Abstract
The synergetic potentials of essential ecosystem services have not been well explored under mountainous farming conditions in southern Africa. Cropping practices that maximize beneficial pollinators and reduce dependency on chemical inputs through efficient crop management in the mountainous environment are needed. The synergetic potentials of insect pollination on soybeans under varying soil fertility during two seasons in Phuthaditjhaba, the Free State, South Africa was examined. We manipulated soil fertility with fertilizer treatments and used exclusion bags to manipulate pollination intensity. High intensity of pollination services increased the seed yield by approximately 0.5 tons per hectare on optimally fertilized soil and 0.3 tons per hectare on minimally fertilized soil. This study found complementary benefits of using appropriate fertilizer rates on crop pollination. It is an efficient way to minimize losses in crop production and improve yields. However, minimal fertilizer application that is common among smallholder farmers still gave substantial yield in insect pollinator-rich environments such as Phuthaditjhaba. This finding gives an immense advantage to farmers in Phuthaditjhaba who tend to minimize the use of fertilizer due to financial issues. Harnessing the prospects from these ecosystem service benefits would help local communities to attain sustainable food production (SDGs 2 and 15).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Mountain regions harbor great biological richness and are centers of biodiversity, but are greatly affected by climate change causing shifts in temporal and spatial insect species distribution and habitat losses (Liu et al. 2019). The interaction of climate change on insect pollinators’ availability and the role of synthetic fertilizers during crop flowering on fragile mountain ecosystems are still unknown. Maintaining insect biodiversity and attaining sustainable food production in mountainous areas which is currently a challenge—contributes to achieving UN Sustainable Development Goal (SDG) 2 and even other SDGs. Therefore, improved crop management practices are essential (Viana et al. 2022).
The smallholding farming system is one of the unique and resilient strategies embraced by the mountainous community of Phuthaditjhaba in the eastern Free State, South Africa (Myeni et al. 2019; Adelabu et al. 2020). In addition, strategies that reduce reliance on chemical inputs and harness the potential of agro-ecological services in crop production could increase food security and sustainability in the community (Garratt et al. 2018; Adelabu et al. 2020). Despite the beneficial roles of ecosystem services in the agricultural landscapes of Phuthaditjhaba (Mukwada and Manatsa 2018), sustainable cropping is still very limited due to resource constraints, such as favourable agricultural policies, the use of agricultural biotechnologies, and the availability of extension expertise and funds, in comparison with other urban farming communities in South Africa (Grebitus et al. 2020).
Crop pollination is a crucial ecosystem service, while the response of crop yield to pollination is interwoven with soil nutrients and water management (Garibaldi et al. 2018). Embracing soil management practices that boost insect visitation may maximize crop yield and influence the usage of external inputs, thus affecting farmers’ income, insect visitors’ health and supporting the attainment of most of the 17 SDG goals, in particular 1, 2, 3, 10, 11, 12, and 15 (Garibaldi et al. 2020; Viana et al. 2022). These interactions have the potential to alter productivity and the performance of flowering crops, and an understanding of them is essential for proper management of multiple ecosystem services (Bartomeus et al. 2014; Kovács-Hostyánszki et al. 2017). Insect visitations are vulnerable to agricultural intensification, which reduces their biodiversity and abundance, consequently causing a substantial reduction in crop productivity and quality (Garibaldi et al. 2020). Studies indicated that yield benefits from common beans and other crops were affected by efficient interactions between soil nutrients and insect pollinations (Otieno et al. 2011; Bartomeus et al. 2014; van Gils et al. 2016) and other factors affecting crop yield such as micro-climate conditions, water, pest or disease status (Viana et al. 2022).
Cropping frequently relies on mineral fertilizers to boost crop productivity because of low soil nutrient availability (Wymann et al. 2014). The residual effects of these fertilizers disturb and alter soil compositions, consequently affecting the quantity and quality of flower resources, insect pollination and reducing the biodiversity hotspots (Campbell and Halama 1993; Ramos et al. 2018). Wagner et al. (2021) reported the abundance of insect pollinators and other native insect visitors to be decreasing globally. A reduction in floral resources available to insect pollinators was hypothesized to be a major reason behind pollinator loss (Hallett et al. 2017). There is an increasing interest in understanding the effects of agro-ecological conditions and soil nutrient management during the production of flowering crops such as soybeans under mountainous ecosystems (Klein et al. 2007; Kovács-Hostyánszki et al. 2017; Garibaldi et al. 2020).
Soybeans (Glycine max [L.] Merr) is a crop that benefits from insect pollination, though it is also capable of autonomous self-pollination. The most frequent insect visitors for nectar and pollen of soybean are honeybees, while Diptera and Coleoptera also play a vital role in its pollination (Santos et al. 2013; Fagúndez 2016; Blettler et al. 2018). Soybean production constitutes an important component in the mountain farming system of Phuthaditjhaba and in the cropping systems of South Africa in general (Liebenberg, 2013; Adelabu et al. 2020). South Africa has been a major producer of soybean for the last four decades, because of soybean’s multiple roles in the livestock feed industry, for human consumption, and its biological nitrogen fixation abilities (Grain 2016; Chigeza et al. 2019). However, average soybean yields of 0.5–1.0 tons/ha were recorded among smallholder farmers in South Africa, which is much lower than the global average yields of 2.5–3.0 tons/ha (Khojely et al. 2018). This gap was attributed to production constraints such as infrastructural problems, vulnerability to climate change, degradation of ecosystem services and poor management of soil nutrients (Mutegi and Zingore 2014).
The use of mineral fertilizer alters the physiology, behaviour and diversity of insect visitors (Ramos et al. 2018). Tamburini et al. (2017) found, in northeast Italy, a strong interaction between pollination and soil nutrient availability affecting sunflower seed production which was maximized at intermediate nutrient levels. Manson et al. (2022) found that pollinator diversity and pollinator visitation time declined with increasing chemical usage in coffee farms of varying management intensity in West Java, Indonesia. Studies by Otieno et al. (2011) and Ramos et al. (2018) showed positive effects of native pollinators on common bean (Phaseolus vulgaris L.) yield that were more pronounced under low nitrogen inputs. Other studies reported few significant interactions between insect pollination and fertilizer application on crop yield. Van Gils et al. (2016) found insect visitation rate on oilseed rape yield did not depend on the level of soil fertilization, suggesting that increased oilseed rape yield was enhanced by pollinator visitation, irrespective of mineral fertilizer application. These variations in findings make it difficult to ascertain the impact of the interaction on the yield of modestly insect-dependent crops such as soybeans. Also, literature that attribute and quantify the role of insect pollination and soil fertility during soybean production at national levels for specific production regions, such as mountainous environments, are scarce. The importance of insect pollination—an ecosystem service—with soil fertility management during soybean production is unclear. The objective of this study was therefore to examine the synergetic response of insect pollination and different levels of soil fertility on soybeans seed yield and productivity under mountainous conditions.
5.2 Materials and Methods
5.2.1 Plant Materials and Crop Management
Soybean seed (Fundacep 65RR cultivar) was purchased from Capstone Seed Company in Free State, South Africa. It is an early cycle hybrid with a maturity group of 5.5–5.9, with medium plant height, white flower and grey pubescence.
5.2.2 Experimental Site Description
The field experiment was conducted from 12 November to 27 April during 2017/18; 28 December to 13 May during the 2018/19 summer season at the research farm of Seotlong Agricultural and Hotel School (28°45’ S; 28°85’ E, 1660 m a s l.) located in Phuthaditjhaba, the Free State, South Africa (Fig. 5.1).
The region is dominated by grassland vegetation, short and tall sour grass with a highly variable climate having warm to hot months (average annual temperature of 18.4 °C) (Brand et al. 2019). The winter months can be cold with temperatures of −9.5 °C. The annual rainfall (650–850 mm) occurs for more than 85% between September and March (Maloti Drakensberg Transfrontier Programme 2015). The trial site was previously used for maize and sorghum production; it has large surrounding patches of natural vegetation (uncultivated areas) at a scale of 1.5 km2 during both seasons. The insect pollinators in the area are native pollinators that depend on the natural grasslands for their existence (Brand et al. 2019). Therefore, no specific permission was required to conduct this study because it did not involve the use of endangered or protected species.
5.2.3 Soil Sample Methodology
The soil at the experimental site was shallow, and classified as loamy-clayey soil (United States Department of Agriculture, 2014). Prior to planting each season, composite soil samples were randomly collected from 0–200 mm topsoil, then submitted for soil textural and fertility analyses at the soil fertility analytical services section, Department of Agriculture and Environmental Affairs, KwaZulu-Natal, South Africa. The soil exchangeable K, Cu and Zn, exchangeable Ca and Mg were analysed using rapid procedures (Farina 1981), while Bray (1948) was used for the available P. The total C and N were determined by Dumas dry combustion method and Walkley–Black method for the determination of organic carbon. Based on the information from the soil chemical analysis (Table 5.1), an optimal soil fertilizer recommendation of 80 kg/ha N and 40 kg/ha P was used.
The planting date in 2017/18 corresponded to the beginning of rainfall at Phuthaditjhaba where there was ≥25 mm rainfall in 7 days prior to planting, which is the conventional period for planting soybeans among South African farmers. However, planting in 2018/19 was late due to a delay in rainfall and a shortage of soil water content. Weeding was done by hand hoeing.
5.2.4 Experimental Design and Layout
In each planting season, the design was a split-plot replicated three times. The main plot comprised of soil fertility levels and sub-plots consisted of pollination rates. Based on soil chemical properties, soil fertility treatments consisted of N and P, where K fertilizer was not applied at all because the soil was not in the deficit of K. The soil fertility levels consisted of control (0 kg ha−1 N and 0 kg ha−1 P), minimal (40 kg ha−1 N and 20 kg ha−1 P) and optimal (80 kg ha−1 N and 40 kg ha−1 P) where urea (N) and triple superphosphate (P) granule fertilizers were used respectively. During both seasons, the trial site was 41.0 m × 12.8 m, with an individual plot size of 2.4 m × 3.6 m (8.64 m2), and 1 m inter plot spacing between the plots given 111,111 plants ha−1. This constituted four rows (planted 300 mm apart and 300 mm within rows) and the sampling units were made of inner rows where six plants were selected at each plot (Fig. 5.2).
Experimental design is 3 × 4 split plot design arranged in RCBD where sunflower,fertilizer levels [control (0 kg ha−1 N and 0 kg ha−1 P), minimal (40 kg ha−1 N: 20 kg ha−1 P) and optimal (80 kg ha−1 N: 40 kg ha−1 P)] and pollination rates (0%, 25%, 50% and 100%) were factors. Note: Soy, P0 = soybeans under 0% pollination rate, Soy, P1 = soybeans under 25% pollination, SoyP2 = soybeans under 50% pollination, SoyP3 = soybeans under 100%pollination
5.2.5 Plant Physiological Growth Measurements
Plant height, leaf number, chlorophyll content index (CCI) and stomatal conductance (gs) were measured on six randomly selected plants from the sampling units. Chlorophyll content index (CCI) was measured using a portable SPAD meter (SPAD-502-PLUS chlorophyll meter, Konica Minolta, Ramsey, New Jersey, USA) on the adaxial leaf surface. gs was measured from the abaxial leaf surface during midday (12:00 p.m. and 14:00 p.m.) using a steady-state leaf porometer (Model SC-1, Decagon Devices, USA).
The pods were manually harvested and threshed and air-dried in the laboratory until they reached a moisture content below 12%, after which moisture content was determined on samples by oven drying. Yield components such as seed yield (kg ha−1), thousand seed weight (kg), fruit weight/head (kg), head diameter (mm) and total biomass (kg ha−1) were recorded. An average of six plant stands per plot was used for each parameter. The harvest index was computed as:
5.2.6 Pollinator Exclusion
Pollination treatments were applied before flowering at the first reproductive stage of soybeans (R1 stage) in each plot. Mesh net placement and removal were performed according to the respective pollination treatments between 08:00 a.m. and 10:00 a.m. Plots were randomly assigned to four levels of insect pollination (0, 25, 50 and 100% pollination levels) at each soil fertility level. The different pollination levels were carried out using pollinator exclusion approach according to Tamburini et al. (2017) where the number of days those flowers were exposed to insect pollination was manipulated. Hence, during a hypothetical flowering period of 21–28 days, insects could visit flower heads 0, 7, 14 and 28 days, for 0%, 25%, 50% and 100% pollination rates respectively. Each of the mesh net was 1.8 × 1.5 m (1.2 mm diameter), fixed to the plant base to prevent insects from pollinating the plant. Newly emerged open flowers were cut from the plants and nets removed when marked flowers had finished flowering to allow undisturbed plant growth.
The 0% pollination rate was achieved by complete exclusion of the plants from insect visitation (no insect visitation) for the 28 days duration of flowering. Here, flowers were enclosed by tying large tulle mesh nets firmly around the stem of the six selected plants within the inner rows of each plot. The crop with 100% pollination rate had its equivalent area completely open to insect visitation (all insects could visit flowers) (Jacobs et al. 2009). Plants with 25% pollination rate consists one-day complete open for insect visitation followed by three days of covering with the mesh (no insect allowed). 50% pollination rate was attained by one day insect visitation where the mesh was removed for 24 h and later covered for another 24 h (no insect allowed). Crop phenology from planting to physiological maturity was followed weekly (Fehr and Caviness 1977).
5.2.7 Insect Visitation and Insect Sampling
The flowering period was from R2 (full bloom) to R4 (full pod) which took between 19 and 28 days. The guild-specific pollination behaviour was assessed following Tamburini et al. (2017), where the number of flowers visited was recorded on a subset of six randomly selected plants during one visitation event, for each pollinator guild (honeybees, beetles, butterflies and hoverflies). The average number of visitation events was calculated for each guild and used to estimate the number of flowers visited per plant. This was done daily by 5 min transect walks in each plot between 10:00 a.m. and14:00 p.m.. All insects touching the plant flowers were recorded at each soil fertility level. Observations were made at a distance of 80 cm from the flower to avoid disturbance, six plants were marked with a small piece of tape in each plot. The visitation rate of insects such as bees (Hymenoptera: Apoidea), hoverflies (Diptera: Syrphidae), ants (Hymenoptera: Formicidea), thrips (Thysanoptera:Thripidae) and butterflies (Lepidoptera:Nymphalidae) were observed and counted. Unidentified specimens were captured on the field and dichotomous keys and identification guides were used to identify insects (Bugg et al. 2008). This was carried out on days when temperatures were at or above 15℃, with no precipitation, dry vegetation, and low wind speed.
5.2.8 Yield Measurements
At the end of the season, all plants with marked racemes (six per study plot) were harvested from the plants and put individually in paper bags. Seed yield was determined after physiological maturity (R8) by cutting all plants at the ground level at each plot, followed by manual threshing. The mean of six plants was used for each parameter measured.
5.2.9 Weather Data
Daily weather data were obtained from an automatic weather station at University of the Free State QwaQwa campus (Lat 28.5, Long 28.8 and 1699 m.asl) managed by the Agricultural Research Council (ARC) and located 1 km from the experimental site. The weather parameters considered were rainfall (mm), maximum (Tx) and minimum (Tn) temperatures (℃), maximum (RHx) and minimum (RHn) relative humidity (%), and evapotranspiration (mm).
5.3 Statistical Analyses
Data were analyzed using linear mixed-effect models (GLM). We analyzed the fixed effect as the pollination rates, soil fertility and their interaction on the proportion of yield components. To account for non-independence in the data, replications and years were included as random effect analyses. For the yield components and insect visitation, ordinary least squares (OLS) models were used. These models include only fixed effects and interactions. The plant physiology parameters, which consisted of plant chlorophyll content index and stomatal conductivity variables, were analyzed using Linear Mixed Effects Models (LME) and a random effect (the weeks after planting). The mixed models were fitted using the standard restricted maximum likelihood (REML) approach. Linear models were used to evaluate the effect of pollination rates, soil fertilization and their interactions on the yield components (fruit head diameter, fruit weight, thousand seed weight, total biomass, seed moisture content and seed yield). Visitation rate for insect types was firstly transformed by adding 1 to all counts and taking the natural logarithm. Where the ANOVA indicated significant differences (P < 0.05), we compared the treatment means using the Tukey test. All statistical analyses were performed using SPSS version 25.
5.4 Results
5.4.1 The Weather and Soil Conditions
During the 2017/18 season, rainfall in March was higher compared to other months which coincided with soybeans’ vegetative growth stage. Rain decreased in April. In the 2018/19 season, plants experienced drought at the seedling stage (January) and an even distribution of rain in February and March, with prolonged rainfall into April. The air temperature and relative humidity in both seasons showed similar trends, while the minimum relative humidity in 2018/19 was a bit higher compared to 2017/18 (Fig. 5.3).
The weather conditions during the critical periods of flowering and pod formation were almost similar in both years, while 2018/19 was drier and experienced higher temperature during seed filling compared with 2017/18 season. The soil pH at both seasons was slightly acidic but still within the suitable pH levels for soybean production in South Africa. The organic carbon, soil density, extractable K+, and total N were comparable in both seasons. The Ca2+, Mg2+ and total cations were higher in the soil during 2017/18 compared to the 2018/19 season (Table 5.1).
5.4.2 Yield Compositions
The crop experienced drought in the 2018/19 season which greatly affected its yield but its seed yield was still improved with pollination rate (F3,48 = 5.294; P = 0.003) and soil fertility (F2,48 = 27.306; P < 0.001). Both had synergetic effects on the yield (F11,48 = 2.274; P = 0.025; Table 5.2).
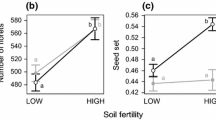
Pollination increased seed yield by approximately 0.53 and 0.32 tons per hectare on optimally and minimally fertilized soil and by approximately 0.16 tons per hectare on poorly fertilized soil (Table 5.2). The pollination rates (F3,, 48 = 1.054; P = 0.377) and soil fertility (F2, 48 = 2.192; P = 0.123) as well as their interaction (F11, 48 = 1.206; P = 0.309; Table 5.1) did not lead to increase total biomass across the years (Table 5.3).
The individual effect of soil fertility (F2,48 = 17.844; P < 0.001) and pollination rate (F3,48 = 9.529; P < 0.001) significantly influenced seed weight, but the interactions of both factors did not influence the seed weight (F11,48 = 0.528; P = 0.875; Table 5.3). The heaviest seed weights were obtained from optimal soil fertility grown under abundant insect visitation. Seed weights were 0.30- and 0.14-times higher compared with seeds from poor soil fertility with poor pollinator visitation (Table 5.3). Soil fertility (F2, 48 = 23.366; P < 0.001) and pollination rate (F3, 48 = 3.648; P = 0.019) influenced the number of pods/plant. However, no interaction effects were observed here. Higher pollination rates increased the number of pod/plants. Pod/plants harvested from minimal and optimal soil fertility conditions with exposure to abundance pollinator visitations gave similar values (Table 5.3). Pollination rate (F3, 48 = 2.688; P = 0.05) and soil fertility (F2, 48 = 54.954; P < 0.001) increased seed number/pod. Moreover, there was an interaction between the two factors (F11, 48 = 3.341; P = 0.002; Table 5.2), where increases in seed number/pod were higher with increases in soil fertility and pollination (Table 5.3).
The synergetic effect of soil fertility and pollination rate significantly increased the number of bees, (Hymenoptera Apidae,) (F(9,72) 4.146; P = 0.008), hoverflies (Diptera: Syrphidae) (F(9,72) 2.493; P = 0.015), ants (Hymenoptera: Formicidae) (F(9,72) 3.615; P = 0.001), visitation to soybean flower across years. However, this interaction did not influence the visitation rate of the butterflies (Lepidoptera Nymhalidae) (F(9,72) 0.910; P = 0.097), beetles Coleoptera (Coccinellidae), (F(9,72) 1.591; P = 0.134), and thrips (F(9,72) 0.247; P = 0.911; Table 5.4).
Optimal soil fertility with an abundance pollination rate experienced the highest number (7.89 bee/plot) of bees’ visitations (Fig. 5.4). Plants under 25% pollination rate experienced a similar number of bees’ visitation at each level of soil fertility. The number of ants visiting the plant at the minimal pollination rate was higher than at 100% pollination rate. Also, the number of hoverflies and butterflies that visited the plants increased with an increase in soil fertility, while the number of beetle/plots increased with an improvement in pollination rate (Fig. 5.4).
Similarly, bee visitation was highest (5.4 bee/plots in 2017/18 and 6.0 bee/plots in 2018/19) at optimal pollination rate. We observed high insect visitation at minimal soil fertility conditions across the years. Also, the minimal pollination rate received an increased number of hoverflies (3.5 hoverflies/plot) and butterflies (2.7 butterflies/plot) in 2017/18 compared to the optimal pollination rate in 2018/19 season. However, plants under minimal soil fertility with 100% pollination rate had a reduced number of thrips visitation at both seasons (Fig. 5.5).
There were no differences in the number of bees’ visitation (4.7 bee/plot) at poor soil fertility conditions. The visitation of hoverflies was highest (3.8 hoverflies/plot) at the optimal pollination rate in 2017/18, while butterflies and ants’ visitation were higher at the minimal pollination rate (1.8 butterflies/plot and 14.3 ants/plot). The number of thrips increased with increases in pollination rate. Also, the insect visitation rates were higher in 2017/18 compared with the 2018/19 season (Fig. 5.6).
5.4.3 Plant Growth and Physiological Parameters
As expected, plant physiological growth indices (plant height, leaf number, stomatal conductance and chlorophyll content index) responded positively to improved soil fertility resulting from fertilizer application (Table 5.5). Plant physiological growth indices measured highlighted the effect of soil fertility on plant growth. Thus, their explicit results were included in Figs. 5.4, 5.5 and 5.6.
5.5 Discussion
The synergetic benefits from insect pollination and soil fertility differ among soybean varieties and growing conditions (Chacoff et al. 2010; Chigeza et al. 2019). Therefore, we examined the beneficial response of the most grown soybean variety in southern Africa under mountainous growing conditions in Phuthaditjhaba. We found that synergetic interactions between insect pollination and soil fertility improved soybean seed production. A significant decline in seed yield, pod number/plant, seed number/pod and seed weight were observed when insect visitors were excluded from soybeans plants. Moreover, the impact of insect pollination was complemented by improved soil fertility. Plants under optimal soil fertility with complete access to insect pollination had improvement in their plant growth physiological parameters and gave the highest seed yield of approximately 0.53 tons per hectare. Plants on minimally fertilized soil with complete access to insect pollination showed 0.32 tons per hectare increment in seed yield. Abundant insect pollinators could only give an increase of approximately 0.16 tons per hectare on poorly fertilized soil. It was noteworthy that minimal soil fertility under abundant insect visitation also led to a substantial yield increment.
This was in concordance with Garratt et al. (2014) who reported insect visitation improving seed set between 60 and 69%, though the soil fertility of growing conditions were not emphasized. Similarly, Huais et al. (2020) explained that small patches of forest encourage native and wild floral insect visitors to ensure greater soybeans yield due to an increase in pollen deposition and seed set. Milfont et al. (2013) observed an increase in soybean yield up to 18% when exposed to honey bees and other native insect pollinators. Our results support these findings insofar as it highlighted the complementary action of insect visitation on soybean flower and soil fertility impacting seed yield, pod/plant and seed weight. This was likely due to the high quality of nectareous resources in the plant from optimally fertilized soil (Adelabu et al. 2021). Soybean has been reported as the most important source of pollen for insect visitors especially bees during the peak flowering periods (Blettler et al. 2018). Soil fertility increases the action of the insect visitors by boosting seed set and relating with a better pollen distribution on the stigmatic surface. This might be due to the intimate contact between the abdominal portion of the insect and the receptive structure of the flower (Milfont et al. 2013). However, the abundance of insect pollination under poor soil fertility did not improve soybean seed production. Thus, restrictive soil management conditions during soybeans growth and the critical period of seed set underlie its yield (Blettler et al. 2018).
The substantial increase in yield and its components (seed yield, seed number, pod number and seed weight) obtained from minimal soil fertility with unrestricted access to insect visitation suggested that minimal soil fertility still enhanced the needed forage resources and diversity of insect visitors and moderately contribute to the increase in soybean production. This yield increase implies that under favourable entomological conditions, smallholder farmers would need to make decisions about the yield and profit implications of applying below-recommended fertilizer rates. Langyintuo (2020) observed that smallholder farmers in Sub Saharan Africa tend to use below-recommended fertilizer rates during crop production due to financial constraints. Ramos et al. (2018) reported that the benefits from insect pollinators for common bean were attained at minimal nitrogen input (≤72 kg N/ha), whereas native insect pollinators had positive effects on crop yield. Otieno et al. (2011) showed that fertilizer application significantly enhanced pollinators’ activity, as well as the abundance of chewing and sucking insect pests in pigeon pea.
Soybean’s crop is an autogamous crop that modestly depends on flower-visitor insects (Klein et al. 2007; Chacoff et al. 2010; Milfont et al. 2013). A range of insect visitors such as bees, butterflies, flies, thrips and wasps were observed to be responsible for soybean pollination, contributing to seed set through the collection of energy-rich nectar. However, the effectiveness of each insect species was not studied. Among the dominant insect visitors of soybean flowers were bees, beetles and thrips, foraging on, inside, or very close to the flower. We found that bees, hoverflies and thrips were among the most abundant insect visitors of soybean flowers. The abundance of these visitors to the flower under complete access to insect pollination contributed to the yield increment. Thrips and bees were considered as more effective pollinators of soybeans, while thrips could also be pests that eat the pollen or leaves (Yoshimura 2011). A high abundance of ants was found on the flowers, but their specific contribution was not studied. Rostás et al. (2018) observed that ant pollination contributes little to crop pollination even with their high abundance and regular visits, because they are nectar robbers, but their ability to forage among flowers under adverse climatic conditions has been suggested to promote crop pollination.
The insect populations in the surroundings of the crop were not disturbed by pesticides because phytophagous insects were below the economic injury threshold, which shows that all insect visitors to the crop have positive contribution to its pollination whether predators, natural enemies, pests or pollinators. Our results indicate complementary benefits of using appropriate fertilizer rate on crop pollination. It is an efficient way to minimize loss and improve nutrient use efficiency in the crop. However, minimal fertilizer application that is common among smallholder farmers still gave substantial yield under insect pollinator-rich environments, such as in Phuthaditjhaba. The richness of insect biodiversity in Phuthaditjhaba may be similar to other montane wetland vegetation of the Maloti-Drakensberg region (Brand et al. 2010). There were large patches of natural vegetation within 500 m of the trial site, which serve as a habitat for the insect visitors. Although we did not focus on the scale of the pollinator community and diversity within the cropping area, flower-visitor assemblages in agroecosystems are increasingly threatened because of increased exposure to inappropriate use of synthetic chemicals which are responsible for yield gaps of pollinator-dependent crops in smallholder farming system (Garibaldi et al. 2016). Mountain farming is characterized by a smallholder farming system, with rich biodiversity but with low soil nutrients, all characteristics found in our Phuthaditjhaba test-site (Adelabu et al. 2020). Maximizing the potential of the native insect populations with efficient soil management practices could help to increase crop production.
5.6 Conclusions
The complementary impact of soil fertility and insect pollination is essential to developing management strategies that harness the potential of the natural environment for crop benefits, particularly in areas where fertilizers are prohibitively expensive. Our findings highlighted the importance of soil fertility and insect pollinators to soybean seed yield in a mountainous farming ecosystem located in Phuthaditjhaba. The benefits of biotic pollinators to improve yield depended on the soil fertility status of the plant. The results of this study encourage soybeans smallholder farmers to maximize the potential of native pollinators in their cultivating areas of Phuthaditjhaba to enhance productivity, thus promoting sustainable management practices and reducing dependence on purchased fertilizers. Embracing this mountainous ecosystem’s interactive benefits would provide an immense advantage for smallholder farmers. Due to limited financial resources, such farmers tend to use below the recommended fertilizer rates, with implications for yield and economic viability. However, ecosystem services such as insect pollination would help local communities to attain sustainable food production, thus attaining the UN SDGs 2 and 15 which are address ‘zero hunger’ through sustainable and resilient food production (SDG 2.4) and ‘life on land’ through the conservation of mountain ecosystems (SDG targets 15.1 and 15.4).
Phuthaditjhaba: Urban Fabric and Population Figures
Jess L. Delves Stefano TerziPhuthaditjhaba can be described as a ‘ruralopolis’ due to its high population density, which exceeds that of many more urbanised areas. This density, combined with an agrarian economy and relatively more traditional social structure, characterizes the area as rural (Qadeer 2000). The city is a sprawling semi-urban space of detached, single story houses, which extends over more than 200 km2. The semi-urban fabric is for the most part homogenous with boundaries between different administrative units not clearly visible. The ‘center’ of the city, towards the northern edge, comprises two shopping malls surrounded by a concentration formal and informal businesses (Fig. 5.7).
The area of contemporary Phuthaditjhaba had a population of 14,000 in 1970 (Krige et al. 1995), which grew rapidly following the designation of Phuthaditjhaba as the capital of the QwaQwa homeland in 1974 (Pickles and Woods 1992). The city expanded outward from the urban centre, with centrally planned urban expansion incorporating road, electricity and water infrastructure. Consequently, much of the centre and surrounding area are characterized by an organized and planned urban structure. This contrasts starkly with the informal settlement structure of the majority of the city. Particularly in steeper areas, houses are not connected to roads and are only reachable by informal footpaths. This contrast can be seen in Figs. 5.8 and 5.9. As the city has expanded over the last 50 years, the municipal administration has not managed to meet the service and infrastructure needs of many areas of the city. This is exacerbated by the semi-formal management of housing construction where chiefs allocate the land available for construction often without being aware of the existing planning from the administration. Houses are often built on top of underground infrastructure or in areas that are not served by basic services. As a consequence, many new constructions are not registered with the municipal authorities and may interfere with, or cannot be connected to, essential infrastructure planned, provided by and requiring maintenance from the municipality (for example water, electricity, sewage).
From an administrative point of view, the urban area commonly referred to as Phuthaditjhaba is in fact a conglomeration of 65 smaller administrative units called Main Places. Only the ‘historic centre’ of the city is officially named Phuthaditjhaba in administrative statistics, and had a population of 54,661 during the 2011 census. The other areas carry the names of the villages which comprise them. This is the first challenge faced when seeking to establish an official population figure for the city, as defining the boundaries of the urban area is very difficult, and inter-village boundaries are unclear or may even be contested.
In addition, informal constructions continue to grow and obtaining data on these is difficult. In fact, it is not clear whether such data is kept. The 2011 census put the population of the Phuthaditjhaba Main Place—the small administrative unit that comprises the ‘city centre’—at 54,661 inhabitants. However, when considering all the administrative units that comprise the ruralopolis, the figure is 274,840 for 2011.
Phuthaditjhaba—the ruralopolis—has undoubtedly expanded spatially since the 2011 census, together with a correlated growth in population. Local researchers and administrators interviewed for this book agree that the actual population is likely far higher than the official number. Estimates range from 300,000 to 1.2 million residents. The 2007 Community Survey put the population of the municipality (including the urban areas of Harrismith and Kestell) at 385,413 (MaP 2011). However, in preparation for its 2010/2011 Integrated Development Plan (IDP), the Maluti-a-Phofung local municipality commissioned a private engineering consultancy (Miletus Consulting Engineers) to estimate the municipality’s population. The resulting study digitally logged 83,000 households in the municipality, and “In line with a generally accepted density of 6 persons/dwelling, the 2007 population of Qwaqwa was estimated at 500 000” (MaP 2011: 21). The IDP then states “total population of 620 000 persons is considered accurate for the planning of water and sanitation services” (idem). This figure is far higher that the official population for the municipality as reported from the 2011 census, which was 335,784 (StatsSA 2011).
These extreme discrepancies in population numbers even from official sources is a concern for research. Establishing a reliable figure for population is absolutely necessary for research and for government planning, in order to develop effective and targeted policies, in particular relating to the distribution and allocation of infrastructure, services and resources.
A census was conducted in February–March 2022 which will provide official data on population in Phuthaditjhaba. However, considering the rapid and informal expansion of the city, together with the potential for error in official statistics due to the difficulty in access to large areas of Phuthaditjhaba, we propose to cross-validate this data using, for example, remote sensing.
References
Adelabu DB, Bredenhand E, van der Merwe S, Franke AC (2021) Soil fertilization synergistically enhances the impact of pollination services in increasing seed yield of sunflower under dryland conditions. J Agric Sci 159:258–271
Adelabu DB, Clark VR, Bredenhand E (2020) Potential for sustainable mountain farming: challenges and prospects for sustainable smallholder farming in the Maloti-Drakensberg mountains. Mt Res Dev 40:1–11
Bartomeus I, Potts SG, Steffan-Dewenter I, Vaissiere BE, Woyciechowski M, Krewenka KM, Tscheulin T, Roberts SP, Szentgyörgyi H, Westphal C (2014) Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2:e328
Blettler DC, FagÚNdez GA, Caviglia OP (2018) Contribution of honeybees to soybean yield. Apidologie 49:101–111
Brand R, Scott-Shaw C, O’Connor T (2019) The alpine flora on inselberg summits in the Maloti-Drakensberg Park, KwaZuluNatal South Africa. Bothalia 49(1):a2386
Brand RF, Brown LR, du Preez PJ (2010) A floristic analysis of the vegetation of Platberg, Eastern Free State, South Africa. Koedoe 52:11
Campbell DR, Halama KJ (1993) Resource and pollen limitations to lifetime seed production in a natural plant population. Ecol Lett 74:1043–1051
Chacoff NP, Morales CL, Garibaldi LA, Ashworth L, Aizen MA (2010) Pollinator dependence of Argentinean agriculture: current status and temporal analysis
Chigeza G, Boahen S, Gedil M, Agoyi E, Mushoriwa H, Denwar N, Gondwe T, Tesfaye A, Kamara A, Alamu OE (2019) Public sector soybean (Glycine max) breeding: advances in cultivar development in the African tropics. Plant Breeding 138:455–464
Fagúndez G (2016) Botanical and geographical characterisation of honeys in Diamante, Entre Ríos, Argentina. Palynology 40:308–321
Fehr WR, Caviness CE (1977) Stages of soybean development
Garibaldi LA, Andersson GK, Requier F, Fijen TP, Hipólito J, Kleijn D, Pérez-Méndez N, Rollin O (2018) Complementarity and synergisms among ecosystem services supporting crop yield. Glob Food Sec 17:38–47
Garibaldi LA, Carvalheiro LG, Vaissière BE, Gemmill-Herren B, Hipólito J, Freitas BM, Ngo HT, Azzu N, Sáez A, Åström J (2016) Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 351:388–391
Garibaldi LA, Sáez A, Aizen MA, Fijen T, Bartomeus I (2020) Crop pollination management needs flower‐visitor monitoring and target values. J Appl Ecol
Garratt MPD, Coston DJ, Truslove CL, Lappage MG, Polce C, Dean R, Biesmeijer JC, Potts SG (2014) The identity of crop pollinators helps target conservation for improved ecosystem services. Biol Cons 169:128–135
Grain SA (2016) South Africa's soybean industry: a brief overview. Grain SA, South Africa
Grebitus C, Chenarides L, Muenich R, Mahalov A (2020) Consumers’ perception of urban farming—an exploratory study. Front Sustain Food Syst 4
Hallett AC, Mitchell RJ, Chamberlain ER, Karron JD (2017) Pollination success following loss of a frequent pollinator: the role of compensatory visitation by other effective pollinators. AoB Plants 9
Huais PY, Grilli G, Amarilla LD, Torres C, Fernández L, Galetto L (2020) Forest fragments influence pollination and yield of soybean crops in Chaco landscapes. Basic Appl Ecol 48:61–72
Jacobs JH, Clark SJ, Denholm I, Goulson D, Stoate C, Osborne JL (2009) Pollination biology of fruit-bearing hedgerow plants and the role of flower-visiting insects in fruit-set. Ann Bot 104:1397–1404
Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci 274:303–313
Kovács-Hostyánszki A, Anahí E, Vanbergen A, Settele J, Kremen C, Dicks L (2017) Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol Lett 20
Langyintuo A (2020) Smallholder farmers’ access to inputs and finance in Africa. The Role of Smallholder Farms in Food and Nutrition Security. Springer, Cham, pp 133–152
Liebenberg F (2013) South African agricultural production, productivity and research performance in the 20th century. University of Pretoria
Liu Y, Su X, Shrestha N, Xu X, Wang S, Li Y, Wang Q, Sandanov D, Wang Z (2019) Effects of contemporary environment and Quaternary climate change on drylands plant diversity differ between growth forms. Ecography 42:334–345
Maluti-a-Phofung IDP Steering Committee (MaP) (2011) Integrated Development Plan 2010/2011 for Maluti a Phofung. National Treasury, Republic of South Africa
Manson S, Nekaris K, Hedger K, Balestri M, Ahmad N, Adinda E, Budiadi B, Imron MA, Nijman V, Campera M (2022) Flower visitation time and number of visitor species are reduced by the use of agrochemicals in coffee home gardens. Agronomy 12:509
Milfont MO, Rocha EEM, Lima AON, Freitas BM (2013) Higher soybean production using honeybee andwild pollinators, a sustainable alternative to pesticides andautopollination. Environ Chem Lett 11:335–341
Mukwada G, Manatsa D (2018) Spatiotemporal analysis of the effect of climate change on vegetation health in the Drakensberg Mountain Region of South Africa. Environ Monit Assess 190:1–21
Mutegi J, Zingore S (2014) Boosting soybean production for improved food security and incomes in Africa
Myeni L, Moeletsi M, Thavhana M, Randela M, Mokoena L (2019) Barriers affecting sustainable agricultural productivity of smallholder farmers in the Eastern Free State of South Africa. Sustainability 11:3003
Otieno M, Woodcock BA, Wilby A, Vogiatzakis IN, Mauchline AL, Gikungu MW, Potts SG (2011) Local management and landscape drivers of pollination and biological control services in a Kenyan agro-ecosystem. Biol Cons 144:2424–2431
Pickles J, Woods J (1992) South Africa's homelands in the age of reform: The case of QwaQwa. Ann Assoc Am Geograph 82:629-652. https://doi.org/10.1111/j.1467-8306.1992.tb01721.x
Ramos DL, Bustamante MMC, Silva FDS, Carvalheiro LG (2018) Crop fertilization affects pollination service provision—common bean as a case study. PLoS ONE 13:e0204460–e0204460
Rostás M, Bollmann F, Saville D, Riedel M (2018) Ants contribute to pollination but not to reproduction in a rare calcareous grassland forb. PeerJ 6:e4369–e4369
Santos E, Mendoza Y, Vera M, Carrasco-Letelier L, Díaz S, Invernizzi C (2013) Aumentoen la producción de semillas de soja (Glycine max) empleando abejas melíferas (Apis mellifera). Agrociencia Uruguay 17:81–90
Statistics South Africa (StatsSA) (2011) Census 2011 Municipal report, Free State, Report 03-01-52. http://www.statssa.gov.za/census/census_2011/census_products/FS_Municipal_Report.pdf
Tamburini G, Lami F, Marini L (2017) Pollination benefits are maximized at intermediate nutrient levels. Proc R Soc B Biol Sci 284:20170729
van Gils S, van der Putten WH, Kleijn D (2016) Can above-ground ecosystem services compensate for reduced fertilizer input and soil organic matter in annual crops? J Appl Ecol 53:1186–1194
Viana CM, Freire D, Abrantes P, Rocha J, Pereira P (2022) Agricultural land systems importance for supporting food security and sustainable development goals: a systematic review. Sci Total Environ 806:150718
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: death by a thousand cuts. Proc Nat Acad Sci 118
Wymann von Dach S, Romeo R, Vita A, Wurzinger M, Kohler T (2014) Mountain farming is family farming: a contribution from mountain areas to the international year of family farming. FAO CDE, Boku, Rome, Italy
Yoshimura Y (2011) Wind tunnel and field assessment of pollen dispersal in soybean [Glycine max (L.) Merr.]. J Plant Res 124:109–114
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Adelabu, D.B., Franke, A.C. (2023). Beneficial Role of Pollination and Soil Fertility for Soybean Production in Mountainous Farming Conditions. In: Membretti, A., Taylor, S.J., Delves, J.L. (eds) Sustainable Futures in Southern Africa’s Mountains. Sustainable Development Goals Series. Springer, Cham. https://doi.org/10.1007/978-3-031-15773-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-15773-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15772-1
Online ISBN: 978-3-031-15773-8
eBook Packages: Social SciencesSocial Sciences (R0)