Abstract
South Africa’s ecosystems are challenged in various ways by anthropogenic effects, such as land-use change, leading to soil erosion in concert with industrial or agricultural pollution, leading to an increase in pollutants in final depositional systems. Here we focus on metals in the marine environment of Richards Bay Harbour. The use for Al, Fe, Rb, Ti and the silt fraction of the sediment as normalisers of Cr, Cu, Co and Pb concentrations in sediment is compared to determine if they provide the same understanding on the enrichment. Baseline metal concentration models were defined and Enrichment Factors calculated to quantify the magnitude of enrichment.
Exceedingly high Cr and Cu concentrations in defined parts of the harbour lead to similar trends rather than a similar effectiveness of the normalisers. Probable biogeochemical processes hinder the effectiveness of Fe and geological background or hydrodynamic properties hinder the effectiveness of Ti as normaliser. Differences in the spatial extent of sediment identified as enriched and the area where metal concentrations exceed guidelines detracts from fully appreciating the extent of metal contamination of sediment using guidelines, with management implications. Furthermore, in the case of Cu, the guidelines for this metal might be underproductive.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Metals are common, and often significant contaminants of sediment in ports (Birch et al. 2020; Mehlhorn et al. 2021) and are the subject of considerable attention in the scientific literature. The focus is founded on valid concerns, including that sediment is a major fate for, and through remobilisation a potential source of metals in aquatic ecosystems (Newman and Watling 2007), and metals are known to present ecological risks when present at elevated concentrations (Chapman and Wang 2001). A major challenge, however, is identifying metal concentrations that reflect the natural state and those that are enhanced through an anthropogenic contribution (i.e. contamination). This is complicated for several reasons. First, metals are a ubiquitous, naturally occurring component of sediment. The mere presence of metals in sediment does not thus infer contamination. Second, metal concentrations in uncontaminated sediment can vary by orders of magnitude over small spatial scales depending on the sediment’s mineralogy, granulometry and organic content amongst other factors (Schropp and Windom 1988; Windom et al. 1989; Loring 1991; Krumgalz et al. 1992; Balls et al. 1997; Grant and Middleton 1998; Thomas and Bendell-Young 1999; Rubio et al. 2000; Kersten and Smedes 2002; Amorosi et al. 2007; Du Laing et al. 2007; Woods et al. 2012). Third, despite input and transport dissimilarities, naturally occurring and anthropogenically introduced metals tend to accumulate in sediment in the same areas (Loring 1991; Hanson et al. 1993). Due to these complexities, similar metal concentrations in two sediment samples from the same system may reflect contamination in one sample but not the other, due to a difference in the sediments granulometry. Similarly, very different metal concentrations in two sediment samples from the same aquatic system might in both cases reflect the natural condition, for the same reasons. Therefore, “high” metal concentrations do not necessarily reflect increased levels of contamination and vice versa (Newman and Watling 2007). The direct comparison of metal concentrations amongst sediment samples is thus important in terms of ecological and human health, if certain thresholds are exceeded, but not in the context of identifying contamination itself.
To properly interpret metal concentrations in sediment, it is necessary to compensate for the factors that control their natural variation before background or baseline concentrations can be distinguished from enriched (higher than “expected”) concentrations. There are two approaches to normalisation, namely: a) using a metal that acts conservatively—geochemical normalisation, or b) using a grain size fraction—granulometric normalisation (Birch and Snowdon 2004; Newman and Watling 2007). In this way, metal concentrations that are atypical of the bulk of the data can be identified. The investigator must then decide if atypically high metal concentrations reflect contamination or can be explained by natural biogeochemical or hydrodynamic processes.
Geochemical normalisation makes use of a metal that acts as a proxy for the grain size variation of sediment, and more specifically for the silt and clay (mud) fraction (Birch and Snowdon 2004). A metal normaliser should (a) be highly refractory, (b) be structurally combined to one or more of the major metal-bearing phases of sediment, (c) co-vary in proportion to the naturally occurring concentrations of the metals of interest, (d) be insensitive to inputs from anthropogenic sources and (e) be stable and not subject to environmental influences such as reduction/oxidation, adsorption/desorption and other diagenetic processes that may alter sediment concentrations (Luoma 1990). Commonly, Al, Fe, Li, Rb or Ti are used as geochemical normalisers (Daskalakis and O’Connor 1995; Santos et al. 2005; Tůmová et al. 2019).
In granulometric normalisation, metal concentrations are either normalised to a specific grain size fraction of sediment, or metal concentrations are analysed in a defined grain size fraction of the sediment after sieving. Metals are predominantly incorporated in and preferentially bind to fine sediment rather than to coarse material, meaning that silt and clay are the most effective normalisers in this approach (Newman and Watling 2007; Szava-Kovats 2008; Koigoora et al. 2013). In addition, Ti is often associated with fine sand to silt or clay, whereas K and Al are often associated with clay type minerals (Watling 1977; Haberzettl et al. 2019), while Zr has been reported as enriched in the silt fraction (Cuven et al. 2010; Kylander et al. 2011; Ohlendorf et al. 2014). Therefore, it is necessary to identify the best-fit grain size range for granulometric normalisation. However, caution is required when a grain size fraction is used as a normaliser, since two separate samples are processed and analysed, one for grain size and one for metals. Errors in sample splitting can lead to errors in normalisation. Similarly, isolating a sediment fraction for metal analysis is usually done by wet sieving and this can lead to the loss of metals weakly adsorbed to the surface of sediment grains.
Site-specific circumstances might alter the usefulness of normalisers. In this study, we present data from Richards Bay Harbour on the northeast coast of South Africa, where different factors need to be considered in the choice of the normaliser. Industries near Richards Bay Harbour include two aluminium smelters and a ferrochrome smelter. These industries import alumina and export aluminium and ferroalloys through the port. The effect of open air bulk handling (e.g. of ferrochrome) can introduce metals to the harbour, and point sources of Cr or Cu at bulk handling terminals were recognised by Mehlhorn et al. (2021). Contamination arising from the spillage during import or export and from the smelters (e.g. through atmospheric deposition) could thus influence the utility of Al and Fe as normalisers. In a previous study (Mehlhorn et al. 2021), we used Al to normalise metal concentrations in sediment sampled in Richards Bay Harbour. However, considering the industrial impact outlined above, there are potential limitations to the use of Al and Fe as normalisers of metal concentrations in sediment in the harbour. Similarly, the catchment geology must be considered. For example Ti-bearing heavy mineral dune sands of the Maputaland Group Sibayi Formation surrounding Richards Bay Harbour (Kelbe 2010; Botha 2018) question the usefulness of certain geochemical normalisers, such as Ti, as heavy minerals might be differently influenced by prevailing hydrodynamic conditions compared to other normalisers. Furthermore, the basin topography of Richards Bay Harbour is optimised to serve marine port operations. To keep the port operational, the basin shape of the harbour is largely controlled by dredging (Greenfield et al. 2011; Dladla et al. 2021). Remobilisation of metals from sediment by seabed disturbances can occur via bioturbation, increased current velocities (storms, tides) or dredging (Förstner 1989; Daskalakis and O’Connor 1995; Saulnier and Mucci 2000). Ship operations, especially propeller wash currents, impact the depositional environment (Mehlhorn et al. 2021) and disturb natural sedimentation processes.
To understand changes in an environment, it is necessary to identify a system’s reference state using various proxies, in this case, the release of (heavy) metals by surrounding industries and their potential health risks. This enables their comparability to past and future changes (cf. Mehlhorn et al. 2021), but also shifts in the spatial extent of contamination, indicating sink and source relationships. However, each basin or catchment is subject to site-specific environmental influences that need special consideration when evaluating (normalising) parameters. In this study, we evaluate the efficacy of several potential normalisers for concentrations of Cr, Cu, Co and Pb in Richards Bay Harbour sediment, by defining baseline models using different normalisers and comparing trends in the enrichment of sediment identified by the models.
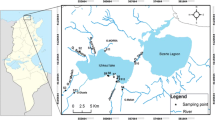
The study area of Richards Bay Harbour. Indicated is the harbour area, which is separated from the Mhlatuze Estuary (sanctuary) by an artificial berm. Also shown are potential contamination sources (red) and sampling locations (black dots). A low altitude dune ridge extending from WNW to ESE divides the harbour and separates the shallow water Mudflats from the deep water Terminal Front
2 Materials and Methods
2.1 Site Description
Richards Bay Harbour (Fig. 27.1) is situated in the province of KwaZulu-Natal, on the subtropical northeast coast of South Africa. Prior to port construction, Richards Bay was a large, shallow estuary of about 30 km2 fed by five rivers (Begg 1978). Harbour development started in the early 1970s and involved the construction of an artificial berm that divided the estuary into two parts. The northern part was developed into Richards Bay Harbour. A new mouth was dredged for the southern part, which was designated a nature sanctuary (Mhlathuze Estuary), and part of the Mhlathuze River was canalised and diverted into this part. A major reason for the construction of Richards Bay Harbour was to facilitate the export of coal through the Richards Bay Coal Terminal, which is now one of the largest coal export terminals in the world (Nel et al. 2007). Other industries that were established in the area to take advantage of the import and export opportunities provided by the harbour are the following: aluminium smelters, a phosphoric acid and fertiliser plant, a ferrochrome smelter and heavy minerals mining and refining operations. A wide range of bulk materials are imported and exported through the harbour in addition to coal, including ferroalloys, sulphur, phosphoric acid, alumina, aluminium, heavy minerals and woodchips.
2.2 Sampling
Sampling was conducted in August and September of 2018. Eighty surface sediment samples were collected (Fig. 27.1) using an Ekman-Birge bottom sampler (HYDROBIOS, Kiel, Germany). Only the topmost 1 cm was sampled with a plastic spoon. Samples were stored in sterile polyethylene Nasco Whirl-Pak’s and cooled until further processing.
2.3 Granulometric and Geochemical Analysis
The sediment was prepared for granulometric analyses by soaking 0.5–2 g aliquots of wet sediment in 2 ml of hydrochloric acid (HCl, 10%) and 5 ml of hydrogen peroxide (H2O2, 10%). The residues were dispersed overnight in 5 ml tetrasodium pyrophosphate (Na4P2O7 10 H2O, 0.1 M) in an overhead shaker. The samples were then analysed using a Laser Diffraction Particle Size Analyser (Fritsch Analysette 22; FRITSCH GmbH, Germany).
Freeze-dried aliquots of sediment were ground to a particle size <60 μm. Subsamples of the sediment were digested at the University of Greifswald using a modified aqua regia treatment. The procedure involved the addition of 1.25 ml of HCl (37%, suprapur) and 1.25 ml of HNO3 (65%, suprapur) to 100 mg of sediment, which was then digested in PTFE crucible pressure bombs in an oven at 160 °C for 3 h. Each batch included a laboratory blank as well as a reference sediment sample of certified estuarine sediment (BCR-667) and indicated a good recovery range of 76.9–98.6% for the measured metals (Co = 86%; Cr = 96%; Cu = 96%; Fe = 99%; Pb = 77%). Elemental concentrations were measured at Friedrich Schiller University Jena using an Agilent 725 ES ICP-OES (Al, Ca Fe, K, Mg, Mn, Na, P, S, Sr, Ti) and a Thermo Fischer Scientific X-Series II ICP-MS (As, Co, Cr, Cu, Ni, Pb, Zn, Rb), as described in Mehlhorn et al. (2021).
2.4 Data Analysis
Scatterplots of the relationship between potential normalisers (Al, Rb, Ti, Fe, Silt) and co-occurring Cu, Cr, Co and Pb concentrations in sediment showed that, apart from Ti, there was a linear relationship between the bulk of the concentrations and the normalisers. Baseline models were thus defined by fitting a linear regression and 95% prediction limits to scatter plots of Cu, Cr, Co and Pb concentrations and the potential normalisers. Cu, Cr, Co and Pb concentrations falling outside the prediction limits were deemed outliers and sequentially trimmed, starting with the concentration with the largest residual, reiterating the regression and proceeding in this manner until all concentrations fell on or within the prediction limits. The resultant regression and associated prediction limits define the baseline model. Apart from Ti, error terms for the regressions approximated normality, but their variance was usually not homogenous. The Cu, Cr, Co and Pb concentrations were not transformed to approximate this assumption. In general, the lack of error term homogeneity does not result in biased estimates of regression parameters, but does result in an increase in variance about these estimates (Hanson et al. 1993). Schropp et al. (1990), Weisberg et al. (2000) and Woods et al. (2012) used a similar approach for defining baseline models, but continued to trim metal concentrations until the error terms were normally distributed and homogenous. This approach was not followed in this study, since it required the trimming of a larger number of Cu, Cr, Co and Pb concentrations than the approach described above, including concentrations that were subjectively considered to be part of the baseline range.
Sediment with Cu, Cr, Co and Pb at a concentration above a baseline model upper prediction limit was interpreted as enriched (i.e. the metal concentration is in excess of the baseline). The upper prediction limit was thus used to discriminate baseline from enriched metal concentrations. The magnitude of enrichment for each metal concentration was quantified by computing an Enrichment Factor (EF), which is calculated by dividing the observed metal/normaliser ratio by the reference (upper prediction limit) metal/normaliser ratio. EF’s >1 represent enriched concentrations, noting that this does not imply an enhancement through anthropogenic contribution, but rather that the concentration is atypical of the data that define the model (Horowitz 1991). Spatial trends for EF’s were plotted using ArcMap v. 10.8.1., using inverse distance weighting with barrier function.
Reaching a conclusion on whether metal enrichment reflects contamination thus requires consideration of ancillary factors, including possible (bio)geochemical processes that can lead to natural enrichment (e.g. diagenetic enhancement), the absolute difference between a metal concentration and the baseline model upper prediction limit, the number of metals in a particular sediment sample at an enriched concentration and the proximity of metal-enriched sediment to known or strongly suspected anthropogenic sources of metals. The larger the difference between a metal concentration and the baseline model upper prediction limit, the closer the enriched sediment sampling site is to known or strongly suspected anthropogenic sources of metals, and the greater the number of metals enriched in sediment at a particular sampling site the more likely the excess concentrations reflect contamination.
3 Results and Discussion
3.1 Evaluation of Normaliser Suitability
In a mineralogically homogenous area, the absolute concentrations of metals in sediment are largely controlled by the sediments grain size (Taylor and McLennan 1981; Horowitz and Elrick 1987; Förstner 1989; Horowitz 1991; Loring 1991; Larrose et al. 2010; Matys Grygar and Popelka 2016; Liang et al. 2019). Aluminosilicates, the dominant natural metal-bearing phase of sediment, predominate in silt and clay (mud). Sand, in contrast, is comprised largely of metal-deficient quartz. Metals naturally adsorb onto Fe/Mn oxides and organic matter in sediment in quantities that are usually proportional to grain size (Kersten and Smedes 2002). As a result, there is usually a strong positive correlation between the concentration of metals and the mud fraction, and between the concentration of different metals in uncontaminated sediment (Rubio et al. 2000; Sabadini-Santos et al. 2009; Coynel et al. 2016; but see Matys Grygar et al. 2013, 2014; Jung et al. 2014, 2016 for evidence of non-linear relationships). These relationships provide the basis for geochemical normalisation, by modelling the linear relationship between metal and co-occurring element (Hanson et al. 1993; Weisberg et al. 2000; Kersten and Smedes 2002). Geochemical normalisation through linear regression is premised on a two-component linear mixing model, one end member representing metal deficient quartz (sand) and the other metal rich aluminosilicates (mud; Hanson et al. 1993). A fundamental requirement for the use of a metal as a geochemical normaliser, therefore, is that the metal must be strongly correlated to the fine-grained fraction of the sediment for which it acts as a proxy.
Richards Bay Harbour includes multiple heterogeneous sedimentary environments (Mehlhorn et al. 2021). To identify the most effective granulometric normaliser we plotted correlation coefficients of individual elements to different grain size classes (Fig. 27.2). Several elements show a high positive correlation with parts of the clay and silt fraction. The curve shape of Al is highlighted as an example in Fig. 27.2. Therefore, this grain size fraction appears to be a good normaliser. As the grain size increases the correlation coefficient decreases and changes to strongly negative at the onset of 250 μm (medium sand). Elements showing a visually similar pattern to Al, but at a lower correlation coefficient of r = 0.5, include Cu, Cr and Mn. Other elements, like Sr, Ti and Ca, show no distinct correlation to any grain size class. The correlation between the Silt and Mud (Clay + Silt, which appear to be good normalisers) fractions of sediment in Richards Bay Harbour is strong (r = 0.993, p < 0.01). Consequently, only Silt is hereafter considered as a normaliser of metal concentrations in sediment in the harbour.
Correlation coefficient versus grain size. The unique distribution curves indicate the varying correlation coefficients of individual elements against grain size classes. Elements displayed by grey lines show a similar behaviour to Al. Coloured curves indicate a differing and individual behaviour when plotted against grain size
Al and Rb concentrations in sediment in Richards Bay Harbour are highly correlated to the silt fraction (r = 0.954, p < 0.001), but less so to the clay fraction (r = 0.782 − 0.792, p < 0.001). The implication is that Al and Rb are closely associated with or incorporated in the silt fraction of the sediment and are thus effective proxies for this fraction. Despite the import of alumina, export of aluminium and presence of two large aluminium smelters near Richards Bay Harbour, sediment in the harbour does not indicate contamination by Al. This conclusion is based on the strong correlation of Al with most of the elements, and by the absence of pronounced outliers in the relationship between Al and the silt fraction of the sediment and elements that are unlikely to be influenced by anthropogenic activities, such as Rb.
Fe concentrations are also highly correlated to the silt fraction of the sediment (r = 0.834, p < 0.001), but less so to the clay fraction (r = 0.798, p < 0.001). The weaker correlation of Fe concentrations to the silt fraction compared to Al and Rb is a result of anomalously high Fe concentrations in sediment at five stations in a shallow area opposite the Richards Bay Coal Terminal, colloquially known as the Mudflats (Fig. 27.1). If the data for sediment sampled on the Mudflats is excluded, the correlation between Fe concentrations and the silt fraction increases (r = 0.928, p < 0.001), but the correlation to the clay fraction remains moderate (r = 0.777, p < 0.001). The implication is that Fe is closely associated with or incorporated into the silt fraction of the sediment and is thus an effective proxy for this fraction across most of the harbour, but not on the Mudflats.
Ti concentrations in sediment of Richards Bay Harbour are not correlated to the silt (r = −0.094, p = 0.405) or clay (r = 0.061, p = 0.589) fractions. Ti is thus not an effective proxy for these fractions of sediment in the harbour, and by implication also not for the concentrations of other metals.
The concentrations/values of normalisers should be strongly positively correlated to the concentrations of metals in uncontaminated sediment. However, in some parts of Richards Bay Harbour, the sediment is metal contaminated (Mehlhorn et al. 2021). The baseline models for Cu, Cr, Co and Pb do not thus represent background concentrations, since it is possible, and for Cu and Cr likely, that certain concentrations included in the baseline models reflect low magnitude contamination of the sediment but are not identified as outliers through the approach used to define the models. Nevertheless, the stronger the relationship between the normalisers and metals, the more effective the baseline model should theoretically be in identifying metal enrichment of sediment.
There is not much difference in the coefficients of determination for baseline models apart from Ti, for which coefficients are consistently very low (Table 27.1). The Fe normalised baseline models provide the highest coefficients for Cu, Cr and Co, and the Rb model for Pb. The baseline model coefficients of determination suggest, therefore, that Fe is the best normaliser for Cu, Cr and Co, and Rb for Pb. However, the Fe normalised baseline models are strongly influenced by the exclusion of Cu and Cr and inclusion of Co and Pb concentrations in sediment on parts of the Mudflats due to the anomalous concentrations of Fe, Co and Pb in this part of the harbour. The anomalous concentrations of Fe might be caused by a local change in redox processes (Wündsch et al. 2014; Zolitschka et al. 2019). The critical Eh for the reduction of Fe3+ to more soluble Fe2+ is 100 mV (Sigg and Stumm 1996). If the redox potential is lowered, Fe ions start to migrate in pore water. When an Eh above 100 mV is encountered, the ions will oxidise and precipitate, leading to the enrichment of sediment that is not necessarily linked to an anthropogenic impact (Haberzettl et al. 2007; Brunschön et al. 2010).
3.2 Baseline Model Comparison
If normalisers are to be considered similarly effective, then (a) they should identify a similar number of sediment samples as enriched by any metal, (b) the spatial extent of the enrichment should be similar, (c) the magnitude of the enrichment should be similar and (d) the enrichment should be logical in the context of known or potential anthropogenic sources of the metal. The baseline models defined for Cu, Cr, Co and Pb using different normalisers are provided in Figs. 27.3, 27.4, 27.5 and 27.6, with outlier concentrations superimposed. Parameters for the baseline models are provided in Table 27.2.
As stated previously, sediment with metals at a concentration above the upper prediction limit was deemed to be enriched by the metal. The number of sediment samples identified as enriched by individual baseline models is provided in Fig. 27.7.
The number of sediment samples identified as enriched by Cu is similar for the Al, Rb and Silt normalised baseline models, but the Fe and Ti models identify a higher number. The Al, Fe, Rb and Ti normalised baseline models identify a similar number of sediment samples as enriched with Cr, but the Silt model identifies a higher number. The number of samples identified as Co and Pb enriched varies widely amongst the normalisers and is most similar for Al, Rb, and Silt and the lowest for Ti. The number of metals identified as enriched by baseline models defined using different normalisers is not particularly convincing as to which of the normalisers is more effective than others. However, we consider those normalisers that identify a comparable number of sediment samples as enriched by a metal are likely more effective than others.
3.3 Spatial Enrichment Trends
The spatial trend for Cu and Cr EFs that are >1 is comparable when computed using Al, Rb or Silt normalised baseline models (Figs. 27.8, 27.9, 27.10 and 27.11). However, the trend for EFs computed using Fe and Ti normalised models is different, especially for Co and Pb (Figs. 27.10 and 27.11). A larger area of sediment is identified as Cu enriched by the Fe and Ti normalised baseline models, the additional area extending from the 700 series berth basin into the northern part of the Richards Bay Coal Terminal basin and also including one or more isolated areas in the latter basin (Fig. 27.8). Fe and Ti identify a similar area of the 600 and 700 series berth basins (part of the Dry Bulk Terminal, Fig. 27.1) as Cr enriched compared to other normalisers but identify little (Fe) or no (Ti) Cr enrichment of sediment on the Mudflats.
Spatial distributions of the copper (Cu) baseline models normalised to (a) Al, (b) Fe, (c) Rb, (d) Ti and (e) Silt. The enrichment factors are plotted to the same scale to achieve visual comparability, note the extended legend of Ti (EFmax = 14.7), maximum values are shown next to the classification. For additional comparison, the distribution map of Cu (f) in surface sediments of Richards Bay Harbour is plotted, making use of guideline concentrations as presented by the Department of Environmental Affairs (2012)
Spatial distributions of the chromium (Cr) baseline models normalised to (a) Al, (b) Fe, (c) Rb, (d) Ti and (e) Silt. The enrichment factors are plotted to the same scale; maximum values are shown next to the classification. For additional comparison, the distribution map of Cr (f) in surface sediments of Richards Bay Harbour is plotted, making use of guideline concentrations as presented by the Department of Environmental Affairs (2012)
Spatial distributions of the cobalt (Co) baseline models normalised to (a) Al, (b) Fe, (c) Rb, (d) Ti and (e) Silt. The enrichment factors are plotted to the same scale; maximum values are shown next to the classification. For additional comparison, the concentration of Co (f) in surface sediments of Richards Bay Harbour is plotted
Spatial distributions of the Lead (Pb) Baseline models normalised to (a) Al, (b) Fe, (c) Rb, (d) Ti and (e) Silt. The enrichment factors are plotted to the same scale; maximum values are shown next to the classification. For additional comparison, the concentration of Pb (f) in surface sediments of Richards Bay Harbour is plotted
There is clear evidence for significant anthropogenic inputs of Cu and Cr in Richards Bay Harbour. Chromium ore and ferrochrome are exported through the harbour. During export, chromium ore and ferrochrome particles are spilled into the harbour and account for the Cr contamination (Mehlhorn et al. 2021). The source of the excess Cu in sediment is less certain. Cu concentrate was historically exported through the harbour and the contamination might reflect the spillage of Cu concentrate particles during export, noting that exports ceased in 2012. The Cu and Cr enrichment of sediment sampled across much of the 600 and 700 series berth basins (dry bulk terminals) is of such a high magnitude that comparable areas of sediment are identified as enriched by baseline models defined using different normalisers, regardless of the effectiveness of the normalisers. The sediment in part of the Small Craft Harbour is identified as Cu enriched by all normalisers (Fig. 27.8), possibly reflecting inputs from antifouling coatings on vessel hulls amongst other possible sources. Some of the baseline models also identify Cu enrichment of sediment in canals joining the north-eastern part of the port, but the source of the Cu is uncertain.
All baseline models identify Cu and Cr enrichment near the 600 and 700 series berths. An extension of the Cu enrichment from the 700 series berth basin to the northern part of the Richards Bay Coal Terminal basin is identified using the Fe and Ti normalised baseline models (Fig. 27.8). This is contradictory as it seems unlikely that Cu but not Cr (Fig. 27.9) would be dispersed to and accumulated in this area considering the strong similarity in parts of the 600 and 700 series berth basins where the sediment is identified as Cu and Cr enriched by Al, Rb and Silt normalised baseline models. The wider area of Cu enrichment identified by the Fe normalised baseline model is probably related to the wider scatter of Cu versus Fe concentrations compared to other normalisers, which led to the trimming of a larger number of concentrations compared to other normalisers (Table 27.2). The Cr enrichment of sediment identified on the Mudflats using the Al, Rb and Silt normalised baseline models, but absence of Cu enrichment also seems illogical for the same reason discussed above for Cu enrichment extending to the Richards Bay Coal Terminal basin. As stated above, the magnitude of Cu and Cr enrichment of sediment at many stations in the 600 and 700 series berth basins is large and it is almost inevitable the sediment will be identified as enriched regardless of the effectiveness of the baseline models defined using different normalisers.
Trends in Co and Pb enrichment of sediment are more revealing on the effectiveness of different normalisers. Sediment on the Mudflats of Richards Bay Harbour is identified as enriched by Co and Pb using Al, Rb and Silt normalised baseline models, but not by Fe and/or Ti normalised baseline models (Figs. 27.10 and 27.11). These elements might share a similar accumulation mechanism or be affected similarly by sediment biogeochemical processes.
Examples of Fe enrichment identified using Al and Silt as the normaliser are provided in Fig. 27.12. The consequence is that Fe normalised Co and Pb concentrations are shifted to the right in scatterplots, to the extent they fall within the prediction limits of baseline models defined using the data (Fig. 27.13). In contrast, the Cu and Cr concentrations in sediment at these stations were trimmed during baseline model definition and fall below the lower prediction limit (Figs. 27.3 and 27.4). The anomalous concentrations do not thus have the same influence on the baseline models as the Co and Pb concentrations.
Relationship between aluminium and iron concentrations and co-occurring cobalt and lead concentrations in sediment in Richards Bay Harbour. Cobalt and lead concentrations in sediment sampled on the Mudflats are highlighted (black filled dots). Note how the anomalously high cobalt and lead concentrations in the aluminium normalised plots are shifted to the right in the iron normalised plots
The differences between Al, Rb and Silt normalised baseline models and the Fe baseline model for Co and Pb suggest there is either an anthropogenic source of Fe, Co and Pb to the Mudflats, or there is a biogeochemical or hydrodynamic process leading to the enrichment of these metals in sediment in this part of the harbour. The Bhizolo Canal connects to the Mudflats and is a potential source of effluents from adjacent aluminium smelters, but it seems unlikely this will be limited largely to Fe, Co and Pb, but rather to the introduction of Al. A more likely explanation is that redox processes resulted in an inhomogeneous Fe distribution (Demory et al. 2005; Brunschön et al. 2010). Support for this hypothesis is provided by Mn, which is also a redox-sensitive metal (Haberzettl et al. 2007) and which is also identified as enriched in sediment on the Mudflats (data not reported here) using Al, Rb and Silt normalised baseline models.
The EF plots presented in Figs. 27.8–27.11 make it easy to visually compare the spatial extent of enrichment of sediment by various metals in Richards Bay Harbour and to identify areas where the highest enrichment is evident, but it is not easy to visually compare areas of low magnitude enrichment. To facilitate such comparison, the relationship between EFs is provided for Cu and Co in Figs. 27.14 and 27.15. The oblique lines in the graphs represent a 1:1 relationship. The more similar the EFs are to one another, the nearer they fall to the 1:1 line. The graphs also reveal if the baseline model defined using a normaliser provides consistently higher or lower EFs compared to baseline models defined using other normalisers depending on whether the EFs fall above or below the 1:1 line. In Fig. 27.14 (and Fig. 27.8), for example, the Ti normalised baseline model provides higher EFs for Cu in a large proportion of sediment samples compared to other baseline models. The most similar EFs for Cu and Cr are for combinations of Al, Fe, Rb and Silt normalised baseline models. For Co (Fig. 27.15) and Pb (not shown), combinations of Al, Rb and Silt normalised baseline models provide similar EFs, but these differ from EFs computed using Fe and Ti normalised models. The differences for Co and Pb reflect the influence of the high Fe, Co and Pb concentrations in sediment on the Mudflats on baseline models defined using Al, Rb and Silt (Figs. 27.12 and 27.13). Thus, while these sediment samples are included in the baseline models for Co and Pb, the Cu and Cr concentrations fall below the lower prediction limit of the Fe normalised models. They do not thus exert a similarly pronounced influence on the baseline model parameters.
A random choice of any of the normalisers considered in this study will thus provide a different understanding on the spatial extent and magnitude of enrichment. The differences will vary depending on the chosen normaliser, but are small when Al, Rb and Silt are used as normalisers. If only Fe was considered for normalisation, then this would have provided a different understanding on metal enrichment of sediment in some parts of the harbour compared to that provided by Al, Rb and Silt normalised baseline models. Ti provides a very different understanding of enrichment for most metals compared to other normalisers, reflecting its unsuitability as a normaliser in the case of Richards Bay Harbour.
3.4 Comparison to Guidelines
A common approach to estimating the toxicological significance of metal concentrations in sediment is to compare them to sediment quality guidelines. A misconception is that sediment quality guidelines indicate the onset of metal contamination of sediment. Although this is usually true for metal concentrations that exceed a guideline, depending on how conservative the guideline is, the sediment might be contaminated by a metal at concentrations well below the guideline. As examples, spatial trends for Cu and Cr concentrations in sediment in Richards Bay Harbour that exceed sediment quality guidelines used by the Department of Environmental Affairs (2012) to manage dredged material in South Africa are included in Figs. 27.8 and 27.9. There are three guidelines, known as the Warning Level, Level I and Level II. The Warning Level provides a warning of incipient metal contamination but is not used for decision-making. Sediment with metals at a concentration below the Level I is considered to pose a low risk and is suitable for open water disposal. Sediment with metals at a concentration between the Level I and Level II is considered cause for concern, with the degree of concern increasing as the concentrations approach the Level II. Sediment with metals at a concentration exceeding the Level II is considered to pose a high risk and is unsuitable for open water disposal unless other evidence (e.g. toxicity testing) shows the metals are not toxic to sediment-dwelling organisms.
There are large differences in the spatial extent of sediment identified as Cu enriched by baseline models defined using different normalisers and the area of sediment with Cu at a concentration exceeding the guidelines (Fig. 27.8). The area of maximum intensity (either concentration or EF) is confined to the 600 series berth basin. However, the area defined by EFs >1 extends into the 700 berth basin (Terminal Front) and to other areas, such as the Small Craft Harbour.
The difference in the spatial extent of sediment identified as metal enriched using the baseline models and the spatial extent of sediment with metals at a concentration exceeding Action List guidelines is often pronounced. This alludes to the guidelines perhaps being under- or over-protective, noting that the guidelines were not derived using empirical data from South African coastal waters but rather were adopted from guidelines used in North America. The guidelines might not, therefore, be appropriate to (all) parts of the South African coastline. Taking Cu as an example, large areas of sediment in the harbour are identified as enriched by this metal, yet the area where Cu concentrations exceed sediment quality guidelines is quite small. The use of the guidelines thus fails to identify sediment that is quite significantly enriched by Cu as potentially problematic, and alludes to management challenges larger than those alluded to by using the guidelines alone. This may mean the Cu guidelines are under-protective, that is the guideline concentrations are too high for sediment in Richards Bay Harbour.
4 Conclusion
This study compares the effectiveness of different normalisers on assorted metal in sediment in Richards Bay Harbour. The aim was to define metal concentration baselines and to identify areas of the harbour that are currently metal enriched, to aid in the tracking of changes in metal enrichment of sediment over time. The baseline models defined using some normalisers provide a powerful tool for this purpose, compensating for grain size influences on natural metal concentrations and reducing subjectivity in deciding if sediment is metal enriched. The models are equally effective in identifying changes in metal concentrations that may result from anthropogenic metal inputs and those that might arise from future climate changes, such as the introduction of increased metal and sediment loads to coastal waters associated with changes in precipitation patterns.
The use of different normalisers provides a different understanding on metal enrichment of sediment in Richards Bay Harbour. Despite the import of alumina and the presence of two large aluminium smelters near Richards Bay harbour, this does not appear to result in a significant Al contamination of sediment in the harbour affecting its use as a normaliser. The EF trends for metals in Richards Bay Harbour, including those not discussed here, are most similar when Al, Rb or Silt are used as normalisers. The EF trends using Fe as the normaliser are similar to the trends for most other metals normalised by Al, Rb and Silt. However, the (potential hydrodynamic or redox-induced) enhancement of Co, Pb and Mn concentrations in sediment across part of the Mudflats influences the Fe normalised baseline model parameters and results in differences in EF trends for these metals in this part of the harbour. Ti is not considered an effective normaliser of metal concentrations in sediment of Richards Bay Harbour, as it is not correlated with the fine-grained fraction of sediment or other metal concentrations. However, if contamination is of a very high magnitude, contaminated areas will be identified using different normalisers, regardless of the effectiveness of the normalisers. The comparison of baseline models to Action List guidelines shows that baseline models reveal a wider area of contamination (e.g. Cu), thus leading to a better understanding of the system`s state.
The findings of this study highlight the need to investigate the utility of different normalisers before a decision is made on the most effective one. Different normalisers should not be expected to provide precisely the same understanding on metal enrichment of sediment, even if this might be an aspirational outcome. Small differences in sample processing and analysis, and biogeochemical processes in sediment, will lead to small differences in the identification of enrichment, especially if it is of a low magnitude. Further, baseline models should not be used as precise boundaries, to clearly delineate enriched from unenriched sediment. Certain normalisers might prove more effective for certain metals than others. This does not imply different normalisers should be used for different metals, but by investigating the utility of different normalisers, anomalies in relationships for metals that allude to important features in the environment that need to be considered may be identified. The implication is that more than one, and preferably several potential normalisers should be analysed in studies focussing on metals in sediment if the aim is to identify enrichment that reflects contamination. Although the inclusion of several potential normalisers has financial implications from an analytical perspective, the ecological or management implications based on conclusions reached using a single (faulty) normaliser that might over- or underestimate enrichment may be significant and warrants such investment.
References
Amorosi A, Sammartino I, Tateo F (2007) Evolution patterns of glaucony maturity: a mineralogical and geochemical approach. Deep-Sea Res II Top Stud Oceanogr 54:1364–1374. https://doi.org/10.1016/j.dsr2.2007.04.006
Balls PW, Hull S, Miller BS, Pirie JM, Proctor W (1997) Trace metal in Scottish estuarine and coastal sediments. Mar Pollut Bull 34:42–50. https://doi.org/10.1016/S0025-326X(96)00056-2
Begg G (1978) The estuaries of Natal: a resource inventory report to the Natal Town and Regional Planning Commission conducted under the auspices of the Oceanographic Research Institute, Durban. Natal Town and Regional Planning Report, pp 1–657
Birch GF, Snowdon RT (2004) The use of size-normalisation techniques in interpretation of soil contaminant distributions. Water Air Soil Pollut 157:1–12. https://doi.org/10.1023/B:WATE.0000038854.02927.1f
Birch GF, Lee J-H, Tanner E, Fortune J, Munksgaard N, Whitehead J, Coughanowr C, Agius J, Chrispijn J, Taylor U, Wells F, Bellas J, Besada V, Viñas L, Soares-Gomes A, Cordeiro RC, Machado W, Santelli RE, Vaughan M, Cameron M, Brooks P, Crowe T, Ponti M, Airoldi L, Guerra R, Puente A, Gómez AG, Zhou GJ, Leung KMY, Steinberg P (2020) Sediment metal enrichment and ecological risk assessment of ten ports and estuaries in the World Harbours Project. Mar Pollut Bull 155:111129. https://doi.org/10.1016/j.marpolbul.2020.111129
Botha GA (2018) Lithostratigraphy of the late Cenozoic Maputaland Group. S Afr J Geol 121:95–108. https://doi.org/10.25131/sajg.121.0007
Brunschön C, Haberzettl T, Behling H (2010) High-resolution studies on vegetation succession, hydrological variations, anthropogenic impact and genesis of a subrecent lake in southern Ecuador. Veg Hist Archaeobotany 19:191–206. https://doi.org/10.1007/s00334-010-0236-4
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22. https://doi.org/10.1002/etc.5620200102
Coynel A, Gorse L, Curti C, Schafer J, Grosbois C, Morelli G, Ducassou E, Blanc G, Maillet GM, Mojtahid M (2016) Spatial distribution of trace elements in the surface sediments of a major European estuary (Loire Estuary, France): source identification and evaluation of anthropogenic contribution. J Sea Res 118:77–91. https://doi.org/10.1016/j.seares.2016.08.005
Cuven S, Francus P, Lamoureux SF (2010) Estimation of grain size variability with micro X-ray fluorescence in laminated lacustrine sediments, Cape Bounty, Canadian High Arctic. J Paleolimnol 44:803–817. https://doi.org/10.1007/s10933-010-9453-1
Daskalakis KD, O’Connor TP (1995) Normalization and elemental sediment contamination in the coastal United States. Environ Sci Technol 29:470–477
Demory F, Oberhänsli H, Nowaczyk NR, Gottschalk M, Wirth R, Naumann R (2005) Detrital input and early diagenesis in sediments from Lake Baikal revealed by rock magnetism. Glob Planet Chang 46(1–4):145–166. https://doi.org/10.1016/j.gloplacha.2004.11.010
Department of Environmental Affairs (2012) National Environmental Management: Integrated Coastal management Act, 2008 (Act No. 24 of 2008). National Action List for the screening of dredged material proposed for marine disposal in term of section 73 of the National Environmental Management: Integrated Coastal management Act, 2008 (Act No. 24 of 2008). Government Gazette, pp 6–9
Dladla NN, Green AN, Cooper J, Mehlhorn P, Haberzettl T (2021) Bayhead delta evolution in the context of late Quaternary and Holocene sea-level change, Richards Bay, South Africa. Mar Geol 441:106608. https://doi.org/10.1016/j.margeo.2021.106608
Du Laing G, Vandecasteele B, de Grauwe P, Moors W, Lesage E, Meers E, Tack FMG, Verloo MG (2007) Factors affecting metal concentrations in the upper sediment layer of intertidal reedbeds along the river Scheldt. J Environ Monit 9:449–455. https://doi.org/10.1039/B618772B
Förstner U (1989) Contaminated sediments. Springer, Berlin
Grant A, Middleton R (1998) Contaminants in sediments: using robust regression for grain-size normalization. Estuaries 21:197. https://doi.org/10.2307/1352468
Greenfield R, Wepener V, Degger N, Brink K (2011) Richards Bay Harbour: metal exposure monitoring over the last 34 years. Mar Pollut Bull 62:1926–1931. https://doi.org/10.1016/j.marpolbul.2011.04.026
Haberzettl T, Corbella H, Fey M, Janssen S, Lücke A, Mayr C, Ohlendorf C, Schäbitz F, Schleser GH, Wille M, Wulf S, Zolitschka B (2007) Lateglacial and Holocene wet—dry cycles in southern Patagonia: chronology, sedimentology and geochemistry of a lacustrine record from Laguna Potrok Aike, Argentina. The Holocene 17:297–310. https://doi.org/10.1177/0959683607076437
Haberzettl T, Kirsten KL, Kasper T, Franz S, Reinwarth B, Baade J, Daut G, Meadows ME, Su Y, Mäusbacher R (2019) Using 210Pb-data and paleomagnetic secular variations to date anthropogenic impact on a lake system in the Western Cape, South Africa. Quat Geochronol 51:53–63. https://doi.org/10.1016/j.quageo.2018.12.004
Hanson PJ, Evans DW, Colby DR, Zdanowicz VS (1993) Assessment of elemental contamination in estuarine and coastal environments based on geochemical and statistical modeling of sediments. Mar Environ Res 36:237–266. https://doi.org/10.1016/0141-1136(93)90091-D
Horowitz AJ (1991) A primer on sediment-trace element chemistry. Lewis Publishers, Chelsea
Horowitz AJ, Elrick KA (1987) The relation of stream sediment surface area, grain size and composition to trace element chemistry. Appl Geochem 2:437–451. https://doi.org/10.1016/0883-2927(87)90027-8
Jung H-S, Lim D, Xu Z, Kang J-H (2014) Quantitative compensation of grain-size effects in elemental concentration: a Korean coastal sediments case study. Estuar Coast Shelf Sci 151:69–77. https://doi.org/10.1016/j.ecss.2014.09.024
Jung H, Lim D, Xu Z, Jeong K (2016) Secondary grain-size effects on Li and Cs concentrations and appropriate normalization procedures for coastal sediments. Estuar Coast Shelf Sci 175:57–61. https://doi.org/10.1016/j.ecss.2016.03.028
Kelbe B (2010) Hydrology and water resources of the Richards Bay EMF area
Kersten M, Smedes F (2002) Normalization procedures for sediment contaminants in spatial and temporal trend monitoring. J Environ Monit 4:109–115. https://doi.org/10.1039/b108102k
Koigoora S, Ahmad I, Pallela R, Janapala VR (2013) Spatial variation of potentially toxic elements in different grain size fractions of marine sediments from Gulf of Mannar, India. Environ Monit Assess 185:7581–7589. https://doi.org/10.1007/s10661-013-3120-8
Krumgalz BS, Fainshtein G, Cohen A (1992) Grain size effect on anthropogenic trace metal and organic matter distribution in marine sediments. Sci Total Environ 116:15–30. https://doi.org/10.1016/0048-9697(92)90362-V
Kylander ME, Ampel L, Wohlfarth B, Veres D (2011) High-resolution X-ray fluorescence core scanning analysis of Les Echets (France) sedimentary sequence: new insights from chemical proxies. J Quat Sci 26:109–117. https://doi.org/10.1002/jqs.1438
Larrose A, Coynel A, Schäfer J, Blanc G, Massé L, Maneux E (2010) Assessing the current state of the Gironde Estuary by mapping priority contaminant distribution and risk potential in surface sediment. Appl Geochem 25:1912–1923. https://doi.org/10.1016/j.apgeochem.2010.10.007
Liang J, Liu J, Xu G, Chen B (2019) Distribution and transport of heavy metals in surface sediments of the Zhejiang nearshore area, East China Sea: sedimentary environmental effects. Mar Pollut Bull 146:542–551. https://doi.org/10.1016/j.marpolbul.2019.07.001
Loring DH (1991) Normalization of heavy-metal data from estuarine and coastal sediments. ICES J Mar Sci 48:101–115. https://doi.org/10.1093/icesjms/48.1.101
Luoma SN (1990) Processes affecting metal concentrations in estuarine and coastal marine sediments. In: Furness RW, Rainbow PS (eds) Heavy metals in the marine environment. CRC Press, Boca Raton
Matys Grygar T, Popelka J (2016) Revisiting geochemical methods of distinguishing natural concentrations and pollution by risk elements in fluvial sediments. J Geochem Explor 170:39–57. https://doi.org/10.1016/j.gexplo.2016.08.003
Matys Grygar T, Nováková T, Bábek O, Elznicová J, Vadinová N (2013) Robust assessment of moderate heavy metal contamination levels in floodplain sediments: a case study on the Jizera River, Czech Republic. Sci Total Environ 452–453:233–245. https://doi.org/10.1016/j.scitotenv.2013.02.085
Matys Grygar T, Elznicová J, Bábek O, Hošek M, Engel Z, Kiss T (2014) Obtaining isochrones from pollution signals in a fluvial sediment record: a case study in a uranium-polluted floodplain of the Ploučnice River, Czech Republic. Appl Geochem 48:1–15. https://doi.org/10.1016/j.apgeochem.2014.06.021
Mehlhorn P, Viehberg F, Kirsten K, Newman B, Frenzel P, Gildeeva O, Green A, Hahn A, Haberzettl T (2021) Spatial distribution and consequences of contaminants in harbour sediments – a case study from Richards Bay Harbour, South Africa. Mar Pollut Bull 172:112764. https://doi.org/10.1016/j.marpolbul.2021.112764
Nel EL, Hill TR, Goodenough C (2007) Multi-stakeholder driven local economic development: reflections on the experience of Richards Bay and the uMhlathuze municipality. Urban Forum 18:31–47. https://doi.org/10.1007/s12132-007-9004-7
Newman BK, Watling RJ (2007) Definition of baseline metal concentrations for assessing metal enrichment of sediment from the south-eastern Cape coastline of South Africa. Water SA 33. https://doi.org/10.4314/wsa.v33i5.184089
Ohlendorf C, Fey M, Massaferro J, Haberzettl T, Laprida C, Lücke A, Maidana NI, Mayr C, Oehlerich M, Ramón Mercau J, Wille M, Corbella H, St-Onge G, Schäbitz F, Zolitschka B (2014) Late Holocene hydrology inferred from lacustrine sediments of Laguna Cháltel (southeastern Argentina). Palaeogeogr Palaeoclimatol Palaeoecol 411:229–248. https://doi.org/10.1016/j.palaeo.2014.06.030
Rubio B, Nombela M, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40:968–980. https://doi.org/10.1016/S0025-326X(00)00039-4
Sabadini-Santos E, Knoppers BA, Oliveira EP, Leipe T, Santelli RE (2009) Regional geochemical baselines for sedimentary metals of the tropical São Francisco estuary, NE-Brazil. Mar Pollut Bull 58:601–606
Santos IR, Silva-Filho EV, Schaefer CEGR, Albuquerque-Filho MR, Campos LS (2005) Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar Pollut Bull 50(2):185–194. https://doi.org/10.1016/j.marpolbul.2004.10.009
Saulnier I, Mucci A (2000) Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord, Canada. Appl Geochem 15:191–210. https://doi.org/10.1016/S0883-2927(99)00034-7
Schropp SJ, Windom HL (1988) A guide to the interpretation of metal concentrations in estuarine sediments coastal zone management section. Florida Department of Environmental Regulation, Florida
Schropp SJ, Lewis FG, Windom HL, Ryan JD, Calder FD, Burney LC (1990) Interpretation of metal concentrations in estuarine sediments of Florida using aluminum as a reference element. Estuaries 13:227. https://doi.org/10.2307/1351913
Sigg L, Stumm W (1996) Aquatische Chemie: eine Einführung in die Chemie wässriger Lösungen und natürlicher Gewässer. vdf, Hochschulverl.-AG an d. ETH Zürich, Zürich
Szava-Kovats RC (2008) Grain-size normalization as a tool to assess contamination in marine sediments: is the <63 micron fraction fine enough? Mar Pollut Bull 56:629–632. https://doi.org/10.1016/j.marpolbul.2008.01.017
Taylor SR, McLennan SM (1981) The composition and evolution of the continental crust: rare earth element evidence from sedimentary rocks. Phil Trans R Soc A 301:381–399. https://doi.org/10.1098/rsta.1981.0119
Thomas C, Bendell-Young L (1999) The significance of diagenesis versus riverine input in contributing to the sediment geochemical matrix of iron and manganese in an intertidal region. Estuar Coast Shelf Sci 48:635–647. https://doi.org/10.1006/ecss.1998.0473
Tůmová Š, Hrubešová D, Vorm P, Hošek M, Grygar TM (2019) Common flaws in the analysis of river sediments polluted by risk elements and how to avoid them: case study in the Ploučnice River system, Czech Republic. J Soils Sediments 19(4):2020–2033. https://doi.org/10.1007/s11368-018-2215-9
Watling RJ (1977) Trace metal distribution in the Wilderness-lakes. CSIR Special Report FIS 147
Weisberg SB, Wilson HT, Heimbuch DG, Windom HL, Summers JK (2000) Comparison of sediment metal:aluminum relationships between the eastern and gulf coasts of the United States. Environ Monit Assess 61:373–385. https://doi.org/10.1023/A:1006113631027
Wepener V, Vermeulen LA (2005) A note on the concentrations and bioavailability of selected metals in sediments of Richards Bay Harbour, South Africa. Water SA 31(4):589–596. https://doi.org/10.4314/wsa.v31i4.5149
Windom HL, Schropp SJ, Calder FD, Ryan JD, Smith RG Jr, Burney LC, Lewis FG, Rawlinson CH (1989) Natural trace metal concentrations in estuarine and coastal marine sediments of the southeastern United States. Environ Sci Technol 23:314–320
Woods AM, Lloyd JM, Zong Y, Brodie CR (2012) Spatial mapping of Pearl River Estuary surface sediment geochemistry: influence of data analysis on environmental interpretation. Estuar Coast Shelf Sci 115:218–233. https://doi.org/10.1016/j.ecss.2012.09.005
Wündsch M, Biagioni S, Behling H, Reinwarth B, Franz S, Bierbaß P, Daut G, Mäusbacher R, Haberzettl T (2014) ENSO and monsoon variability during the past 1.5 kyr as reflected in sediments from Lake Kalimpaa, Central Sulawesi (Indonesia). The Holocene 24:1743–1756. https://doi.org/10.1177/0959683614551217
Zolitschka B, Fey M, Janssen S, Maidana NI, Mayr C, Wulf S, Haberzettl T, Corbella H, Lücke A, Ohlendorf C, Schäbitz F (2019) Southern Hemispheric Westerlies control sedimentary processes of Laguna Azul (south-eastern Patagonia, Argentina). The Holocene 29:403–420. https://doi.org/10.1177/0959683618816446
Acknowledgements
This work was supported by the German Federal Ministry of Education and Research [grant number: 03F0798C] and is part of project TRACES (Tracing Human and Climate impact in South Africa) within the SPACES II Program (Science Partnerships for the Assessment of Complex Earth System Processes). The field work support by Andrew Green (University of KwaZulu-Natal), Peter Frenzel, Olga Gildeeva (both Friedrich-Schiller-University Jena) and George Best is gratefully acknowledged. We also thank Tammo Meyer (University of Greifswald) for providing help for sample digestion and Dirk Merten (Friedrich-Schiller-University Jena) for ICP-MS and -OES analyses.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Mehlhorn, P., Newman, B., Haberzettl, T. (2024). Comparison of Different Normalisers for Identifying Metal Enrichment of Sediment: A Case Study from Richards Bay Harbour, South Africa. In: von Maltitz, G.P., et al. Sustainability of Southern African Ecosystems under Global Change. Ecological Studies, vol 248. Springer, Cham. https://doi.org/10.1007/978-3-031-10948-5_27
Download citation
DOI: https://doi.org/10.1007/978-3-031-10948-5_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10947-8
Online ISBN: 978-3-031-10948-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)