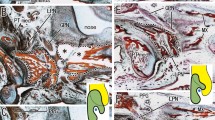
Abstract
The purpose of this communication is to explore in detail the developmental anatomy of the soft palate, its pathologies, and strategies for management. Despite the voluminous literature regarding complete cleft palate in its usual presentation, little attention has been paid to the developmental biology of palatal bone structures. Defects in individual bone fields create a spectrum of pathologies, ranging in severity from the submucous variant, with nothing notable save a groove and a palpable defect of the posterior spine, all the way to a complete disruption of the soft tissue envelope and the horizontal palatine shelves. All these presentations are but variations of a common pathologic mechanism: stem cell failure of individual angiosomes.
In this chapter, we consider the embryologic events that generate the mesenchymal building blocks from which the posterior palate is constructed: the palatine bone, oral and nasal mucosa, the palatine aponeurosis, and muscle slings. Palate structures develop from neural crest (NC) and mesoderm; these tissues originate at specific sites along the axis of the embryo and they can be mapped according to the developmental units of the CNS from which they are innervated. These units, called neuromeres, are specific zones within the neural tube, the boundaries of which are established by the expression pattern of homeotic genes. The forebrain (prosencephalon) consists of a telencephalon and 3 prosomeres, the midbrain (mesencephalon) has 1–2 mesomeres, and the hindbrain (rhombencephalon) has 12 rhombomeres. Each neuromere has a specific neuroanatomic content and is hardwired to specific tissues outside the brain. The learning objective of this chapter is to understand the neuromeric construction of the hard palate.
We next consider a model of the palate which is analogous to a pinball machine that consists of a platform (bone) and a mobile “flippers” or lever arms (the velum). The osseous platform is discussed in detail, with neural crest bones being coded by the sensory innervation of their surrounding soft tissue envelope. Maxilla, the palatine bone, and the vomers are all derivatives of hindbrain neural crest arising from rhombomere 2 but distributed according to various neurovascular pedicles of the V2 stapedial system, the anatomy of which will be explained in detail. Next, the evolution of palate will be presented as a series of innovations favoring increased metabolic capacity. A final appendix presents a functional classification of cranial nerves which I have endeavored to make straightforward. This will prove useful when reading the second part of this manuscript, which has to do with the neuromuscular apparatus of the soft palate.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
O’Rahilly R, Müller F. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs. 2010;192:73–84.
Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–76.
Puelles L, Harrison M, Paxinos G, Watson C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 2013;36:570–8.
Tomás-Roca L, Corral-San-Miguel R, Aroca P, Puelles L, Marín F. Crypto-rhombomeres of the mouse medulla oblongata, defined by molecular and morphological features. Brain Struct Funct. 2016;221:815–38.
Puelles L. Forebrain development: the prosomeric model. 2009. http://www.nslc.wustl.edu/courses/bio3411/woolsey/Readings/Lecture7/Puelles%202009.pdf.
Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–218.
Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–15.
Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207(5):501–61.
Hall BK. The neural crest in vertebrate development and evolution. 2nd ed. New York: Springer; 2009.
Trainor P. Neural crest cells: evolution, development, and disease. Cambridge: Academic Press; 2014.
Barry A. The aortic arch derivatives in human adult. Anat Rec. 1951;111:221–38.
Noden DM. Origins and assembly of avian embryonic blood vessels. Ann N Y Acad Sci. 1990;588:236–49.
Diamond MK. Homologies of the meningeal-orbital arteries of humans: a reappraisal. J Anat. 1991a;178:223–41.
Hansen L, Nolting D, Holm G, Hansen BF, Kjaer I. Abnormal vomer development in human fetuses with isolated cleft palate. Cleft Palate Craniofac J. 2004;41:470–3.
Kimes KR, Mooney MP, Siegel MI, Todhunter JS. Growth rate of the vomer in normal and cleft lip and palate human fetal specimens. Cleft Palate Craniofac J. 1992;29:38–42.
Sandikcioglu M, Mølsted K, Kjaer I. The prenatal development of the human nasal and vomeral bones. J Craniofac Genet Dev Biol. 1994;14:124–34.
Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, et al. A new function of BMP4: dual role for BMP4 in regulation of sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–43.
Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–46.
Mohri M, Amatsu M. Congenital defects of the vomer. Ann Otol Rhinol Laryngol. 2000;109:497–9.
Miller IJ, Spangler KM. Taste bud distribution and innervation on the palate of the rat. Chem Senses. 1982;7(1):99–108.
Benton M. Vertebrate paleontology. 4th ed. Oxford: Wiley-Blackwell; 2015.
Ewings E, Carstens MH. Neuroembryology and functional anatomy of craniofacial clefts. Indian J Plast Surg. 2009;42(Suppl):S19–34. https://doi.org/10.4103/0970-0358.57184.
Tuncbilek G. Congenital isolated absence of the nasal cartilaginous septum. J Plast Reconstr Aesthet Surg. 2008;61:e1–4.
Bocconi L, Boschetto C, Cerani F, Kustermann A. Fetal breathing movements. In: Piontelli A, editor. Development of normal fetal movements. Milan: Springer Italia; 2010.
Talmant JC, Talmant JC. Cleft rhinoplasty, from primary to secondary surgery. Ann Chir Plast Esthet. 2014;59(6):555–84. https://doi.org/10.1016/j.anplas.2014.08.004.
Yan DJ, Lenoir V, Chatelain S, Stefanelli A, Becker M. Congenital vomer agenesis a rare and poorly understood condition revealed by cone beam Ct. Diagnostics. 2018;8:15–9.
Naidu P, Yao CA, Chong DK, Magee WP III. Cleft palate repair: a history of techniques and variations. Plast Reconstr Surg Glob Open. 2022;10(3):e4019. https://doi.org/10.1097/GOX.0000000000004019.
Bennun RD, Monasterio AL. Chap. 11: Cleft palate repair. In: Bennun RB, Harfin JF, Sandor GKB, Genecov D, editors. Cleft lip and palate management: a comprehensive atlas. New York: Wiley Blackwell; 2015. p. 163–73.
Bennun RD. Cleft palate repair: predictive factors of difficulty and planned strategies to solve it. J Craniofac Surg. 2020;31:1664–7.
Astrada S, Bennun RD. Cleft palate repair: a study between two surgical procedures. J Craniofac Surg. 2020;31:2280–4.
Carstens MH. Chap. 1: Mechanisms of cleft palate: developmental field analysis. In: Bennun RB, Harfin JF, Sandor GKB, Genecov D, editors. Cleft lip and palate management: a comprehensive atlas. New York: Wiley Blackwell; 2015. p. 3–21.
Moggi LE, Ventorutti T, Bennun RD. Cleft palate repair: a new maxillary nerve approach. J Craniofac Surg. 2020;31:1547–50.
Rivelli RA, Casadio V, Bennun RD. Audiological alterations in patients with cleft palate. J Craniofac Surg. 2018;29:1486–9.
Harfin JF, Bennun RD. Chap. 15: Strengthening surgical/orthodontic interrelationships. In: Bennun RB, Harfin JF, Sandor GKB, Genecov D, editors. Cleft lip and palate management: a comprehensive atlas. New York: Wiley Blackwell; 2015. p. 227–41.
Harfin JF. Chap. 16: To what extent dental alveolar osteogenesis can be achieved solely with orthodontic treatment in cleft patient? In: Bennun RB, Harfin JF, Sandor GKB, Genecov D, editors. Cleft lip and palate management: a comprehensive atlas. New York: Wiley Blackwell; 2015. p. 245–52.
Further Reading
Benninger B, McNeil J. Transitional nerve: a new and original classification of a peripheral nerve supported by the nature of the accessory nerve (CN XI). Neurol Res Int. 2010;2010:476018.
Burdi AR. The premaxillary-vomerine junction: an anatomic viewpoint. Cleft Plate J. 1971;8:364–70.
Carlson BM. Human embryology and developmental biology. 5th ed. New York: Elsevier; 2013.
Celebi AA, Uncar FI, Sekerici AE, Caglaroglu M, Tan E. Effects of cleft lip and palate on the development of permanent upper central incisors: a cone-beam computed tomography study. Eur J Orthod. 2015;37:544–9. https://doi.org/10.1093/ejo/cju082.
Diamond MK. Homologies of the meningeal-orbital arteries of humans: a reappraisal. J Anat. 1991;178:223–41.
Diogo R, Kelly RG, Christiaen L, Levine M, Ziermann JM, Molnar JL, Noden DM, Tzahor E. A new heart for a new head in vertebrate craniofacial evolution. Nature. 2015;520(7548):466–73. https://doi.org/10.1038/nature14435.
Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. In: Madame Curie bioscience database. Austin: Landes Bioscience; 2000.
Garib DG, Rosae JP, Sahler LR, Ozawa TG. Dual embryonic origin of maxillary lateral incisors: clinical implications in patients with cleft lip and palate. Dental Press J Orthod. 2015;20(5):118–25. https://doi.org/10.1590/2177-6709.20.5.118.128.sar.
Gilbert S, Barresi MJ. Developmental biology. 11th ed. Sunderland: Sinauer Assoc; 2016.
Hiruma T. Formation of the ocular arteries in the chick embryo: observations of corrosion casts by scanning electron microscopy. Anat Embryol (Berl). 1996;193:585–92.
Hiruma T, Hirakow R. Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy. Anat Embryol (Berl). 1995;191:415–23.
Hiruma T, Nakajima Y, Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. J Anat. 2002;201:15–29.
Hovoraskova M, Lesot H, Peterkova R, Peterka M. Origin of the deciduous upper lateral incisor and its clinical aspects. J Dent Res. 2006;85(2):167–71.
Kardong K. Vertebrates: comparative anatomy, function, evolution. 7th ed. New York: McGraw Hill; 1997.
LeDourain NM, Kalchaim C. The neural crest. 2nd ed. Oxford: Cambridge University Press; 2009.
Liem K, Bemis W, Walker WF, Grande L. Functional anatomy of the vertebrates. 3rd ed. Boston: Cengage; 2000.
Liem KF, Bemis WE, Walker WF, Grande L. Functional anatomy of the vertebrates. Belmont: Brooks-Cole; 2001. p. 99–101.
Mavelli ME, Ayurek M. Congenital isolated absence of the nasal columella: reconstruction with internal nasal vestibular skin flap and bilateral labial mucosa flaps. Plast Reconstr Surg. 2000;106:393–6.
Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601.
O’Rahilly R, Müller F. Human embryology and teratology. 3rd ed. Somerset: Wiley-Liss; 2001.
Okazaki M, Sarukawa S, Fukuda N. A patient with congenital defect of nasal cartilaginous septum and vomeral bone reconstructed with costal cartilaginous graft. J Craniofac Surg. 2005;16(5):819–22.
Padget DH. The development of the cranial arteries in the human. Contrib Embryol. 1948;32:205–61.
Standring S, editor. Gray’s anatomy. 40th ed. New York: Elsevier; 2006.
Tada MN, Kuratani S. Evolutionary and developmental understanding of the spinal accessory nerve. Zool Lett. 2015;1:4.
Talmant JC, Talmant JC, Lumineau JP. Primary treatment of cleft lip and palate, its fundamental principles. Ann Chir Plast Esthet. 2016;61(5):348–59. https://doi.org/10.1016/j.anplas.2016.06.007.
Talmant JC, Talmant JC, Lumineau JP. Secondary treatment of cleft lip and palate. Ann Chir Plast Esthet. 2016;61(5):360–70. https://doi.org/10.1016/j.anplas.2016.06.012.
Valencia MP, Castillo M. Congenital and acquired lesions of the nasal septum: a practical guide for differential diagnosis. Radiographics. 2008;28(1):205–23. https://doi.org/10.1148/rg.281075049.
Wei X, Senders C, Owrn GO, Liu X, Wei Z-N, Dillard-Telm L, McClure HM, Hendricks AG. The origin and development of the upper lateral incisor and premaxilla in normal and cleft lip/palate monkeys induced with cyclophosphamide. Cleft Palate Craniofac J. 2000;37(6):571–83.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Commentary: Ricardo Bennun
Commentary: Ricardo Bennun
Alveolar Extension Palatoplasty: Technical Notes
Introduction
Successful palate closure became possible in the mid-nineteenth century with the development of the mucoperiosteal flaps by Dieffenbach and von Langenbeck [27].
The fact that simple closure of the palate cleft did not necessarily ensure normal speech was recognized a little later and led to the development of palate-lengthening procedures and various forms of pharyngoplasty.
This chapter serves as a logical extension to the multidisciplinary approach to cleft palate management and reconstruction from genetic factors that precipitate cleft to a thorough discussion of today’s approaches.
Surgical Procedure
A complete report of the alveolar extension palatoplasty (AEP) was precisely illustrated in a chapter of our Atlas published in 2015 [28]. The predictive factors of difficulty before reconstruction and the planned strategies were also described by the author [29]. A comparative study between Veau–Wardill–Kilner and the AEP procedure was also published [30].
Technical Recommendations
A short incision of the oral mucosa over the alveolar border must be carried out. Utilizing a Gillies retractor, under direct vision, and with the blade in an oblique direction, the cut must be completed, leaving all the dental germs in their position.
Minimal lateral incisions are planned in simple cases, where the soft palate major gap distance (SPMGD) is inferior to 6 mm, with symmetrical bilateral soft palate length (BSPL) superior to 20 mm. This strategy will also be useful in the presence of easily isolated cleft palates.
When the SPMGD is between 7 and 11 mm, one alveolar extended palatal flap elevation is considered. In that case, the author’s choice will be the palatal flap from the sick side. Some surgeons utilizing the AEP procedure will prefer to select the normal-side palatal flap. Outcomes seem to be similar.
In bilateral cleft cases, the suggestion for a complete and cautious hard and soft palate closure will be using both AEP flaps. This recommendation is also valid in patients with SPMGD greater than 12 mm, but having a symmetrical BSPL superior to 20 mm.
In very difficult cases with a SPMGD superior to 12 mm, plus symmetrical/asymmetrical BSPL under 16 mm, or even a short bilateral hard palate length with depressions (BHL&D), our indication would be a nasal plane reconstruction utilizing a vomer flap, complemented with a superior pharyngeal flap [29].
Results
Statistically significant differences were found when comparing the total percentage of complications between both groups (Veau–Wardill–Kilner and AEP). The amount and severity of each complication by groups were also established as significant [30]:
-
Bite alterations and transversal collapse with dental malpositioning in group A: 29.84% and group B: 23.155% (P value < 0.009)
-
Presence of fistulas in group A: 4.11% and group B: 5.0% (P value < 0.02)
-
Patients with VPI in group A: 6.12% and group B: 0.11% (P value < 0.14)
Complications
No additional complications have been reported utilizing this procedure. Outcomes and follow-up during the last 12 years have proved no teeth alteration, in all patients (pictures).
Conclusions
Since 2009, the author has selected the alveolar extension palatoplasty variant, plus the complete muscle dissection and retropositioning, and the posterior pillar’s elongation with hemi-uvula rotation/reconstruction, as the technique of choice for primary cleft palate repair [28].
The utilization of the pre-op cited parameters to identify cleft palate diversity and severity seems to be a useful methodology to select the correct surgical strategy [29].
Moving and reducing incisions to protect blood supply, following Carstens’ suggestion, allow us to reduce the use of electric coagulation and blood loss. The presence of less raw areas prevents the incidence of retractile scars [31].
Employing regional blocking [32] joint to general and local anesthesia not only decreased intra- and post-op pain and baby neurotoxicity but also allowed us to initiate oral feed and discharge the baby early.
Having bigger palatal flaps in width and length allows us to decrease the incidence of anterior/medial fistulas, velopharyngeal incompetence, and maxillary alterations. Dental malpositioning and misalignment, as medial otitis, were also present in an inferior percentage [33,34,35].
Note on the Clinical Series
Bilateral cases 4, 5, and 7 and unilateral cases 5, 7, 11, 12, 13, and 14 demonstrate class 1 occlusion.
A Note from Dr. Carstens
Ricardo Bennun
Alveolar extension palatoplasty as a concept is uniquely South American, both in its design and in its surgical verification. AEP, in concept of the palatal version of subperiosteal tissue transfer for cleft lip repair, was drawn out on a paper napkin during an airplane flight to Ecuador. Although the operation was described in 1999, it was subsequently picked up by Dr. Luis Monasterio, a distinguished cleft surgeon in Santiago, Chile. Lucho invited me to do some cases with him at the Fundación Gantz. Since the incisions required to reposition the entire embryonic field of the hard palate were made on the alveolar ridge, the effect of these on dental eruption was an issue of debate. Dr. Monasterio did his own series, following dental development for several years. He determined that eruption was unaffected (which he subsequently reported to the 12th American Cleft Palate Association meeting). He also introduced me to Dr. Ricardo Bennun from the University of Buenos Aires and director of the Fundación Piel. Ricardo subsequently took the operation to the next level, beginning a case series which now extends to over a decade. Ricardo is a true biologic surgeon, reflecting his long-time commitment to burn care; he keeps on asking questions and seeking answers. I had the opportunity to contribute two chapters for his 2015 work, “Cleft Lip and Palate Management: A Comprehensive Atlas”; in the process, his attention to detail and insistence of quality forced me to think about the issues more deeply than before. It is his influence that really pushed me over the edge, daring me to write this book, a task that seemed overwhelming to me at the time. True to form, his atlas of AEP cases faithfully recorded herein will stand the test of time as a surgical proof that developmental biology can win out over dogma for better patient outcomes. For this, all cleft surgeons will be grateful.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Carstens, M.H. (2023). Pathologic Anatomy of the Hard Palate. In: Carstens, M.H. (eds) The Embryologic Basis of Craniofacial Structure. Springer, Cham. https://doi.org/10.1007/978-3-031-15636-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-15636-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15635-9
Online ISBN: 978-3-031-15636-6
eBook Packages: MedicineMedicine (R0)