerumbone (ZER) is a cyclic ketone and a sesquiterpene compound, a cytotoxic component obtained by steam distillation of the Zingiber zerumbet Smith. ZER is used in food and herbal medicine, and it also has anti-inflammatory, antiproliferative, and antitumor properties, as observed in many tumor types (including breast, pancreas, colon, lung, and skin). In addition, the radiosensitizing effects of ZER on tumors, by means of its regulatory activities on DNA DSB repair, cell cycle, and apoptotic pathways, have been highlighted too [203].
ZER was able to significantly increase radiation-induced cell death in human lung adenocarcinoma cells by inhibiting heat-shock proteins (HSP), increasing caspase 3 and PARP cleavage, and inhibiting HSP27 binding to apoptotic molecules such as PKCδ and cytochrome C [203].
In addition, the radiosensitizing effect was also observed in human glioblastoma cells. The same authors showed an IR-induced decrease of cell survival on human prostate cancer cells, associated with a reduced expression of proteins involved in the DNA damage repair pathway, such as γH2AX and ATM [203].
Moreover, in human colon-rectal cancer cells, ZER pretreatment is able to induce apoptosis and enhance radiation-induced G2/M arrest and reduction of activation of the DSB DNA repair machinery.
11.4.1.7 Caffeic Acid Phenethyl Esther
CAPE is an active component of honeybee propolis, a phenolic compound, and a structural derivative of flavonoids. It was described for its antiviral, bactericidal, anti-inflammatory, and antioxidant properties. CAPE compound is also able to change the redox state by perturbing the activation of GSH and to induce apoptosis. Furthermore, it has been shown to be more toxic to cancer cells than normal cells, as well as to amplify the action of RT in a variety of cancers.
CAPE has been shown to improve radiation-induced cell cycle arrest and death in human medulloblastoma DAOY cells. In particular, the combined treatment with CAPE and 2 Gy of IR caused an ROS enhancement production, a significant inhibition of NF-kB activity, apoptosis activation, and downregulation of cyclin B1 protein expression. In line with these data, a strong reduction of cell survival, in a concentration-dependent manner, was described in the same cell line pretreated with CAPE (0.1–10 M) for 24 h before exposure to γ-ray irradiation at various doses (0–8 Gy), associated with cell cycle progression inhibition, by arresting cells in the S phase [203].
The CAPE pretreatment radiosensitizing effect was also shown in mouse CT26 adenocarcinoma cells, using both in vitro and in vivo approaches showing decreased cell survival rate and reduced NF-kB activation. CAPE-induced decrease of survival rate was also described in breast and lung cancer cell lines. In particular, in MDA-MB-231 and T47D breast cancer cell lines, CAPE and X-ray combined treatments decreased cell growth and delayed the DNA repair process for up to 60 min after exposure [203].
11.4.1.8 Emodin
Traditional Chinese medicine uses emodin (6-methyl-1,3,8-trihydroxyanthraquinone), a natural phenolic derived from the roots and rhizomes of numerous plants (e.g., Polygonum cuspidatum and Cascara buckthorn).
Emodin is chemically similar to the mitochondrial ubiquinone named DMNQ (2,3-dimethoxy-1,4-naphthoquinone), an endogenous ROS inductor, as it is able to transfer electrons. It is also known to have antibacterial, antiviral, anti-inflammatory, and anticancer effects. The emodin’s antitumor effect has been observed in several types of cancer (leukemia, breast, colon, and lung cancer), also in combination with RT schedules, although its mechanism of action still remains unclear.
Under hypoxic conditions, emodin treatment enhanced the radiosensitivity of CNE-1 NPC human nasopharyngeal carcinoma cell line. In particular, treatment with 3.9 and 7.8 g/mL emodin 24 h before 2 Gy IR induced an increase in the apoptosis ratio and cell cycle arrest in the G2/M phase. Moreover, an increase of ROS production in tandem with a downregulation of HIF-1 levels (both mRNA and protein) was also described. These data were also confirmed by using CNE-1 xenograft models where a tumor growth delay was observed after emodin and IR combined treatments [203].
The radiosensitizing effect of emodin has also been observed in the HeLa cervical cancer cell line, where pretreatment with different concentrations of aloe emodin (AE) before X-ray irradiation (0–10 Gy) leads to decrease in the mean lethal dose (D0) in a concentration-dependent manner, as well as an enhancement in the percentage of cells in the G2/M phase and a sub-G1 peak at 24, 48, and 72 h, using 50 M and 4 Gy IR. In addition, an increased expression of cyclin B, γ-H2AX, and alkaline phosphatase (ALP) activity was also described. Similar data regarding a decrease of cell growth and viability were observed also in human HepG2 hepatocellular carcinoma cell line treated with 10 Gy of γ-irradiation and AE, under hypoxic conditions. This combined treatment leads to higher increase in both G2/M and apoptotic populations [203].
11.4.1.9 Flavopiridol
Flavopiridol is a flavone originating from the Dysoxylum binectariferum plant commonly used in Indian medicine. This molecule is able to arrest cell cycle by acting on cyclin-dependent kinases (CDKs) during the G1/S or G2/M phases, which is confirmed in several cancer cell types (chronic lymphocytic leukemia, squamous cancer, breast cancer cells). In addition, flavopiridol is able to induce the transcriptional suppression of genes involved in the proliferation pathways, to stimulate apoptosis, to inhibit angiogenesis, and to increase the chemotherapeutic effects [203].
The power of flavopiridol to affect cell radiosensitivity, in tandem with docetaxel, was described in H460 human lung carcinoma, by using both in vitro and in vivo approaches. Multiple treatments with docetaxel (10 M), γ-irradiation (0–5 Gy), and flavopiridol (120 M) are able to augment radiation effects by inducing cell cycle arrest in the G1 and G2/M phases. On the other hand, in esophageal squamous carcinoma cell lines, cell cycle arrest after irradiation was described with the decrease of cyclin D1 and retinoblastoma protein (Rb) levels. Additionally, in the SEG-1 esophageal cancer cell line, treatment with flavopiridol 24 h before γ-radiation (2–6 Gy) increased radiosensitivity compared to the control, due to inhibition of several CDKs, cell cycle redistribution in G1 and G2 phases, and induction of apoptosis [203].
The experimental evidences show that cells containing mutated p53 or overexpressed Bcl-2 are more radioresistant than wild type. However, flavopiridol increased the cytotoxic effects of radiation in cells with altered status of p53 and Bcl-2, confirming the hypothesis according to which these two pathways are targeted by radiosensitizer mechanism exerted by flavopiridol [203]. Moreover, the radiosensitizing effects of flavopiridol were evaluated in vivo on glioma xenograft models using GL261 cells. The interaction of γ-radiation (5 Gy), fractionated for 10 days, with flavopiridol (5 mg/kg) resulted in a decrease in cell proliferation, which was mainly mediated by the flavopiridol’s antiangiogenic activity, which also inhibited the HIF-1 pathway [203].
On the other hand, as described in OCA-I ovarian carcinoma cells, the radiosensitizing action of flavopiridol could be sustained also by the downregulation of Ku70 and Ku80 proteins, known to be involved in DNA repair mechanisms after radiation exposure, by the redistribution of the cell cycle with a greater accumulation of cells in the two more radiosensitive G1 and G2 phases [203].
11.4.1.10 Berberine
Berberine is an alkaloid which can be extracted from the roots of many plants like the barberry, the tree turmeric, and the California poppy. Berberine is used to treat health problems like hypercholesterolemia and type 2 diabetes mellitus.
Berberine works by inhibiting cell cycle progression, thereby exerting, in vitro, an antitumor activity in a large array of tumors, and its radiosensitizing properties were investigated on lung, esophageal, and breast cancer cells. Since berberine interferes with the expression and activity of RAD51, involved in DNA damage repair response, its radiosensitizing mechanism is based on hindering DNA damage recovery after X-ray irradiation. In vitro and in vivo experimental data has revealed the ability of berberine to inhibit HIF-1α and suppress VEGF. For example, in an in vitro nasopharyngeal carcinoma study, berberine when combined with γ-rays demonstrated a reduction of cancer cell proliferation, viability, and Sp1 decreased expression, a protein involved in tumor motility and invasion [203].
11.4.1.11 Genistein
As expected, genistein also acts as a radiosensitizing agent, if combined with γ-irradiation, as shown in vitro in cervical cancer cells, where the growth inhibition was associated with survivin downregulation, a prosurvival protein. Again, in cervical neoplasms, genistein enhanced RT effects in multiple ways: by inhibiting G2/M phase of cell cycle; by reducing the expression of two prosurvival proteins, Mcl-1 and AKT; and by triggering cell apoptosis via cytochrome c release, cleavage of caspase-3 and -8, inhibition of Bcl-2, and enhancement of Bax expression. Similar results were also shown on breast and non-small cell lung cancers, where the radiosensitizing ability was associated with the inhibition of Bcl-x, ROS production enhancement, and antioxidant molecule downregulation [203].
11.4.1.12 BP-C2
BP-C2, a lignin-derived polymer containing benzene polycarboxylic acids complexed with ammonium molybdate, is an antioxidant that promotes the release of prorepair cytokines (IL-4 and IL-10) and suppresses the release of proinflammatory cytokines (TNF-α and IL-6). Orally administered BP-C2 was found to have radioprotective and mitigative activity in H-ARS and GI-ARS [208]. Topical BP-C2 was found to have radiomitigative activity in a cutaneous radiation injury model (CRI-ARS) [209].
11.4.1.13 Sodium Selenite
Several studies have revealed the prooxidant and cytotoxic properties of sodium selenite, with respect to other selenium compounds, recognized for their antioxidant activity. In particular, the effect on natural killer (NK) cell activation is known, as well as the inhibition of the disulfide exchange on cell surface, a remodeling process, which drives cancer to uncontrolled cell division [203].
Schueller et al. [210] tested a 14-day pretreatment of C6 rat glioma cell line with selenite in the range concentration of 2–3.6 mM, before applying 0–20 Gy of γ-rays. The results showed a significant difference between the 0 mM and 3 mM survival curves applying 5 Gy (p = 0.02) and 10 Gy (p = 0.009). Also, the vehiculated sodium selenite nanoparticles (nano-Se) were tested as radiosensitizers, using the 0–3 mg/mL range concentrations pretreatment, before treating with 0–8 Gy of X-rays. In this case, the authors showed the effect on MCF7 breast cancer cells, observing that combined treatment generated a higher mortality rate of the IR or nano-Se single treatments, inducing block at the G2/M phase of cell cycle, autophagy activation, and ROS generation. Moreover, A375 melanoma cells were subjected to 4-h pretreatment with a selenium nanosystem, using 0–15 mM coated hemocompatible erythrocyte membrane combined with bevacizumab (RBCs@Se/Av) and 2–8 Gy of X-rays. This study showed a strong cell survival reduction, an increase in the sub-G1 cell proportion, apoptotic pathway activation, and ROS generation. In addition, as expected by the bevacizumab treatment, decreased VEGF and VEGF2 levels were observed as tumor angiogenesis reduction [203].
11.4.2 Corticosteroids
Corticosteroids are a group of hormones, produced by the cortex of the adrenal glands, having the characteristic steroid nucleus and derived from subsequent degradations of the cholesterol side chain. They include numerous molecules with different actions, including sex hormones. However, they are divided into glucocorticoids, such as cortisol which controls the metabolism, and mineralocorticoids, such as aldosterone which controls the concentration of electrolyte and water in the blood.
Among these molecules, many are used for their potent anti-inflammatory and immunosuppressive properties, such as corticosterone (C21H30O4) and cortisone (C21H28O5, 17-hydroxy-11-dehydrocorticosterone).
In the context of clinical RT, corticosteroids are currently used as mitigators of side effects caused by irradiation [211]. However, some researchers have highlighted the radiosensitizing effects of these molecules, used in the pretreatment phase.
Glucocorticoids (GCs), acting on stress pathways, are well known in the treatment of different types of tumors. They have a strong inhibitory action on the proinflammatory cytokine production, although their action mechanisms need deeper investigation, if used in combination with IR.
An in vitro study has investigated the role of dexamethasone (Dex), a synthetic glucocorticoid, in DNA damage response (DDR) pathway, on three astrocytoma cell lines (CT2A, APP.PS1 L.1, and APP.PS1 L.3). The results showed increased basal levels of γ-H2AX foci, keeping them higher 4 h after irradiation (IR) of the cells, while no effect was shown on the 53BP1 foci formation, compared to untreated cells. The high-level expression of γ-H2AX was reversed by ascorbic acid administration, a strong inhibitor of reactive oxygen species, showing that DEXA induces DNA damage by oxidative stress [203].
In addition, in a preclinical study on rat model, the effect of 1 mg Dex was studied alone or in combination with radioprotective molecules turpentine oil (TO), α2-macroglobulin (α2-M), or amifostine, before the administration of 6.7 Gy (LD50/30) of RI, evaluating survival and blood inflammatory markers. The results showed that Dex alone was lethal for 45% and 55% of control and irradiated rats, respectively. On the other hand, from the combination of pretreatments, it emerged that 1 mg Dex reduced the radioprotective efficacy of TO and Ami to 30% and 40%, respectively, even if, given together, TO and Ami provided 70% protection to rats receiving Dex. Instead, TO and α2-M enhanced the rate of survival from 50% to 90% and 100%, respectively [203].
11.4.3 Nanoparticles
A crucial question for cancer treatment is how to increase the therapeutic window, enhancing radiation damage in tumors, while preserving the surrounding healthy tissues. One promising strategy is the accumulation of nanoparticles composed of high-Z materials (e.g., gold, palladium, platinum, gadolinium) in the tumor cells.
High-atomic-number (Z) compounds have long been used as image contrast agents due to their high X-ray attenuation properties compared to soft tissues. The higher energy absorption of elements such as iodine and barium can enhance the contrast of the organs and tissues in which they are injected. The concentration of the compounds and the radiation doses used for diagnostic applications are usually so low that radiation effects and risks can be neglected. However, the same differential energy absorption principle can be exploited for therapeutic use. Recent developments in nano-manufacturing have provided reasonable and affordable methods to produce high-Z structures with dimensions smaller than 100 nm, which can be loaded in tumor volumes and in tumor cells. Their small size allows the nanostructures to escape the leaky vasculature system of tumor regions, providing a natural method for passive tumor accumulation. The majority of work has been concentrated on gold thanks to its biocompatibility and easy functionalization. The former means that considerable concentrations of gold nanostructures can be administered without toxicity effects, while the latter allows for the development of bespoke products able to accumulate in specific tissues/cells (active accumulation). Gold’s high atomic number (Z = 79) provides excellent radiation absorption contrast as indicated in Fig. 11.23. Other materials such as gadolinium (Z = 64) and more recently superparamagnetic iron oxide nanoparticles (SPION, ZFe = 26) have also been suggested and explored.
Early work by Hainfeld [212] demonstrated the potential of high-Z nanostructures to enhance the effect of radiation and improve tumor control in mice treated with kilovoltage X-rays minutes after injection of gold nanoparticles (GNP). In vitro work using a wide range of cell lines and radiation qualities confirmed that the presence of GNP can enhance the effect of radiation by 10–100% [213].
Interestingly, the radiation sensitization observed in in vitro and in vivo work is often significantly greater than that predicted from simple macroscopic dose models. Furthermore, the size, shape, and surface coating of the nanoparticle as well as the radiation quality and cell line have been shown to affect the radiation response observed. The discrepancy between dosimetric and experimental results regarding the radiosensitization effect emphasizes that complex physical, chemical, and biological interactions are involved in high-Z nanoparticle-mediated radiosensitization, which still need to be fully elucidated in order to extrapolate the nanoparticle radiosensitization concept to patient cancer RT. Physical, chemical, and biological mechanisms of nanoparticle radiosensitization are shown in Fig. 11.24.
11.4.3.1 Physical Radiosensitization
The physical processes driving the enhancement in radiation effectiveness in the presence of nanoparticles strongly depend on the radiation quality used. For medium-energy X-rays (<300 kVp), the radiation-nanoparticle interaction is dominated by the photoelectric effect, especially for photons with energies around the L- and K-shell excitation edges. This causes the emission of inner shell electrons from the high-Z material, resulting in a cascade of low-energy Auger electrons (10–20 electrons per interaction). The majority of these low-energy electrons are reabsorbed by the nanoparticle. Only a few Auger electrons escape the nanoparticle, releasing their energy in a few 10 s of nm around the nanoparticle [214]. As a result, the presence of high-Z nanoparticles increases the dose absorbed by the volume in which the nanoparticles are loaded, and it changes the spatial distribution of the ionizations concentrating the energy deposition around the nanoparticles.
At higher photon energies (such as MV which is routinely used for cancer treatment), the dominant process in the interaction between radiation and nanoparticles is Compton scattering. At these energies, the Compton cross sections for high-Z materials are similar to that of soft tissues, and therefore no differences should be expected from the presence of high-Z nanoparticles. However, MV photon beams usually contain a considerable fraction of lower keV photons and electrons, which can also increase due to scattering processes (e.g., at 10 cm depth, the fraction of <150 keV photons from a flattening filter-free beam is between 13% and 20%). MV photon beams can therefore produce a considerable amount of Auger electrons from interaction with high-Z nanoparticles and alter both the macroscopic and the microscopic dose absorbed.
With protons and charged particles, the probability of interaction between the primary beam particle and the nanoparticles is considerably smaller than that for photon beams due to the lower number of primary tracks required to deliver a given dose. The dominant interaction process is the production of secondary electrons from the nanoparticle via small-angle scattering, which is proportional to the density and therefore higher for high-Z nanoparticles than for soft tissues. In contrast to the photon interactions, these secondary electrons are produced from the outer atomic orbital of the nanoparticle and contribute only a few percentages of the additional absorbed dose.
In all cases, the presence of high-Z nanoparticles causes an increase in the overall macroscopic dose absorbed and high localized energy deposition spikes. The former was initially thought to be the main cause of the observed radiobiological enhancement. However, calculations clearly show that the additional absorbed macroscopic dose by itself is not enough to explain the increased effectiveness observed in experimental studies. The localized energy deposition spikes, caused by the presence of nanoparticles, are similar to those produced by the traversal of charged particles, and their impact on the biological effects can therefore be estimated using radiobiological models based on microscopic dose distributions. The local effect model (LEM) is widely used for charged particles and has been employed to demonstrate that higher radiosensitization enhancement ratios can be expected when local energy distribution is taken into account. Radiosensitization prediction by the LEM-based model strongly depends on the location of the nanoparticles and the radiation quality, with the closer the nanoparticles are to the critical structures of the cell (e.g., DNA), the larger the effect.
11.4.3.2 Chemical Radiosensitization
A key property of nanoparticles is the increased interaction with the surrounding environment (due to the high surface-to-volume ratio). The intracellular nanoparticle concentration is an important determinant for radiation sensitization. Numerous studies have investigated the importance of the nanoparticle size on cellular uptake, and an optimum diameter appears to range between 10 nm and 50 nm, showing a strong correlation between radiosensitization and nanoparticle concentration [215]. However, the precise value also depends on the coating and on the cell line. High-Z nanostructures are generally coated with a layer of polyethylene glycol (PEG) to provide a hydrophilic nature, preventing the nanostructures from aggregating and increasing their cellular internalization by macrophage recognition. Moreover, nanoparticles are often also conjugated with other biomolecules to achieve specific cellular and subcellular targeting. By using such an approach, it is possible to manufacture nanoparticles that dock to specific cell surface proteins or distinct subcellular compartments such as mitochondria using specific peptide sequences. The presence of the chemical coating and the high-Z element itself also change the chemical environment of the cell.
Reactive oxygen species (ROS) play an important role in mediating DNA damage produced by radiation. In fact, 50–70% of the DNA lesions in standard RT upon X-ray irradiation are attributed to the hydroxyl radical (OH). By altering the chemical environment of the cell, the nanoparticles can in principle affect both the yield and the spectrum of ROS produced, which in turn has consequences for the DNA damage. Competing mechanisms may be at play depending on the composition of the nanoparticles and their coating. For instance, certain chemical compounds, such as PEG, can act as ROS scavengers and actually detoxify radicals formed by the interaction of radiation with water molecules. Studies aiming at quantifying the G-value (i.e., the number of molecules of a specific radical produced per 100 eV of energy absorbed) have indicated three possible mechanisms through which nanoparticles can affect the ROS production by radiation and therefore affect the radiation effectiveness (Fig. 11.25). Increased radicals can be produced as a result of direct interaction between radiation and nanoparticles through the production of electrons and low-energy photons emitted by the nanoparticles. As the energy spectrum of the secondary radiation emitted by the nanoparticle is different from the primary radiation beam, a different spectrum of radicals is to be expected and the yield is generally higher due to their lower energy and cascade of secondary electrons. The presence of the nanoparticles, however, can also affect the yield of radicals produced by the direct interaction of radiation with the water molecules. This may occur through scavenging action from the nanoparticle-coating elements or by chemical interaction between the nanoparticles and the radiolysis products.
11.4.3.3 Biological Radiosensitization
When nanoparticles reach the cell surface, they are usually internalized via endocytosis and end up in intracellular vesicles, also called endosomes. This endosomal pathway is often a barrier hindering biological or therapeutic effects of nanoparticles. Nevertheless, nanoparticles are able to escape the endosomal transport system, accessing the cytoplasm and organelles by destabilizing the endosomal membrane, inducing osmotic swelling and membrane rupture, or passively crossing the plasma membrane or the endosomal membrane [216]. Once internalized, the nanoparticles can affect different cellular and molecular processes. For instance, apart from creating additional DNA damage, nanoparticles directly or indirectly affect the functioning of DNA repair proteins by preventing their synthesis, posttranslational modification, or recruitment to the site of damage. For instance, due to the large specific surface area, nanoparticles are very efficient in capturing a large amount of proteins, including DNA repair proteins, eventually leading to their deprivation and hence reducing the DNA repair efficiency [217]. On the other hand, the release of metal ions could imbalance the metal homeostasis in the cells, which is critical for protein folding and could replace the metallic cofactor in active sites of enzymes that are involved in the antioxidant defense system, altering their structure and inhibiting their activity.
Besides affecting the DNA damage repair machinery and causing antioxidant enzyme inhibition, multiple in vitro studies showed that nanoparticles can cause cell cycle disruption. The radiosensitivity of cells can vary depending on their cell cycle phase, with cells in the late G2 and mitosis (G2/M) phases being the most radiosensitive, presumably because the condensed chromatin in mitotic cells is more susceptible to radiation-induced double-strand breaks, which are commonly repaired by the error-prone NHEJ mechanism. On the other hand, cells in the late S phase are the most radioresistant, due to the diffused chromatin regions and the fact that during the S phase, DNA damage is usually repaired by the accurate HR mechanism. Multiple research groups reported an elevated proportion of cells in the G2/M phase and a decreased cell number in the G0/G1 phase after gold nanoparticle exposure [218]. As a result, the radiosensitizing effects of gold nanoparticles can also be attributed to stalling of the cell cycle in the radiosensitive G2/M phase.
Finally, mitochondria, located in the cytoplasm and having their own DNA, are another potential target for nanoparticle-mediated radiosensitization as the same processes as described above for nuclear DNA may affect mitochondrial (mt) DNA. However, nanoparticles are hardly ever detected in mitochondria and are often accumulated in endosomes and lysosomes. In general, although hypothetical, nanoparticles accumulated and irradiated within lysosomes or other organelles may decrease cell survival by directly harming these organelles and their functions [219]. Even milder damage to organelles could potentially lead to altered signaling and eventually increased cell death.
Cytoplasmic organelles might thus represent a parallel or even dominant target to nuclear DNA. It is obvious from the previous paragraphs that nanoparticle-mediated radiosensitization is not fully understood. The research is complicated by extreme complexity and variability of the systems studied, including different materials, sizes, shapes, and modifications of nanoparticles; different cell types; different types of radiation and irradiation conditions, etc. Under certain experimental conditions, a plethora of biological processes may be induced so that it might not be excluded that different types and sizes of nanoparticles do interact according to specific mechanisms and their combinations (Box 11.6).
Box 11.6: Nanoparticle-Mediated Radiosensitization
-
The physical mechanisms of nanoparticle radiosensitization are related to both the higher attenuation cross section of high-Z materials compared to soft tissues and an increased clustering of ionizations from the secondary electrons emitted by the nanoparticle.
-
The chemical mechanisms are related to production and/or scavenging of reactive radical species mainly from the chemical compounds surrounding the nanoparticle.
-
The biological mechanisms are related to the interaction of the nanoparticles with the cellular and molecular processes, including antioxidant enzyme activities, the DNA repair pathways, the cell cycle and organelle functioning (e.g., mitochondria, lysosomes, ER, Golgi apparatus).
11.4.4 Autophagy Inhibitors
Autophagy is a basic catabolic mechanism that involves cell degradation of unnecessary or dysfunctional cell components, such as damaged endoplasmic reticulum (ER) and other cytoplasmic constituents through lysosome action.
The autophagy is mediated by protein complexes, such as class III PI3K, autophagy-related gene (Atg) proteins, and others containing microtubule-associated protein 1 light-chain subunit 3 (LC3), recruited to the membrane favoring membrane expansion and phagophore elongation. Particularly, autophagy can be activated by multiple signaling pathways, mainly through energy signals via AMPK. AMPK activation can phosphorylate ULK1, inhibit mTOR signaling, and activate Beclin-1 and Vps34 molecules resulting in the upregulation of autophagy intensity. Thus, the AMPK-mTOR-ULK1 pathway plays an important role in autophagy. Recently, the autophagy-related protein BECN1 has been shown to regulate radiation-induced G2/M arrest. In the context of a disease, autophagy has been described as an adaptive response to survival, a strategy to maintain metabolic homeostasis. In cancer cells, autophagy is a double-edged sword. In early stages, it could limit tumorigenesis. However, it could also provide a prosurvival function for adaptation and detoxification in a stressful environment, such as starvation, hypoxia, and chemotherapy/radiotherapy.
Some studies show that the autophagy preventing is radiosensitive, while the autophagy promoting is radioprotective, suggesting that IR-induced autophagy may represent an adaptive response to maintain tumor growth and survival. In order to improve IR tumor responses, several sensitization agents to radiation-induced autophagy are currently being studied. The molecular machinery involved in IR-induced autophagy is still not clear. Recent studies show that p53 and PARP-1, a DNA repair enzyme triggered by DNA damage, exert essential roles in starting the autophagy process regulating the PI3K/PKB/AKT/mTOR signaling pathway that represents an autophagy key regulator. Reports by investigators have recently shown that autophagy activity increased after IR and chemotherapy. It is an escape mechanism for cell survival in response to cytotoxic agents, including IR and temozolomide (TMZ) in glioblastoma (GBM) treatment [220]. In radioresistant breast cancer (BC) cells, a strong postirradiation autophagy induction has been observed as a protective and prosurvival mechanism of radioresistance after exposure to IR. Studies have also shown that induced autophagy in some radioresistant cancers, such as GBM, causes IR sensitization and increased cell death.
In normal conditions, microautophagy and chaperone-mediated autophagy permit the breakdown of abnormal proteins, cellular debris, or damaged organelles, maintaining cellular homeostasis and/or as tools to recycle biological constituents (e.g., amino acid, fatty acid, and energy in the form of ATP). After stress stimuli, such as nutrient starvation, protein aggregation, organelle damage, and oxidative or genotoxic stress, including by IR, the autophagy hyperactivation promotes cell death, via nonapoptotic and caspase-independent mechanisms, and this case is also called macroautophagy [221].
As described above, AMPK promotes the activation of autophagy. Among factors activated by AMPK, an interesting and not well-described role in the autophagy process, it was supposed to be for glucose transporter GLUT-1, often upregulated in cancer cells. In this sense, the cross talk among GLUT1, curcumin, and AMPK pathway in LC remains vague. Interestingly, it was recently described that the treatment with GLUT1 siRNA alone or in combination with curcumin resulted in profound improvement of the radiosensitivity of LC cells after irradiation. In particular, curcumin and GLUT1 siRNA combined treatment not only promoted apoptosis of LC cells, but also induced autophagy-associated cell death through activation of AMPK/mTOR/ULK1 signaling-mediated autophagy with or without irradiation treatment [222].
Taking all these observations, we can speculate that the role of autophagy in cancer cells depends upon certain factors, such as cell type, specific characteristics of tumor cells, microenvironment of the tumor, and type of treatment applied. In addition, a variety of radiation-resistant molecules, such as PI3K/AKT, EGFR, NF-κB, and p53, may play an important role in the regulation of autophagy, thus indicating that the mechanisms that regulate autophagy are very complex. Thus, many questions and contradictory findings have yet to be clarified.
11.4.5 Metformin (MTF)
In cancer patients, MTF may affect tumor growth and treatment response in two different ways: directly by inhibiting mitochondrial metabolism and activating downstream cell signaling pathways in cancer cells or indirectly by keeping low the levels of glucose, insulin, and other factors which can activate cancer cell proliferation [223]. MTF has also shown anticancer effects in nondiabetic patient populations (described in Sect. 11.1.9).
With regard to the relationship between MTF and IR, MTF is able to increase intrinsic radiosensitivity, as tested in vitro in many cell lines of different tumors, including lung, head and neck, breast, liver, prostate, and pancreas cancers and murine fibrosarcoma, with specific cell line-dependent radiosensitizing effect [203, 223].
Several clinical data on the effect of MTF on patient outcome, focused particularly on patient populations undergoing RT, have shown that these patients have better outcomes if they are treated with MTF. These and other data show the significant role of MTF in enhancing the RT efficacy and suggest an interaction between MTF and IR [223].
In vitro and in vivo studies, concerning the MTF radiosensitizing effect, have showed an enhanced DNA damage after MTF treatment combined with RT, as tested by phosphorylation of histone γH2AX, a well-known marker for DNA damage [203].
In particular, MTF may increase initial DNA damage or inhibit repair processes, being able to reduce the expression of DNA repair protein Ku70 and hamper radiation-induced activation of EGFR and DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Considering that the primary targets of MTF are the mitochondrial complex I and mGPD (glycerol-3-phosphate dehydrogenase), it may induce an increase of ROS generation and oxidative damage to lipids, protein, and DNA that could strengthen the effects of IR. Indeed, several data have demonstrated that MTF can increase ROS in cancer cells when delivered alone or in combination with IR. In general, as regards the main known MTF mechanisms of action when combined with RT, in vitro and in vivo data show a significant role of MTF in affecting at least four different parameters at radiobiological level, including intrinsic cell radiosensitivity (SF2, i.e., the fraction of cells surviving after a single IR dose of 2 Gy), cancer stem cell fraction, tumor proliferation rates, and tumor hypoxia [223].
11.4.6 PARP Inhibitors
Poly-(adenosine diphosphate-ribose)-polymerase (PARP) are cellular enzymes that play crucial roles in various cell processes like replication, transcription, cellular repair, and also death [224]. PARP detects single-strand breaks and triggers the activation of cellular machinery involved in the repair of the single-strand break. Studies have shown that PARP inhibitors (PARPi) exhibit enhanced radiosensitivity when combined with IR. Radiation works by damaging the DNA, which can activate the single-strand break (SSB) repair pathway like base excision repair (BER) or double-strand break (DSB) pathways like the HR and the NHEJ pathway (more details in Chap. 3). SSB, if unrepaired, gets converted to double-stranded breaks, which consequently hinder normal cellular processes. PARPi imparts radiosensitivity through the SSB and base excision pathway, which substantially increases the risk to collapsed replication fork, thereby producing a stable DSB [225]. Due to the crucial role of PARP in the DNA repair pathway, PARPi have proved as effective radiosensitizers, especially in tumors harboring DNA repair deficiencies like the BRCA mutation. The replication-reliant operations of PARPi facilitate the establishment of differential outcomes in tumor and healthy tissues. Other mechanisms that are known to induce the radiosensitization effects include inhibition of chromatin remodeling, G2/M arrest, vasodilatory effect induced by PARPi, etc. Characteristically, factors that affect radiosensitivity are the capability of tumors to repair the damage, redistribution of the cell cycle, process of reoxygenation, vascular endothelial damage process, tumor immunity, and repopulation of the tumor tissue. A radiosensitizer should have the potential not only to influence these processes but also prevent the increase in the toxicity. Since PARPi possess most of these qualities necessary for being a potential radiosensitizer (Fig. 11.26), it has gained interest in the medical community [226].
11.4.6.1 Nitroxides
Nitroxides are a class of stable free radical compounds that exhibit antioxidant mechanisms, thereby safeguarding the cells from several lethal agents like superoxide and hydrogen peroxide (also described in Sect. 11.1.2). However, the rationale to use nitroxides in cancer RT comes from the role of free radicals in tumor development and capacity of inhibitors of radical reactions to suppress tumorigenesis. The underlying mode of action of nitroxyls exhibiting the radiosensitization effect can be attributed to cell signaling, enhanced blood flow to the tumor, consequences on the cellular respiration, and generation of reactive oxygen and nitrogen species that can operate as metabolite radiosensitizers. The effects of nitroxides with radiation were found conflicting, leading to uncertainty about their radiosensitizing nature.
Tempol (TPL) is a piperidine nitroxide that possesses an unpaired electron and goes through swift reversible transfer among the three forms: nitroxide, hydroxylamine, and oxoammonium cation. Based on its concentration in the cell, it can act as an oxidative or reductive agent. In cancer cells, TPL favorably inhibits growth by increasing the generation of cellular ROS [227]. When used with RT and chemotherapy, TPL exhibits a differential effect of protecting the normal healthy cells from RT and cisplatin-mediated damage, whereas in cancer cells, tempol is reduced to its hydroxylamine form that is unable to protect the cells from radiation and cisplatin-mediated damage. This differential or selective acting on cancer cells while sparing the normal cells is particularly of significance in cancer radio- as well as chemotherapy [228]. It was observed that the anticancer effects of cisplatin increased due to the prooxidant activity of TPL via the increased ROS-mediated cell apoptosis [227]. In several cancer cell lines, TPL was able to free radical-dependent apoptosis. On 24-h exposure to TPL, human promyelocytic leukemic cell line (HL-60) showed reduced levels of mitochondrial and intracellular glutathione, failure in the oxidative phosphorylation process, and diminished mitochondrial membrane potential. TPL also particularly targeted the respiratory chain complex I and showed some insignificant effects on the complexes II and IV. This can be attributed to the role of mitochondria in apoptosis and it being a free radical resource and target. In HL-60, TPL works by targeting the mitochondria, which subsequently leads to mitochondria associated with oxidative stress and apoptosis. This in turn can sensitize the tumor cells to the proapoptotic effects of cytotoxic agents [229]. When human breast cancer cells (MDA-MB 231) were treated with tempo, another nitroxide, it exhibited considerable levels of tyrosine phosphorylation of numerous unknown proteins when evaluated with the equimolar concentration, i.e., 10 mm TPL. The compounds tempo and TPL lead to the phosphorylation of tyrosine and trigger the Raf-1 protein kinase (30 min, two- to threefold) [230]. Nevertheless, TPL leads to augmented extracellular signal-regulated kinase 1 activity. Tempo also activated the stress-associated protein kinase (2 h, threefold) and induced apoptosis (2 h, >50%). The ceramide levels significantly increase (54% over control) at 30 min and (71% over control) at 1 h after treatment, prior to activation of stress-activated protein kinase and cell death via apoptosis [230]. TPL protects normal cells and tissues from oxidative damage and remarkably hampers the proliferation of cancer cells. These clearly imply that the nitroxide TPL shows the potential to be a good prooxidant and can be a potent radiosensitizing agent for cancer treatment.
11.5 Exercises and Self-Assessment
-
Q1.
How do radioprotectors work?
-
Q2.
What is the purpose of radioprotectors in RT?
-
Q3.
What is TRUE: All the antioxidants are radioprotectors or all the potential radioprotectors are often antioxidants?
-
Q4.
What are radiomitigators?
-
Q5.
Can immunomodulators be classified as radiomitigators?
-
Q6.
What is the purpose of a radiosensitizing agent in cancer RT?
-
Q7.
Describe the curcumin action mechanism when administered with irradiation.
-
Q8.
Generate a graph similar to Fig. 11.1 for gadolinium nanoparticles and estimate the mass attenuation coefficient ratio at 100 keV.
11.6 Exercise Solutions
-
SQ1.
The detailed mechanisms are described in Sect. 11.1.1. The possible mechanisms are listed in Box 11.2 as well.
-
SQ2.
Radioprotectors, which should be safe and nontoxic for human health, are used to protect the normal cells or nontumor cells from the harmful insults of ionizing irradiation and to increase survival rate in patients when administered before the exposure to radiation or at the time of RT for their effectiveness.
-
SQ3.
The potential radioprotectors are often antioxidants, but not all the antioxidants are radioprotectors.
-
SQ4.
Radiomitigators are used to minimize the toxicity or damage caused by ionizing irradiation in noncancerous cells even after radiation has been delivered and thus able to improve the effectiveness of radiation therapy.
-
SQ5.
Yes. Radioprotective agents with potency to stimulate the proliferation and modify the function of hematopoietic and immunopoietic stem cells, often referred to as immunomodulators, can be considered as radiomitigators. The mode of action (MOA) of radiomitigators is depicted in the figure (figure number will be written).
-
SQ6.
Radiosensitizer is a chemical or pharmaceutical agent which enhances the killing effect on tumor cells by making them more susceptible/sensitive to radiation therapy and at the same time having less effect on normal tissues/cells.
-
SQ7.
Curcumin has been shown to have a double-face mechanism. Indeed, on the one hand, it is an antioxidant and anti-inflammatory molecule, useful in limiting the IR-induced ROS generation and IR side effects. On the other, it acts as an anticancer molecule, interacting with cellular processes, such as cell cycle, proliferation, apoptosis, and autophagy.
-
SQ8.
Gd/water mass attenuation coefficient ratio @ 100 keV = 72.6.
References
Ando K. Chemical radioprotectors. Gan No Rinsho. 1987;33(13):1679–83.
Raviraj J, Bokkasam VK, Kumar VS, Reddy US, Suman V. Radiosensitizers, radioprotectors, and radiation mitigators. Indian J Dent Res. 2014;25(1):83–90.
Mishra KN, Moftah BA, Alsbeih GA. Appraisal of mechanisms of radioprotection and therapeutic approaches of radiation countermeasures. Biomed Pharmacother. 2018;106:610–7.
Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899(1):44–60.
Singh VK, Seed TM. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin Drug Saf. 2019;18(11):1077–90.
Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15(4):360–71.
Huang, Eng-Yen, Feng-Sheng Wang, Yu-Min Chen, Yi-Fan Chen, Chung-Chi Wang, I-Hui Lin, Yu-Jie Huang, Kuender D. Yang. Amifostine alleviates radiation-induced lethal small bowel damage via promotion of 14-3-3σ-mediated nuclear P53 accumulation. Oncotarget 2014. 5 (20): 9756–9769.
Hofer M, Falk M, Komůrková D, Falková I, Bačíková A, Klejdus B, Pagáčová E, Štefančíková L, Weiterová L, Angelis KJ, Kozubek S, Dušek L, Galbavý Š. Two new faces of amifostine: protector from DNA damage in normal cells and inhibitor of DNA repair in cancer cells. J Med Chem. 2016;59(7):3003–17.
Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014;9(5):e95968.
Yang C, Tang H, Wang L, Peng R, Bai F, Shan Y, Yu Z, Zhou P, Cong Y. Dimethyl sulfoxide prevents radiation-induced oral mucositis through facilitating DNA double-strand break repair in epithelial stem cells. Int J Radiat Oncol Biol Phys. 2018;102(5):1577–89.
Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43(2):143–81.
Estrela JM, Ortega A, Mena S, Sirerol JA, Obrador E. Glutathione in metastases: from mechanisms to clinical applications. Crit Rev Clin Lab Sci. 2016;53(4):253–67.
Estrela JM, Obrador E, Navarro J, Lasso De la Vega MC, Pellicer JA. Elimination of Ehrlich tumours by ATP-induced growth inhibition, glutathione depletion and X-rays. Nat Med. 1995;1(1):84–8. https://doi.org/10.1038/nm0195-84. PMID: 7584960.
Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat Res. 2010;173(5):579–89.
Elkady AA, Ibrahim IM. Protective effects of Erdosteine against nephrotoxicity caused by gamma radiation in male albino rats. Hum Exp Toxicol. 2016;35(1):21–8.
Patyar RR, Patyar S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur J Pharmacol. 2018;819:207–16.
Willis J, Epperly MW, Fisher R, Zhang X, Shields D, Hou W, Wang H, et al. Amelioration of head and neck radiation-induced mucositis and distant marrow suppression in Fanca−/− and Fancg−/− mice by intraoral administration of GS-nitroxide (JP4-039). Radiat Res. 2018;189(6):560–78.
Epperly MW, Sacher JR, Krainz T, Zhang X, Wipf P, Liang M, Fisher R, Li S, Wang H, Greenberger JS. Effectiveness of analogs of the GS-nitroxide, JP4-039, as total body irradiation mitigators. In Vivo (Athens, Greece). 2017;31(1):39–43.
Luo G, Sun L, Li H, Chen J, He P, Zhao L, Tang W, y Hongdeng Qiu. The potent radioprotective agents: novel nitronyl nitroxide radical spin-labeled resveratrol derivatives. Fitoterapia. 2021;155:105053.
Mastromarino A, Wilson R. Antibiotic radioprotection of mice exposed to supralethal whole-body irradiation independent of antibacterial activity. Radiat Res. 1976;68:329–38.
Kim K, Pollard JM, Norris AJ, McDonald JT, Sun Y, Micewicz E, Pettijohn K, Damoiseaux R, Iwamoto KS, Sayre JW, Price BD, Gatti RA, McBride WH. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin Cancer Res. 2009;15(23):7238–45.
Alok A, Chaudhury NK. Tetracycline hydrochloride: A potential clinical drug for radioprotection. Chem Biol Interact. 2016;245:90–9.
Lapeyre M, Charra-Brunaud C, Kaminsky MC, Geoffrois L, Dolivet G, Toussaint B, Maire F, Pourel N, Simon M, Marchal C, Bey P. Management of mucositis following radiotherapy for head and neck cancers. Cancer Radiother. 2001;5(1):121s–30s.
Mehrotra S, Pecaut MJ, Gridley DS. Analysis of minocycline as a countermeasure against acute radiation syndrome. Vivo Athens Greece. 2012;26:743–58.
Ma S, Jin Z, Liu Y, Liu L, Feng H, Li P, Tian Z, Ren M, Liu X. Furazolidone increases survival of mice exposed to lethal total body irradiation through the antiapoptosis and antiautophagy mechanism. Oxidative Med Cell Longev. 2021b;2021:6610726.
Dowlath MJH, Karuppannan SK, Sinha P, Dowlath NS, Arunachalam KD, Ravindran B, Chang SW, Nguyen-Tri P, Nguyen DD. Effects of radiation and role of plants in radioprotection: A critical review. Sci Total Environ. 2021;779:146431.
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. 2009;2(5):270–8.
Liu J, Bai R, Liu Y, Zhang X, Kan J, Jin C. Isolation, structural characterization and bioactivities of naturally occurring polysaccharide-polyphenolic conjugates from medicinal plants. A review. Int J Biol Macromol. 2018a;107(Pt B):2242–50.
Liu Z, Lei X, Li X, Cai JM, Gao F, Yang YY. Toll-like receptors and radiation protection. Eur Rev Med Pharmacol Sci. 2018b;22(1):31–9.
Obrador E, Salvador R, Villaescusa JI, Soriano JM, Estrela JM, Montoro A. Radioprotection and radiomitigation: from the bench to clinical practice. Biomedicine. 2020;8:461.
Begum N, Prasad NR, Kanimozhi G, Hasan AQ. Apigenin ameliorates gamma radiation-induced cytogenetic alterations in cultured human blood lymphocytes. Mutat Res. 2012;747:71–6.
Begum N, Rajendra Prasad N, Kanimozhi G, Agilan B. Apigenin prevented gamma radiation-induced gastrointestinal damages by modulating inflammatory and apoptotic signalling mediators. Nat Prod Res. 2021;5:1–5.
Rithidech KN, Tungjai M, Reungpatthanaphong P, Honikel L, Simon SR. Attenuation of oxidative damage and inflammatory responses by apigenin given to mice after irradiation. Mutat Res. 2012;749(1–2):29–38.
Jagetia GC. Radioprotection and radiosensitization by curcumin. Adv Exp Med Biol. 2007;595:301–20.
Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65(3):890–8.
Jagetia GC, Rajanikant GK. Curcumin stimulates the antioxidant mechanisms in mouse skin exposed to fractionated γ-irradiation. Antioxidants (Basel). 2015;4(1):25–41.
Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26(5A):3601–6.
Ahire V, Kumar A, Mishra KP, Kulkarni G. Ellagic acid enhances apoptotic sensitivity of breast cancer cells to γ-radiation. Nutr Cancer. 2017;69(6):904–10.
Das SKR, Sinha M, Khan A, Das K, Manna K, Dey S. Radiation protection by major tea polyphenol, epicatechin. Int J Hum Genet. 2013;13(1):59–64.
West JD, Marnett LJ. Endogenous reactive intermediates as modulators of cell signalling and cell death. Chem Res Toxicol. 2006;19:173–94.
Shimura T, Koyama M, Aono D, Kunugita N. Epicatechin as a promising agent to countermeasure radiation exposure by mitigating mitochondrial damage in human fibroblasts and mouse hematopoietic cells. FASEB J. 2019;33:6867–76.
Zhu W, Xu J, Ge Y, Cao H, Ge X, Luo J, Xue J, Yang H, Zhang S, Cao J. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J Radiat Res. 2014;55:1056–65.
Xie LW, Cai S, Zhao TS, Li M, Tian Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic Biol Med. 2020;161:175–86.
Davis TA, Mungunsukh O, Zins S, Day RM, Landauer MR. Genistein induces radioprotection by hematopoietic stem cell quiescence. Int J Radiat Biol. 2008;84(9):713–26.
Zhang J, Pang Z, Zhang Y, Liu J, Wang Z, Xu C, He L, Li W, Zhang K, Zhang W, Wang S, Zhang C, Hao Q, Zhang Y, Li M, Li Z. Genistein from fructus sophorae protects mice from radiation-induced intestinal injury. Front Pharmacol. 2021;12:655652.
Song L, Ma L, Cong F, Shen X, Jing P, Ying X, Zhou H, Jiang J, Fu Y, Yan H. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 2015;366(1):100–11.
Manna K, Das U, Das D, Kesh SB, Khan A, Chakraborty A, Dey S. Naringin inhibits gamma radiation-induced oxidative DNA damage and inflammation, by modulating p53 and NF-κB signaling pathways in murine splenocytes. Free Radic Res. 2015;49(4):422–39.
Das RN, Balupillai A, David E, Santhoshkumar M, Muruhan S. Naringin, a natural flavonoid, modulates UVB radiation-induced DNA damage and photoaging by modulating NER repair and MMPS expression in mouse embryonic fibroblast cells. J Environ Pathol Toxicol Oncol. 2020;39(2):191–9.
Kumar S, Tiku AB. Biochemical and molecular mechanisms of radioprotective effects of naringenin, a phytochemical from citrus fruits. J Agric Food Chem. 2016;64(8):1676–85.
Koohian F, Shanei A, Shahbazi-Gahrouei D, Hejazi SH, Moradi MT. The radioprotective effect of resveratrol against genotoxicity induced by γ-irradiation in mice blood lymphocytes. Dose Response. 2017;15(2):1559325817705699.
Carsten RE, Bachand AM, Bailey SM, Ullrich RL. Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiat Res. 2008;169(6):633–8.
Zhang H, Yan H, Zhou X, Wang H, Yang Y, Zhang J, Wang H. The protective effects of resveratrol against radiation-induced intestinal injury. BMC Complement Altern Med. 2017;17(1):410.
Obrador E, Salvador-Palmer R, Jihad-Jebbar A, López-Blanch R, Dellinger TH, Dellinger RW, Estrela JM. Pterostilbene in cancer therapy. Antioxidants (Basel). 2021;10(3):492.
Sirerol JA, Feddi F, Mena S, Rodriguez ML, Sirera P, Aupí M, Pérez S, Asensi M, Ortega A, Estrela JM. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic Biol Med. 2015;85:1–11.
Obrador E, Salvador-Palmer R, Pellicer B, López-Blanch R, Sirerol JA, Villaescusa JI, Montoro A, Dellinger RW, Estrela JM. Combination of natural polyphenols with a precursor of NAD+ and a TLR2/6 ligand lipopeptide protects mice against lethal γ radiation. J Adv Res. 2023.
Farooqi Z, Kesavan PC. Radioprotection by caffeine pre- and post-treatment in the bone marrow chromosomes of mice given whole-body gamma-irradiation. Mutat Res. 1992;269:225–30.
Sim HJ, Bhattarai G, Lee J, Lee JC, Kook SH. The long-lasting radioprotective effect of caffeic acid in mice exposed to total body irradiation by modulating reactive oxygen species generation and hematopoietic stem cell senescence-accompanied long-term residual bone marrow injury. Aging Dis. 2019;10(6):1320–7.
Khayyo N, Taysı ME, Demir E, Ulusal H, Çınar K, Tarakçıoğlu M, et al. Radioprotective effect of caffeic acid phenethyl ester on the brain tissue in rats who underwent total-head irradiation. Eur J Ther. 2019;25(4):265–72.
Kumar A, Selvan TG, Tripathi AM, Choudhary S, Khan S, Adhikari JS, Chaudhury NK. Sesamol attenuates genotoxicity in bone marrow cells of whole-body γ-irradiated mice. Mutagenesis. 2015;30(5):651–61.
Khan S, Kumar A, Adhikari JS, Rizvi MA, Chaudhury NK. Protective effect of sesamol against 60Co γ-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic Res. 2015;49:1344–61.
Majdaeen M, Banaei A, Abedi-Firouzjah R, Ebrahimnejad Gorji K, Ataei G, Momeni F, Zamani H. Investigating the radioprotective effect of sesamol oral consumption against gamma irradiation in mice by micronucleus and alkaline comet assays. Appl Radiat Isot. 2020;159:109091.
Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68(6):1927–34.
Chen H-X, Xiang H, Wen-Huan X, Li M, Yuan J, Liu J, Sun W-J, et al. Manganese superoxide dismutase gene-modified mesenchymal stem cells attenuate acute radiation-induced lung injury. Hum Gene Ther. 2017;28(6):523–32.
Fan S, Meng Q, Xu J, Jiao Y, Zhao L, Zhang X, Sarkar FH, Brown ML, Dritschilo A, Rosen EM. DIM (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci U S A. 2013;110(46):18650–5.
Changizi V, Haeri SA, Abbasi S, Rajabi Z, Mirdoraghi M. Radioprotective effects of vitamin A against gamma radiation in mouse bone marrow cells. MethodsX. 2019;6:714–7.
Bakshi HA, Zoubi MSA, Hakkim FL, Aljabali AAA, Rabi FA, Hafiz AA, Al-Batanyeh KM, Al-Trad B, Ansari P, Nasef MM, Charbe NB, Satija S, Mehta M, Mishra V, Gupta G, Abobaker S, Negi P, Azzouz IM, Dardouri AAK, Dureja H, Prasher P, Chellappan DK, Dua K, Webba da Silva M, El Tanani M, McCarron PA, Tambuwala MM. Dietary crocin is protective in pancreatic cancer while reducing radiation-induced hepatic oxidative damage. Nutrients. 2020;12(6):E1901.
Zhang C, Chen K, Wang J, Zheng Z, Luo Y, Zhou W, Zhuo Z, Liang J, Sha W, Chen H. Protective effects of crocetin against radiation-induced injury in intestinal epithelial cells. Biomed Res Int. 2020;2020:2906053.
Sindhu ER, Preethi KC, Kuttan R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J Exp Biol. 2010;48(8):843–8.
Vasudeva V, Tenkanidiyoor YS, Radhakrishna V, Shivappa P, Lakshman SP, Fernandes R, Patali KA. Palliative effects of lutein intervention in gamma-radiation-induced cellular damages in Swiss albino mice. Indian J Pharmacol. 2017;49(1):26–33.
Motallebnejad M, Zahedpasha S, Moghadamnia AA, Kazemi S, Moslemi D, Pouramir M, Asgharpour F. Protective effect of lycopene on oral mucositis and antioxidant capacity of blood plasma in the rat exposed to gamma radiation. Caspian J Intern Med. 2020;11(4):419–25.
Mortazavi SMJ, Sharif-Zadeh S, Mozdarani H, Foadi M, Haghani M, Sabet E. Future role of vitamin C in radiation mitigation and its possible applications in manned deep space missions: survival study and the measurement of cell viability. Phys Med. 2014;30:e97.
Sato T, Kinoshita M, Yamamoto T, Ito M, Nishida T, Takeuchi M, Saitoh D, Seki S, Mukai Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS One. 2015;10(2):e0117020.
Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54(6):973–88.
Aykin-Burns N, Pathak R, Boerma M, Kim T, Hauer-Jensen M. Utilization of vitamin E analogs to protect normal tissues while enhancing antitumor effects. Semin Radiat Oncol. 2019;29(1):55–61.
Lee S-G, Kalidindi TM, Lou H, Gangangari K, Punzalan B, Bitton A, Lee CJ, et al. γ-tocotrienol-loaded liposomes for radioprotection from hematopoietic side effects caused by radiotherapeutic drugs. J Nucl Med. 2021;62(4):584–90.
Vasil’eva IN, Bespalov VG, Baranenko DA. Radioprotective and apoptotic properties of a combination of α-tocopherol acetate and ascorbic acid. Bull Exp Biol Med. 2016;161(2):248–51.
Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing radiation as a source of oxidative stress—the protective role of melatonin and vitamin D. Int J Mol Sci. 2020;21(16):5804.
Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437(1):7–11.
Kaminskyi OV, Pankiv VI, Pankiv IV, Afanasyev DE. Vitamin d content in population of radiologically contaminated areas in Chernivtsi oblast (pilot project). Probl Radiac Med Radiobiol. 2018;23:442–51. English, Ukrainian
Ji MT, Nie J, Nie XF, Hu WT, Pei HL, Wan JM, Wang AQ, Zhou GM, Zhang ZL, Chang L, Li BY. 1α,25(OH)2D3 radiosensitizes cancer cells by activating the NADPH/ROS pathway. Front Pharmacol. 2020;11:945.
Hosseinimehr SJ. The protective effects of trace elements against side effects induced by ionizing radiation. Radiat Oncol J. 2015;33(2):66–74.
Bartolini, Desirée, Kenneth D. Tew, Rita Marinelli, Francesco Galli, y Gavin Y. Wang. Nrf2-modulation by Seleno-Hormetic agents and its potential for radiation protection. BioFactors (Oxford, England) 2020. 46 (2): 239–245.
Kunwar A, Bansal P, Jaya Kumar S, Bag PP, Paul P, Reddy ND, Kumbhare LB, et al. In vivo radioprotection studies of 3,3′-diselenodipropionic acid, a selenocystine derivative. Free Radic Biol Med. 2010;48(3):399–410.
Du J, Zhanjun G, Liang Y, Yong Y, Yi X, Zhang X, Liu J, et al. Poly(Vinylpyrrolidone)- and selenocysteine-modified Bi2Se3 nanoparticles enhance radiotherapy efficacy in tumors and promote radioprotection in normal tissues. Adv Mater (Deerfield Beach, Fla.). 2017;29(34):1701268.
Muecke R, Micke O, Schomburg L, Buentzel J, Kisters K, Adamietz IA, AKTE. Selenium in radiation oncology-15 years of experiences in Germany. Nutrients. 2018;10(4):483.
Kunwar A, Indira Priyadarsini K, Jain VK. 3,3′-diselenodipropionic acid (DSePA): a redox active multifunctional molecule of biological relevance. Biochim Biophys Acta Gen Subj. 2021;1865(1):129768.
Wang G, Ren X, Yan H, Gui Y, Guo Z, Song J, Zhang P. Neuroprotective effects of umbilical cord-derived mesenchymal stem cells on radiation-induced brain injury in mice. Ann Clin Lab Sci. 2020a;50(1):57–64.
Wang L, Ziyu Wang Y, Cao WL, Kuang L, Hua D. Strategy for highly efficient radioprotection by a selenium-containing polymeric drug with low toxicity and Long circulation. ACS Appl Mater Interfaces. 2020b;12(40):44534–40.
Crescenti E, Croci M, Medina V, Sambuco L, Bergoc R, y Elena Rivera. Radioprotective potential of a novel therapeutic formulation of oligoelements Se, Zn, Mn plus Lachesis Muta Venom. J Radiat Res. 2009;50(6):537–44.
Ertekin MV, Koç M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58(1):167–74.
Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer. 2010;127(8):1984–90.
Doctrow SR, Lopez A, Schock AM, Duncan NE, Jourdan MM, Olasz EB, Moulder JE, Fish BL, Mäder M, Lazar J, Lazarova Z. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J Invest Dermatol. 2013;133(4):1088–96. https://doi.org/10.1038/jid.2012.410. Epub 2012 Nov 29. Erratum in: J Invest Dermatol. 2013 Jun;133(6):1691
Batinic-Haberle I, Tovmasyan A, Spasojevic I. Mn porphyrin-based redox-active drugs: differential effects as cancer therapeutics and protectors of normal tissue against oxidative injury. Antioxid Redox Signal. 2018;29(16):1691–724.
Anderson CM, Lee CM, Saunders DP, Curtis A, Dunlap N, Nangia C, Lee AS, Gordon SM, Kovoor P, Arevalo-Araujo R, Bar-Ad V, Peddada A, Colvett K, Miller D, Jain AK, Wheeler J, Blakaj D, Bonomi M, Agarwala SS, Garg M, Worden F, Holmlund J, Brill JM, Downs M, Sonis ST, Katz S, Buatti JM. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe Oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J Clin Oncol. 2019;37(34):3256–65.
Mapuskar KA, Anderson CM, Spitz DR, Batinic-Haberle I, Allen BG, Oberley-Deegan RE. Utilizing superoxide dismutase mimetics to enhance radiation therapy response while protecting normal tissues. Semin Radiat Oncol. 2019;29(1):72–80.
Weitzel DH, Tovmasyan A, Ashcraft KA, Rajic Z, Weitner T, Liu C, Li W, et al. Radioprotection of the brain white matter by Mn(III) n-butoxyethylpyridylporphyrin-based superoxide dismutase mimic MnTnBuOE-2-PyP5+. Mol Cancer Ther. 2012;14(1):70–9.
Fahl WE. Complete prevention of radiation-induced dermatitis using topical adrenergic vasoconstrictors. Arch Dermatol Res. 2016;308(10):751–7.
Vasin MV, Semenov LF, Suvorov NN, Antipov VV, Ushakov IB, Ilyin LA, Lapin BA. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J Radiat Res. 2014;55(6):1048–55.
Grabenbauer GG, Holger G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2016;30(4):655–64.
Fu Q, Berbée M, Wang W, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. Preclinical evaluation of Som230 as a radiation mitigator in a mouse model: postexposure time window and mechanisms of action. Radiat Res. 2011;175(6):728–35.
Amini P, Mirtavoos-Mahyari H, Motevaseli E, Shabeeb D, Musa AE, Cheki M, Farhood B, Yahyapour R, Shirazi A, Goushbolagh NA, Najafi M. Mechanisms for radioprotection by melatonin; can it be used as a radiation countermeasure? Curr Mol Pharmacol. 2019;12(1):2–11.
Wang Q, Wang Y, Liqing D, Chang X, Liu Y, Liu Q, Fan S. Quantitative proteomic analysis of the effects of melatonin treatment for mice suffered from small intestinal damage induced by γ-ray radiation. Int J Radiat Biol. 2021;97(9):1206–16.
Sheikholeslami S, Aryafar T, Abedi-Firouzjah R, Banaei A, Dorri-Giv M, Zamani H, Ataei G, Majdaeen M, Farhood B. The role of melatonin on radiation-induced pneumonitis and lung fibrosis: A systematic review. Life Sci. 2021;281:119721.
Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, Mirtavoos-Mahyari H, et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin Transl Oncol. 2019;21(3):268–79.
Aras S, Efendioğlu M, Wulamujiang A, Ozkanli SS, Keleş MS, Tanzer İO. Radioprotective effect of melatonin against radiotherapy-induced cerebral cortex and cerebellum damage in rat. Int J Radiat Biol. 2021;97(3):348–55.
Alonso-González C, González A, Menéndez-Menéndez J, Martínez-Campa C, Cos S. Melatonin as a radio-sensitizer in cancer. Biomedicine. 2020;8(8):E247.
Adalsteinsson JA, Muzumdar S, Waldman R, Rong W, Ratner D, Feng H, Ungar J, et al. Metformin is associated with decreased risk of basal cell carcinoma: A whole-population case-control study from Iceland. J Am Acad Dermatol. 2021;85(1):56–61.
Keywan M, Shabeeb Dheyauldeen E, Ahmed M, Masoud N, Bagher F. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol. 2019;14(1):41–53.
Kolivand S, Motevaseli E, Cheki M, Mahmoudzadeh A, Shirazi A, Fait V. The anti-apoptotic mechanism of metformin against apoptosis induced by ionizing radiation in human peripheral blood mononuclear cells. Klin Onkol: Casopis Ceske a Slovenske Onkologicke Spolecnosti. 2017;30(5):372–9.
Kelly B, Tannahill GM, Murphy MP, O’Neill LAJ. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290(33):20348–59.
Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C, Clementi E, Shirazi A. Metformin: prevention of genomic instability and cancer: A review. Mutat Res Genet Toxicol Environ Mutagen. 2018;827:1–8.
Karam HM, Radwan RR. Metformin modulates cardiac endothelial dysfunction, oxidative stress and inflammation in irradiated rats: A new perspective of an antidiabetic drug. Clin Exp Pharmacol Physiol. 2019;46(12):1124–32.
Yahyapour R, Amini P, Rezapoor S, Rezaeyan A, Farhood B, Cheki M, Fallah H, Najafi M. Targeting of inflammation for radiation protection and mitigation. Curr Mol Pharmacol. 2018a;11(3):203–10.
Yahyapour R, Amini P, Saffar H, Rezapoor S, Motevaseli E, Cheki M, Farhood B, et al. Metformin protects against radiation-induced heart injury and attenuates the upregulation of dual oxidase genes following Rat’s chest irradiation. Int J Mol Cell Med. 2018b;7(3):193–202.
Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13.
Sharygin VL, Pulatova MK, Shliakova TG, Mitrokhin II, Todorov IN. Activation of deoxyribonucleotide synthesis by radioprotectants and antioxidants as a key stage in formation of body resistance to DNA-damaging factors. Izv Akad Nauk Ser Biol. 2005;4:401–22.
Mun GI, Kim S, Choi E, Kim CS, Lee YS. Pharmacology of natural radioprotectors. Arch Pharm Res. 2018;41:1033–50.
Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, Hauer-Jensen M, Huff LA, Lillis-Hearne P, Maekawa K, Nemhauser J, Powles R, Schünemann H, Shapiro A, Stenke L, Valverde N, Weinstock D, White D, Albanese J, Meineke V. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5(3):202–12.
Dainiak N. Medical management of acute radiation syndrome and associated infections in a high-casualty incident. J Radiat Res. 2018;59(suppl_2):ii54–64. https://doi.org/10.1093/jrr/rry004. PMID: 29509947; PMCID: PMC5941165
Dainiak N, Albanese J. Assessment and clinical management of internal contamination. J Radiol Prot. 2022;42(4) https://doi.org/10.1088/1361-6498/aca0a7.
Singh VK, Romaine PLP, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic National Stockpile. Health Phys. 2015;108(6):607–30.
Hu D, Zhang Y, Cao R, Hao Y, Yang X, Tian T, Zhang J. The protective effects of granulocyte-macrophage colony-stimulating factor against radiation-induced lung injury. Transl Lung Cancer Res. 2020a;9(6):2440–59.
Hu Q, Zhou Y, Shijie W, Wei W, Deng Y, Shao A. Molecular hydrogen: A potential radioprotective agent. Biomed Pharmacother. 2020b;130:110589.
Vadhan-Raj S, Goldberg JD, Perales MA, Berger DP, van den Brink MR. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med. 2013;17(11):1371–84.
DiCarlo AL, Horta ZP, Aldrich JT, Jakubowski AA, Skinner WK, Case CM Jr. Use of growth factors and other cytokines for treatment of injuries during a radiation public health emergency. Radiat Res. 2019;192(1):99–120.
Singh VK, Seed TM. Repurposing pharmaceuticals previously approved by regulatory agencies to medically counter injuries arising either early or late following radiation exposure. Front Pharmacol. 2021;12:624844.
Horta ZP, Case CM Jr, DiCarlo AL. Use of growth factors and cytokines to treat injuries resulting from a radiation public health emergency. Radiat Res. 2019;192(1):92–7.
Farhood B, Samadian H, Ghorbani M, Zakariaee SS, Knaup C. Physical, dosimetric and clinical aspects and delivery systems in neutron capture therapy. Rep Pract Oncol Radiother. 2018;23(5):462–73.
Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18(1):21.
Bazinet A, Popradi G. A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr Oncol. 2019;26(3):187–91.
Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6(9):e24072.
Kunadt D, Stölzel F. Effective immunosurveillance after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Cancer Manag Res. 2021;13:7411–27.
Cavallero S, Riccobono D, Drouet M, François S. MSC-derived extracellular vesicles: new emergency treatment to limit the development of radiation-induced hematopoietic syndrome? Health Phys. 2020;119(1):21–36.
Accarie A, L’Homme B, Benadjaoud MA, Lim SK, Guha C, Benderitter M, Tamarat R, Sémont A. Extracellular vesicles derived from mesenchymal stromal cells mitigate intestinal toxicity in a mouse model of acute radiation syndrome. Stem Cell Res Ther. 2020;11(1):371.
Schoefinius J-S, Brunswig-Spickenheier B, Speiseder T, Krebs S, Just U, Lange C. Mesenchymal stromal cell-derived extracellular vesicles provide long-term survival after total body irradiation without additional hematopoietic stem cell support. Stem Cells. 2017;35(12):2379–89.
Laube M, Kniess T, Pietzsch J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy—A hypothesis-driven review. Antioxidants. 2016;5(2):14.
Alok A, Agrawala PK. Repurposing sodium diclofenac as a radiation countermeasure agent: A cytogenetic study in human peripheral blood lymphocytes. Mutat Res Genet Toxicol Environ Mutagen. 2020;856-857:503220.
Stokman MA, Spijkervet FK, Burlage FR, Roodenburg JL. Clinical effects of flurbiprofen tooth patch on radiation-induced oral mucositis. A pilot study. Support Care Cancer. 2005;13(1):42–8.
Cheki M, Yahyapour R, Farhood B, Rezaeyan A, Shabeeb D, Amini P, Rezapoor S, Najafi M. COX-2 in radiotherapy: A potential target for radioprotection and Radiosensitization. Curr Mol Pharmacol. 2018;11(3):173–83.
Hofer M, Pospíšil M, Hoferová Z, Weiterová L, Komůrková D. Stimulatory action of cyclooxygenase inhibitors on hematopoiesis: A review. Molecules (Basel, Switzerland). 2012;17(5):5615–25.
Hoggatt J, Singh P, Stilger KN, Artur Plett P, Sampson CH, Chua HL, Orschell CM, Pelus LM. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis. 2013;50(3):147–53.
Yamasaki MC, Nejaim Y, Roque-Torres GD, Freitas DQ. Meloxicam as a radiation-protective agent on mandibles of irradiated rats. Braz Dent J. 2017;28(2):249–55.
Xu X, Huang H, Tu Y, Sun J, Xiong Y, Ma C, Qin S, Hu W, Zhou J. Celecoxib alleviates radiation-induced brain injury in rats by maintaining the integrity of blood-brain barrier. Dose Response. 2021;19(2):15593258211024393.
Pietzsch J, Laube M, Bechmann N, Pietzsch F-J, Kniess T. Protective effects of 2,3-diaryl-substituted indole-based cyclooxygenase-2 inhibitors on oxidative modification of human low density lipoproteins in vitro. Clin Hemorheol Microcirc. 2016;61(4):615–32.
Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M, Chan TA, Beal K, Bishop AJ, Barker CA, Jones TS, Hentschel B, Gorlia T, Schlegel U, Stupp R, Weller M, Holland EC, Hambardzumyan D. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–71.
Kumagai T, Rahman F, Smith AM. The microbiome and radiation induced-bowel injury: evidence for potential mechanistic role in disease pathogenesis. Nutrients. 2018;10(10):1405.
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40(5):409–21.
Jian Y, Zhang D, Liu M, Wang Y, Zhi-Xiang X. The impact of gut microbiota on radiation-induced enteritis’ Frontiers in cellular and infection. Microbiology. 2021;11:586392.
Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiation Oncology (London, England). 2010;5:31.
Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V, Thotala D, Ciorba MA, Stenson WF. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68(6):1003–13.
Moreno D, de LeBlanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P, Bermúdez-Humarán LG, LeBlanc JG. Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract. 2015;2015:146972.
Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4(4):CD004827.
Picó-Monllor JA, Mingot-Ascencao JM. Search and selection of probiotics that improve mucositis symptoms in oncologic patients. a systematic review. Nutrients. 2019;11(10):2322.
Garcia-Peris P, Velasco C, Hernandez M, Lozano MA, Paron L, de la Cuerda C, Breton I, Camblor M, Guarner F. Effect of inulin and fructo-oligosaccharide on the prevention of acute radiation enteritis in patients with gynecological cancer and impact on quality-of-life: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2016;70(2):170–4.
Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, Zheng Q, Dong J, Zhao Y, Zhang X, Zhang J, Lu L, Wang H, Fan S. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med. 2017;9(4):448–61.
Ding X, Li Q, Li P, Chen X, Xiang L, Bi L, Zhu J, Huang X, Cui B, Zhang F. Fecal microbiota transplantation: A promising treatment for radiation enteritis? Radiother Oncol. 2020;143(February):12–8.
Zhao Z, Cheng W, Qu W, Shao G, Liu S. Antibiotic alleviates radiation-induced intestinal injury by remodeling microbiota, reducing inflammation, and inhibiting fibrosis. ACS Omega. 2020;5(6):2967–77.
Sun F, Sun H, Zheng X, Yang G, Gong N, Zhou H, Wang S, Cheng Z, Ma H. Angiotensin-converting enzyme inhibitors decrease the incidence of radiation-induced pneumonitis among lung cancer patients: A systematic review and meta-analysis’ journal of. Cancer. 2018;9(12):2123–31.
Medhora M, Gao F, Qingping W, Molthen RC, Jacobs ER, Moulder JE, Fish BL. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 2014;182(5):545–55.
Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Curr Pharm Des. 2007;13(13):1317–25.
Mungunsukh O, George J, McCart EA, Snow AL, Mattapallil JJ, Mog SR, Panganiban RAM, et al. Captopril reduces lung inflammation and accelerated senescence in response to thoracic radiation in mice. J Radiat Res. 2021;62(2):236–48.
McCart EA, Lee YH, Jha J, Mungunsukh O, Rittase WB, Summers TA, Muir J, Day RM. Delayed captopril administration mitigates hematopoietic injury in a murine model of total body irradiation. Sci Rep. 2019;9(1):2198.
Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, Lawton CA, Moulder JE. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83(1):292–6.
Day RM, Davis TA, Barshishat-Kupper M, McCart EA, Tipton AJ, Landauer MR. Enhanced hematopoietic protection from radiation by the combination of genistein and captopril. Int Immunopharmacol. 2013;15(2):348–56.
Turnquist C, Harris BT, Harris CC. Radiation-induced brain injury: current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol Adv. 2020;2(1):vdaa057.
Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ (Clinical Research Ed). 2010;340:b5349.
Pathak R, Kumar VP, Hauer-Jensen M, Ghosh SP. Enhanced survival in mice exposed to ionizing radiation by combination of gamma-tocotrienol and simvastatin. Mil Med. 2019;184(Supplement_1):644–51.
Werner E, Alter A, Deng Q, Dammer EB, Wang Y, Yu DS, Duong DM, Seyfried NT, Doetsch PW. Ionizing radiation induction of cholesterol biosynthesis in lung tissue. Sci Rep. 2019;9:12546.
Zhao X, Yang H, Jiang G, Ni M, Deng Y, Cai J, Li Z, Shen F, Tao X. Simvastatin attenuates radiation-induced tissue damage in mice. J Radiat Res. 2014;55(2):257–64.
Bajaj MS, Ghode SS, Kulkarni RS, Limaye LS, Kale VP. Simvastatin improves hematopoietic stem cell engraftment by preventing irradiation-induced marrow adipogenesis and radio-protecting the niche cells. Haematologica. 2015;100(8):e323–7.
Xu L, Yang X, Chen J, Ge X, Qin Q, Zhu H, Zhang C, Sun X. Simvastatin attenuates radiation-induced salivary gland dysfunction in mice. Drug Des Devel Ther. 2016;10:2271–8.
Wedlake LJ, Silia F, Benton B, Lalji A, Thomas K, Dearnaley DP, Blake P, Tait D, Khoo VS, Jervoise H, Andreyev N. Evaluating the efficacy of statins and ACE-inhibitors in reducing gastrointestinal toxicity in patients receiving radiotherapy for pelvic malignancies. Eur J Cancer. 2012;48(14):2117–24.
Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, Finkelstein JN. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161(5):560–7.
Doi H, Matsumoto S, Odawara S, Shikata T, Kitajima K, Tanooka M, Takada Y, Tsujimura T, Kamikonya N, Hirota S. Pravastatin reduces radiation-induced damage in normal tissues. Exp Ther Med. 2017;13(5):1765–72.
Long S, Wang G, Shen M, Zhao N, Wan H, Xu Y, Wang S, et al. DTMP-GH fusion protein therapy improves survival after radiation injury combined with skin-burn trauma in mice. Radiat Res. 2019;191(4):360–8.
Kandaz M, Ertekin MV, Karslıoğlu İ, Erdoğan F, Sezen O, Gepdiremen A, Gündoğdu C. Zinc sulfate and/or growth hormone Administration for the Prevention of radiation-induced dermatitis: A placebo-controlled rat model study. Biol Trace Elem Res. 2017;179(1):110–6.
Caz V, Elvira M, Tabernero M, Grande AG, Lopez-Plaza B, de Miguel E, Largo C, Santamaria M. Growth hormone protects the intestine preserving radiotherapy efficacy on tumors: a short-term study. PLoS One. 2015;10(12):e0144537.
Mahran YF, El-Demerdash E, Nada AS, El-Naga RN, Ali AA, Abdel-Naim AB. Growth hormone ameliorates the radiotherapy-induced ovarian follicular loss in rats: impact on oxidative stress, apoptosis and IGF-1/IGF-1R axis. PLoS One. 2012;10(10):e0140055.
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K-I, Katayama Y, Asoh S, y Shigeo Ohta. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–94.
Hirano SI, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int J Mol Sci. 2021;22(9):4566.
Qian L, Shen J, Chuai Y, Cai J. Hydrogen as a new class of radioprotective agent. Int J Biol Sci. 2013;9(9):887–94.
Shin MH, Park R, Nojima H, Kim H-C, Kim YK, Chung JH. Atomic hydrogen surrounded by water molecules, H(H2O)m, modulates basal and UV-induced gene expressions in human skin in vivo. PLoS One. 2013;8(4):e61696.
Kang K-M, Kang Y-N, Choi I-B, Yeunhwa G, Kawamura T, Toyoda Y, Nakao A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1:11.
Cheda A, Nowosielska EM, Gebicki J, Marcinek A, Chlopicki S, Janiak MK. A derivative of vitamin B3 applied several days after exposure reduces lethality of severely irradiated mice. Sci Rep. 2021;11(1):7922.
Mishra K, Alsbeih G. Appraisal of biochemical classes of radioprotectors: evidence, current status and guidelines for future development. 3 Biotech. 2017;7(5):292.
EPR- Internal Contamination 2018 (IAEA). Medical Management of Persons Internally Contaminated with Radionuclides in a Nuclear or Radiological Emergency
Rump A, Eder S, Hermann C, Lamkowski A, Kinoshita M, Yamamoto T, Abend M, Shinomiya N, Port M. A comparison of thyroidal protection by iodine and perchlorate against radioiodine exposure in Caucasians and Japanese. Arch Toxicol. 2021;95(7):2335–50.
World Health Organization. Iodine thyroid blocking: guidelines for use in planning for and responding to radiological and nuclear emergencies. Geneva: World Health Organization; 2017.
Strahlenschutzkommission (SSK). Verwendung von Jodtabletten zur Jodblockade der Schilddrüse bei einem Notfall mit Freisetzung von radioaktivem Jod Empfehlung der Strahlenschutzkommission. Verabschiedet in der 294. Sitzung der Strahlenschutzkommission am 26 April 2018. https://www.ssk.de/SharedDocs/Beratungsergebnisse_PDF/2018/2018-04-26Jodmerk.pdf?__blob=publicationFile. Accessed 9 Nov 2020.
Yantasee W, Sangvanich T, Creim JA, Pattamakomsan K, Wiacek RJ, Fryxell GE, Addleman RS, Timchalk C. Functional sorbents for selective capture of plutonium, americium, uranium, and thorium in blood. Health Phys. 2010;99:413–9.
Ogawa K, Fukuda T, Han J, Kitamura Y, Shiba K, Odani A. Evaluation of chlorella as a decorporation agent to enhance the elimination of radioactive strontium from body. PLoS One. 2016;11(2):e0148080.
Brigant L, Rozen R, Apfelbaum M. Tritium dilution space measurement is not modified by a doubling in fluid intake. J Appl Physiol (1985). 1993;75(1):412–5. https://doi.org/10.1152/jappl.1993.75.1.412. PMID: 8376293.
Sonawane VR, Jagtap VS, Pahuja DN, Rajan MGR, Samuel AM. Difficulty in dislodging in vivo fixed radiostrontium. Health Phys. 2004;87:46–50.
Ohmachi Y, Imamura T, Ikeda M, Shishikura E, Kim E, Kurihara O, Sakai K. Sodium bicarbonate protects uranium-induced acute nephrotoxicity through uranium-decorporation by urinary alkalinization in rats. J Toxicol Pathol. 2015;28(2):65–71.
Ansoborlo É, Amekraz B, Moulin C, Moulin V, Taran F, Bailly T, Burgada R, Hengé-Napoli M-H, Jeanson A, Den Auwer C, Bonin L, Moisy P. Review of actinide decorporation with chelating agents. Comptes Rendus Chim. 2007;10(10-11):1010–9.
Gusev I, Guskova A, Mettler FA. Medical management of radiation accidents. Boca Raton, FL: CRC Press; 2001. ISBN 978-1-4200-3719-7.
Morgan C, Bingham D, Holt DC, Jones DM, Lewis NJ. Therapeutic whole lung lavage for inhaled plutonium oxide revisited. J Radiol Prot. 2010;30(4):735–46.
Gosselin-Acomb TK. Principles of radiation therapy. In: Yarbro CH, Goodman M, Frogge MH, editors. Cancer nursing: principles and practice. 6th ed. Sudbury: Jones and Bardett Publishers; 2005. p. 230–49.
Adams GE. Chemical radiosensitization of hypoxic cells. Br Med Bull. 1973;29(1):48–53.
Fowler JF, Adams GE, Denekamp J. Radiosensitizers of hypoxic cells in solid tumors. Cancer Treat Rev. 1976;3(4):227–56.
Juzenas P, Chen W, Sun Y-P, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008;60(15):1600–14.
Cline B, Delahunty I, Xie J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1541.
Calvaruso M, Pucci G, Musso R, Bravatà V, Cammarata FP, Russo G, Forte GI, Minafra L. Nutraceutical compounds as sensitizers for cancer treatment in radiation therapy. Int J Mol Sci. 2019;20:5267.
Minafra L, Porcino N, Bravatà V, Gaglio D, Bonanomi M, Amore E, Cammarata FP, Russo G, Militello C, Savoca G, Baglio M, Abbate B, Iacoviello G, Evangelista G, Gilardi MC, Bondì ML, Forte GI. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Nat Sci Rep. 2019;9:11134.
Verma V. Relationship and interactions of curcumin with radiation therapy. World J Clin Oncol. 2016;7:275–83.
Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35:S224–s243.
Yang Y, Jiang M, Hu J, Lv X, Yu L, Qian X, Liu B. Enhancement of radiation effects by ursolic acid in BGC-823 human adenocarcinoma gastric cancer cell line. PLoS One. 2015;10:e0133169.
Bykov VN, Drachev IS, Kraev SY, Maydin MA, Gubareva EA, Pigarev SE, Anisimov VN, Baldueva IA, Fedoros EI, Panchenko AV. Radioprotective and radiomitigative effects of BP-C2, a novel lignin-derived polyphenolic composition with ammonium molybdate, in two mouse strains exposed to total body irradiation. Int J Radiat Biol. 2018;94(2):114–23.
Pigarev SE, Trashkov AP, Panchenko AV, Yurova MN, Bykov VN, Fedoros EI, Anisimov VN. Evaluation of the genotoxic and antigenotoxic potential of lignin-derivative BP-C2 in the comet assay in vivo. Environ Res. 2021;192:110321.
Schueller P, Puettmann S, Micke O, Senner V, Schaefer U, Willich N. Selenium influences the radiation sensitivity of C6 rat glioma cells. Anticancer Res. 2004;24(5A):2913–7.
Viani GA, Pavoni JF, De Fendi LI. Prophylactic corticosteroid to prevent pain flare in bone metastases treated by radiotherapy. Rep Pract Oncol Radiother. 2021;26:218–25.
Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–15.
Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531–9.
McMahon SJ, Hyland WB, Muir MF, Coulter JA, Jain S, Butterworth KT, et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci Rep. 2011;1:18.
Butterworth KT, Coulter JA, Jain S, Forker J, McMahon SJ, Schettino G, Prise KM, Currell FJ, Hirst DG. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology. 2010;21(29):295101.
Martens T, Remaut K, Demeester J, De Smedt S, Braeckmans K. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today. 2014;9:344–64.
Carriere M, Sauvaigo S, Douki T, Ravanat JL. Impact of nanoparticles on DNA repair processes: current knowledge and working hypotheses. Mutagenesis. 2017;32(1):203–13.
Dobešová L, Gier T, Kopečná O, Pagáčová E, Vičar T, Bestvater F, Toufar J, Bačíková A, Kopel P, Fedr R, Hildenbrand G, Falková I, Falk M, Hausmann M. Incorporation of low concentrations of gold nanoparticles: complex effects on radiation response and fate of cancer cells. Pharmaceutics. 2022;14(1):166.
Stefancikova L, Lacombe S, Salado D, Porcel E, Pagacova E, Tillement O, Lux F, Depes D, Kozubek S, Falk M. Effect of gadolinium-based nanoparticles on nuclear DNA damage and repair in glioblastoma tumor cells. J Nanobiotechnol. 2016;14(1):63.
Liu EK, Sulman EP, Wen PY, Kurz SC. Novel therapies for glioblastoma. Curr Neurol Neurosci Rep. 2020;20:19.
Minafra L, Bravatà V. Cell and molecular response to IORT treatment. Transl Cancer Res. 2014;3:32–47.
Dai LB, Zhong JT, Shen LF, Zhou SH, Lu ZJ, Bao YY, Fan J. Radiosensitizing effects of curcumin alone or combined with GLUT1 siRNA on laryngeal carcinoma cells through AMPK pathway-induced autophagy. J Cell Mol Med. 2021.
Koritzinsky M. Metformin: A novel biological modifier of tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:454–64.
Lesueur P, Chevalier F, Austry JB, Waissi W, Burckel H, Noël G, et al. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies. Oncotarget. 2017;8(40):69105–24.
Angel M, Zarba M, Sade JP. PARP inhibitors as a radiosensitizer: a future promising approach in prostate cancer? Ecancermedicalscience. 2021:15.
Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254–62.
Wang M, Li K, Zou Z, Li L, Zhu L, Wang Q, et al. Piperidine nitroxide tempol enhances cisplatin-induced apoptosis in ovarian cancer cells. Oncol Lett. 2018;16(4):4847–54.
Treatment of Radiation and Cisplatin Induced Toxicities With Tempol | Clinical Research Trial Listing (Mucositis | Ototoxicity | Nephrotoxicity) (NCT03480971). https://www.centerwatch.com/clinical-trials/listings/223105/mucositis-treatment-radiation-cisplatin-induced/
Monti E, Supino R, Colleoni M, Costa B, Ravizza R, Gariboldi MB. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J Cell Biochem. 2001;82(2):271–6.
Suy S, Mitchell JB, Ehleiter D, Haimovitz-Friedman A, Kasid U. Nitroxides tempol and tempo induce divergent signal transduction pathways in MDA-MB 231 breast cancer cells*. J Biol Chem. 1998;273:17871–8.
Further Reading
Du J, Zhang P, Cheng Y, Liu R, Liu H, Gao F, Sh C, Liu C. General principles of developing novel radioprotective agents for nuclear emergencies. Radiat Med Protect. 2020;1(3):120–6.
Moertl S, Mutschelknaus L, Heider T, Atkinson MJ. MicroRNAs (miRNAs) as novel elements in personalized radiotherapy. Transl Cancer Res. 2016;5(Supplement 6):S1262–9.
Ryan JL, Krishnan S, Movsas B, Coleman CN, Vikram B, Yoo SS. Decreasing the adverse effects of cancer therapy: an NCI workshop on the preclinical development of radiation injury mitigators/protectors. Radiat Res. 2011;176(5):688–91.
Wang B, Tanaka K, Morita A, Ninomiya Y, Maruyama K, Fujita K, Hosoi Y, Nenoi M. Sodium orthovanadate (vanadate), a potent mitigator of radiation-induced damage to the hematopoietic system in mice. J Radiat Res. 2013;54(4):620–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Annex 1
Annex 1
Contamination by radionuclides and medical countermeasures (MCMs)
Radiation type | Element | Radioactive half-life | Major exposure pathways | Focal accumulation | Medical countermeasure for internal contamination | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
α | Americium (Am-241) | 458 years | Inhalation, Skin | Lungs, Liver, Bone, Bone marrow | DTPA(Ca-DTPA/Zn-DTPA) Calcium or zinc diethylenetriaminepentaacetate | Chelating agents | FDA approved |
Uranium (U-235) | 7.1 × 108 years | Inhalation, Ingestion | Kidneys, bone | Sodium bicarbonate | Facilitates increased renal excretion | NCRP preferred | |
Polonium (Po-210) | 138.4 days | Inhalation, Ingestion Skin | Spleen, Kidneys, Lymph nodes, Bone marrow, Liver, Lung mucosa | Succimer (DMSA) (DailyMed), dimercaprol (BAL), d-penicillamine | Chelating agents | NCRP suggested | |
Plutonium (Pu-239) | 24,100 years | Inhalation (limited absorption) | Lung, Bone, Bone marrow, Liver, Gonads | DTPA (Ca-DTPA/Zn-DTPA), deferoxamine (DFOA) | Chelating agent | FDA approved | |
β | Phosphorus (P-32) | 4.3 days | Inhalation, Ingestion, Skin | Bone, Bone marrow, Rapidly replicating cells | Aluminum carbonate, sodium glycerophosphate, sodium phosphate, Potassium Phosphate, potassium phosphate, dibasic, sevelamer, aluminum hydroxide | Phosphate binders | NCRP suggested |
Strontium (Sr-90) | 28 years | Inhalation, Ingestion | Bone | Stabile strontium, aluminum Phosphate, ammonium Chloride, calcium gluconate, calcium carbonate, calcium phosphate, sodium alginate, barium sulfate Aluminum, hydroxide | Competes for bone-binding sites Phosphate binder, increases excretion, blocks intestinal absorption | NCRP suggested | |
Tritium (H-3) | 12.5 years | Inhalation, Ingestion Skin | Whole body | Water | Facilitates excretion | NCRP preferred | |
β, γ | Cesium (Cs-137) | 30 years | Inhalation, Ingestion | Follows potassium Renal excretion | Prussian blue | Ion exchange Inhibits enterohepatic recirculation in the GI tract | FDA approved |
Cobalt (Co-60) | 5.26 years | Inhalation | Liver | EDTA, dimercaprol, DTPA (Ca-DTPA/Zn-DTPA) | Chelating agents | NCRP suggested | |
Iodine (I-131) | 8.1 days | Inhalation, Ingestion Skin | Thyroid | KI (potassium Iodide), propylthiouracil | Blocking agents | FDA approved | |
Iridium (Ir-192) | 74 days | N/A | Spleen | EDTA, DTPA (Ca-DTPA/Zn-DTPA) | Chelating agents | NCRP suggested | |
α, γ, neutron | Curium (Cm-244) | 18 years | Inhalation, Ingestion | Liver, Bone | DTPA (Ca-DTPA/Zn-DTPA) | Chelating agent | FDA approved |
α, β, γ | Radium (Ra-226) | 1602 years | Ingestion | Bone | Aluminum hydroxide, calcium gluconate, calcium carbonate, calcium phosphate, sodium alginate, barium sulfate | Blocks intestinal absorption, competes for bone-binding sites Phosphate binder, increases excretion, blocks intestinal absorption | NCRP suggested |
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Montoro, A. et al. (2023). Radioprotectors, Radiomitigators, and Radiosensitizers. In: Baatout, S. (eds) Radiobiology Textbook. Springer, Cham. https://doi.org/10.1007/978-3-031-18810-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-18810-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18809-1
Online ISBN: 978-3-031-18810-7
eBook Packages: MedicineMedicine (R0)