Abstract
The obstruction of the upper urinary tract represents a common medical condition which could be related to significant and life-threating complications such acute renal failure and urosepsis. Ureteral stents are commonly used to prevent and manage such complications. However, the use of standard stents involves significant comorbidities, including stent-associated infection, encrustation, migration, urothelial hyperplasia reaction. Also, urethral strictures represent a common cause of lower urinary tract obstruction with the characteristic of frequent recurrence. Patients suffering from urethral strictures can be treated by minimally invasive techniques such as mechanical dilatation with balloon or placing of urethral stents. In attempt to address the any stent-related complications, the urological research considered ideas and concepts used in interventional cardiology and radiology. Percutaneous transluminal coronary angioplasty (PTCA) is the gold standard for coronary revascularization, even if restenosis complications exist in concerning rates. To address this complication, stents bearing drugs agents (most commonly immunosuppressive agents) have been used. These drug-eluting stents (DESs) release single or multiple bioactive agents, which are deposited on adjacent tissues. The immunosuppressive substances reduce benign tissue proliferation and their use has significantly reduced restenosis rates after PTCA. In a similar fashion, the drug-coated balloons (DCBs) are used as a new alternative instead of DESs in selected cases and offer important advantages. Their drug is released directly at the site of the stricture while avoiding any foreign material at the site of the stricture. Unfortunately, possibility of using DCBs in endourology is also under research.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
The obstruction of the upper urinary tract represents a common medical condition which could be related to significant and life-threating complications such acute renal insufficiency and urosepsis. Ureteral stents are commonly used to prevent and manage such complications. These stents provide non-surgical decongestion of the pelvicalyceal system by achieving unobstructed inflow of urine in the bladder. However, the use of conventional stents involves significant comorbidities, including stent-associated infection, encrustation, migration, hyperplastic urothelial reaction [1].
Urethral strictures represent a common cause of lower urinary tract obstruction with the characteristic of frequent recurrence. Patients suffering from urethral strictures can be treated by minimally invasive techniques [2] such as mechanical dilatation with balloon or placing of urethral stents [3].
In attempt to address the any stent-related complications, the urological research considered ideas and concepts used in interventional cardiology and radiology (Table 1). Percutaneous transluminal coronary angioplasty (PTCA) is the gold standard for coronary revascularization, even if restenosis complications exist in concerning rates. To address this complication, stents bearing pharmaceutical agents (most commonly immunosuppressive agents) have been used [4]. These drug-eluting stents (DESs) release single or multiple bioactive agents, which are deposited on adjacent tissues. The immunosuppressive substances reduce benign tissue proliferation and their use has significantly reduced restenosis rates after PTCA [5, 6]. In a similar fashion, the drug-coated balloons (DCBs) are used as a new alternative instead of DESs in selected cases and offer important advantages (Fig. 1). Their drug is released directly at the site of the stricture while avoiding any foreign material at the site of the stricture. Moreover, DCBs could manage vascular stricture sites inappropriate for stent placement [7].
The impressive impact of the DESs to avoid vascular restenosis proposed the drug-eluting idea to be used for the improvement of urological urinary stents. Thus, the effect of DESs to reduce the existing complications of the indwelling ureteral stents has been investigated [8] (Table 2). The possibility of using DCBs in endourology is also under research (Table 3).
2 Drug Eluting Devices
Drug eluting devices can be classified in three major categories based on the pharmaceutical agent they carry: antibiotic, anti-inflammatory agents and drugs inhibiting the cell proliferation.
2.1 DES Delivering Antibiotics
Infections of the urinary tract are related with the presence of foreign materials such as catheters and nephrostomy tubes. These cases consist the most frequent hospital infections. Any foreign bodies (catheters or urinary stent) offer a suitable surface for the formation of a highly resistant biofilm [9,10,11,12]. Coating the ureteral stents with antibiotics could limit bacterial growth on the foreign bodies and prevent urinary infections. Thus, several antibiotic agents have proposed for urinary stents [9,10,11,12].
2.1.1 Triclosan
The antimicrobial agent Triclosan inhibits the fatty acids synthesis and disrupts the integrity of the bacterial cell’s wall. Chew et al. tested the efficiency of Triclosan eluting stents against common uropathogens in artificial urine. In terms of bacterial growth and adherence, Triclosan DESs was efficient against most of the uropathogens apart from P. aeruginosa [9]. An ex vivo study, Cadieux et al., using the curls of a Triclosan-eluting stent sutured in a rabbit urethra verified the in vitro result, showing also reduced inflammation in comparison to the control group [13]. The same research team tested the long-term effect of using the Triclosan-eluting stent in a small patient group. The patients kept the DES for three months and received oral antibiotics when having UTI symptoms [14]. The results showed no significant difference in the number of the bacteria in the urine cultures. The encrustation rate of the Triclosan-eluting stents was compared to conventional stents in a randomized control trial including 20 patients. These patients were treated with short duration stenting [15]. No significant difference in encrustation rate was observed between the two groups but the Triclosan-eluting stent was associated with reduced incidents of symptomatic UTIs, abdominal and urethral pain and subsequently a reduced need for antibiotic therapy. Overall, the promising results of the in vitro and preclinical in vivo models were not observed in patients. Only the overall antibiotic need and patient discomfort were reduced. Despite the beneficial effects observed in the primary experience of this DES, it has been removed from the market.
2.1.2 Quinolones
Quinolones are commonly used for the management of urinary tract infections. The effect of quinolone-eluting stents has been examined. Stents with a mixture of ofloxacin and ornidazole were evaluated in terms of efficacy against E. coli and S. epidermidis in a study performed by Balasubramanian et al. [16]. The stents were found to be effective against these uropathogens in an agar diffusion test and microbial adhesion was significantly reduced on them in artificial urine environment compared to conventional stents. Studies with DESs containing ciprofloxacin showed that they have reliable antibacterial effect against common uropathogens such as S. aureus and E. coli without damaging the Human Foreskin Fibroblasts [12].
2.1.3 Silver and Nitrofurazone
Urethral catheters containing silver or nitrofurazone were evaluated for their efficacy in the inhibition of resistant E. coli and P. aeruginosa [11]. Nitrofurazone-coated stents offered longer inhibition of all E.coli strains compared to conventional stents, while the silver-coated showed no significant inhibition. Neither the nitrofurazone-coated, nor the silver-coated stent had any effect against P. aeruginosa. The fact that silver has antimicrobial effect but the silver-coated stents did not have any effect, perhaps indicates that the concentration of silver released in the urethra was not high enough to reach its antimicrobial potential. This indicates that while the ability of a stent to release a substance from its surface is important, high enough concentrations are necessary so that the drug eluting stent is effective.
2.1.4 RNA-Inhibiting Peptides and Teicoplanin
Researchers using combination of RNAIII-inhibiting peptides and teicoplanin in a drug-eluting stent managed to significantly reduce the microbial colonization. Interestingly, urine cultures were negative for bacterial growth [17].
2.1.5 Heparin
Stents coated with heparin were used to examine the possibility of decreasing the encrustation of the stent. Because heparin is a negatively charged molecule, an assumption was made that the negatively charged crystals would repel each other resulting in less encrustation. The layer of encrustation on the heparin-eluting stents was thinner and more restricted than the encrustation on the conventional ones, Nonetheless, the two groups did not differ significantly in terms of bacterial adhesion on the stents [18].
2.2 DES Delivering Anti-Inflammatory Substances
The patient discomfort that follows the insertion of a stent is mainly caused by the inflammatory response of the tissue in contact with the stent. Several anti-inflammatory agents have been embedded on stents in an attempt to prevent patient discomfort.
2.2.1 Ketorolac
Several studies involved devices that contained Ketorolac to ease the pain and reduce the inflammation [19,20,21,22]. While the in vitro and animal in vivo studies showed promising results, in the clinical trials Ketorolac stents failed to show significant difference in pain management compared to conventional stents. Only in the category of men under 45 years, significant difference was identified. Ketorolac did not have detectable plasma levels of the patients and led to the assumption that the concentrations were not high enough to achieve the therapeutic levels.
2.2.2 Indomethacin, Dexamethasone and Simvastatin
Dexamethasone was one of the first anti-inflammatory drugs that has been used to cover stents. Antimisiaris et al. [23] applied large multi-lamellar liposomes containing dexamethasone to metallic stents to evaluate in vitro the rate of release of dexamethasone. Absorbable urethral stents containing indomethacin, dexamethasone and simvastatin were used in rabbit urethras to examine the potential reaction of the components and their potential effect in the degradation process [24]. In vivo animal studies using absorbable indomethacin-eluting stents revealed that the delivery of the drug did not intervene with the degradation of the stent and the use of the stents led to lower inflammation and calcification rate during the degradation [25, 26]. The potential role of the indomethacin-stent after urethrotomy should be examined.
2.2.3 EW-7197
Han et al. investigated a nano fiber-self expendable stent that contained the TGF-β type 1 inhibitor EW-7197 in a canine model [27]. The aim of the study was to compare the formation of granulation tissue between a control and a DES stent after an 8-week period. The use of DES resulted in wider urethral luminal diameters, thinner layers submucosal fibrosis and papillary projection and less epithelial layers and collagen deposition in comparison to the control group.
2.2.4 Halofungione (HF)
Krane et al. [28] eluted halofungione on a urethral stent. This alkaloid acts as a selective inhibitor of collagen type I. the stent was inserted in in rat urethras with strictures created by electrocautery. The use of halofungione prevented the formation of new type I collagen in the rat urethra.
2.3 DES and DCB Containing Anti-Cancer Drugs
Stents releasing anti-proliferative agents has been used in great extent in interventional cardiology. The combination of limiting the cell proliferation and avoiding the formation of fibrosis made this drug category especially suitable for preventing coronary vessel restenosis. The advantages in treating restenosis led to the creation of ureteral and urethral stents and balloons.
2.3.1 Paclitaxel
Paclitaxel inhibits the mitosis by stabilizing the microtubules of the cell. It is the most commonly used antiproliferative drug in studies evaluating devices for urinary stenosis.
Aiming to investigate the ability of antiproliferative drugs to permeate membrane models, Barros et al. [29] used ureteral stents with doxorubicin and paclitaxel. The researchers concluded that both drugs were restricted in the ureter and only a fraction of the total drug passed all the layers. Kram et al. [30] used a DES containing paclitaxel in rat ureters to examine its inhibitory effect in hyperplastic proliferation. The rats underwent ureteroureterostomy followed by the insertion of a drug-eluting or a conventional stent. The DES was found significantly more effective in reducing the cell proliferation in the side of the anastomosis. In a similar manner, Liatsikos et al. [31] compared DES to conventional stents in terms of inflammation and the tissue hyperplasia occurring in porcine ureter. The ureters with the indwelling DES showed increased patency in urography compared to the ureters with the conventional stent. Shin et al. came to the same conclusions after examining a custom made paclitaxel stent in canine urethra [32] (Fig. 2).
Biodegradable stents have been also evaluated in an experimental study. Wang et al. used a biodegradable paclitaxel DES in rabbit urethra and observed the absorption of the stent in 12 weeks. Moreover, the treated urethral mucosa showed no signs of fibrosis while the urethras of the control group showed signs of fibrosis [33].
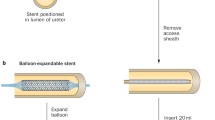
In addition to DES, DCBs with paclitaxel have been investigated (Fig. 3). Barbalias et al. [3] studied how paclitaxel is distributed in layers of rabbit urethra. The study included the dilation of the posterior urethra and subsequent dilation with a paclitaxel-coated balloon. Histological analysis showed that paclitaxel penetrates the urethral layers and especially the urothelial barrier. A similar study by Liourdi et al. [34] proved that dilation with DCBs containing paclitaxel resulted in distribution of the drug in every layer of the ureter. While the forementioned studies proved that paclitaxel could be distributed in through the urothelium to the all the layers of the urethra and the ureter, these experiments were performed in animal models which did not have strictures.
ROBUST I is a multi-centered study prospective study examining the safety and efficacy of Optilume™ Drug Coated Balloon (DCB; Urotronic, Plymouth, MN) in patients with recurrent bulbar strictures. Visasoro et al. published the first-year results results of 53 patients that have been treated in their center using the paclitaxel-coated balloon mentioning 70% anatomic success, which was defined as diameter of lumen equal or greater than 14 Fr [35]. The same research group published the 2 years results announcing 70% success in having at least 50% improved IPSS sore for 2 years after the surgery [36]. Flow rate and post-void residual urine volumes were also improved. There researchers did not encounter any severe adverse effects. The long-term results of the study are expected after a 5-year follow up has been completed. However, it is crucial to bear in mind that every patient in this study prior to use of the DCB balloon received either an uncoated balloon or direct visualization internal urethrotomy treatment until their urethral diameter was increased by 50%. Consequently, safe assumptions for the use of paclitaxel-balloons as monotherapy for the treatment of bulbar strictures cannot be made yet Fig. 4.
Apart from paclitaxel, the efficacy of other anti-proliferative agents such as zotarolimus and sirolimus have been evaluated. Kallidonis et al. [37] using DESs containing zotarolimus in pigs and rabbits showed that zotarolimus-eluting stents reduce inflammation and tissue hyperplasia in comparison to the control conventional stents. Kim et al. used two types of sirolimus-eluting stents containing different concentrations of the agent in male rat models [38]. The researchers found that the use of sirolimus stents reduced the formation of granulation tissue compared to the conventional stents and that the use of stents with sirolimus concentration of 450 μg/cm2 resulted to less layers of epithelial growth and greater rate of apoptosis in comparison to the 90 μg/cm2 stent and the conventional stent.
Many pre-clinical studies testing drug eluting devices have shown impressive results. The ability to prevent fibrosis, tissue proliferation or bacterial adhesion while reducing the symptoms and improving the patient’s quality of life underline the great potential of these devices. That said, achieving a stable delivery of the drug, unaffected by the urine flow could be a key factor to lead to significant results in the human trials. Assumptions for the efficacy of the devices in humans cannot be made safely due to the lack of large clinical studies.
References
Al-Aown A, Kyriazis I, Kallidonis P, Kraniotis P, Rigopoulos C, Karnabatidis D, et al. Ureteral stents: new ideas, new designs. Ther Adv Urol. 2010;2(2):85–92.
Vyas J, Ganpule A, Muthu V, Sabnis R, Desai M. Balloon dilatation for male urethral strictures “revisited”. Urol Ann. 2013;5(4):245–8.
Barbalias D, Lappas G, Ravazoula P, Liourdi D, Kyriazis I, Liatsikos E, et al. Evaluation of the distribution of paclitaxel after application of a paclitaxel-coated balloon in the rabbit urethra. J Endourol. 2018;32(5):381–6.
Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003;361(9353):247–9.
Ni L, Chen H, Luo Z, Yu Y. Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis. J Cardiothorac Surg. 2020;15(1):26.
Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–65.
Nestelberger T, Kaiser C, Jeger R. Drug-coated balloons in cardiovascular disease: benefits, challenges, and clinical applications. Expert Opin Drug Deliv. 2020;17(2):201–11.
Kallidonis PS, Georgiopoulos IS, Kyriazis ID, Al-Aown AM, Liatsikos EN. Drug-eluting metallic stents in urology. Indian J Urol. 2014;30(1):8–12.
Chew BH, Cadieux PA, Reid G, Denstedt JD. In-vitro activity of triclosan-eluting ureteral stents against common bacterial uropathogens. J Endourol. 2006;20(11):949–58.
Elayarajah E, Rajendran R, Venkatrajah V, Sreekumar S. Biopolymer tocopherol acetate as a drug carrier to prevent bacterial biofilm formation on silicone ureteral stents. Int J Pharm Sci Rev Res. 2011;7(2):96–103.
Johnson JR, Johnston BD, Kuskowski MA, Pitout J. In vitro activity of available antimicrobial coated Foley catheters against Escherichia coli, including strains resistant to extended spectrum cephalosporins. J Urol. 2010;184(6):2572–7.
Ma X, Xiao Y, Xu H, Lei K, Lang M. Preparation, degradation and in vitro release of ciprofloxacin-eluting ureteral stents for potential antibacterial application. Mater Sci Eng C Mater Biol Appl. 2016;66:92–9.
Cadieux PA, Chew BH, Knudsen BE, Dejong K, Rowe E, Reid G, et al. Triclosan loaded ureteral stents decrease Proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175(6):2331–5.
Cadieux PA, Chew BH, Nott L, Seney S, Elwood CN, Wignall GR, et al. Use of triclosan-eluting ureteral stents in patients with long-term stents. J Endourol. 2009;23(7):1187–94.
Mendez-Probst CE, Goneau LW, MacDonald KW, Nott L, Seney S, Elwood CN, et al. The use of triclosan eluting stents effectively reduces ureteral stent symptoms: a prospective randomized trial. BJU Int. 2012;110(5):749–54.
Balasubramanian E, Rajendran R, Venkatrajah SS. Biopolymer tocopherol acetate as a drug carrier to prevent bacterial biofilm formation on silicone ureteral stents. Int J Pharm Sci Rev Res. 2011;7:96–103.
Cirioni O, Ghiselli R, Minardi D, Orlando F, Mocchegiani F, Silvestri C, et al. RNAIII-inhibiting peptide affects biofilm formation in a rat model of staphylococcal ureteral stent infection. Antimicrob Agents Chemother. 2007;51(12):4518–20.
Cauda F, Cauda V, Fiori C, Onida B, Garrone E. Heparin coating on ureteral double J stents prevents encrustations: an in vivo case study. J Endourol. 2008;22(3):465–72.
Barros AA, Oliveira C, Reis RL, Lima E, Duarte AR. Ketoprofen-eluting biodegradable ureteral stents by CO2 impregnation: in vitro study. Int J Pharm. 2015;495(2):651–9.
Krambeck AE, Walsh RS, Denstedt JD, Preminger GM, Li J, Evans JC, et al. A novel drug eluting ureteral stent: a prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183(3):1037–42.
Chew BH, Davoudi H, Li J, Denstedt JD. An in vivo porcine evaluation of the safety, bioavailability, and tissue penetration of a ketorolac drug-eluting ureteral stent designed to improve comfort. J Endourol. 2010;24(6):1023–9.
Lin YC, Liu KS, Lee D, Li MJ, Liu SJ, Ito H. In vivo and in vitro elution of analgesics from multilayered poly(d,l)-lactide-co-glycolide nanofibers incorporated ureteral stents. J Nanomater. 2018;2018:8829.
Antimisiaris SG, Siablis D, Liatsikos E, Kalogeropoulou C, Tsota I, Tsotas V, et al. Liposome-coated metal stents: an in vitro evaluation of controlled-release modality in the ureter. J Endourol. 2000;14(9):743–7.
Kotsar A, Isotalo T, Uurto I, Mikkonen J, Martikainen P, Talja M, et al. Urethral in situ biocompatibility of new drug-eluting biodegradable stents: an experimental study in the rabbit. BJU Int. 2009;103(8):1132–5.
Kotsar A, Nieminen R, Isotalo T, Mikkonen J, Uurto I, Kellomäki M, et al. Biocompatibility of new drug-eluting biodegradable urethral stent materials. Urology. 2010;75(1):229–34.
Kotsar A, Nieminen R, Isotalo T, Mikkonen J, Uurto I, Kellomaki M, et al. Preclinical evaluation of new indomethacin-eluting biodegradable urethral stent. J Endourol. 2012;26(4):387–92.
Han K, Park JH, Yang SG, Lee DH, Tsauo J, Kim KY, et al. EW-7197 eluting nano-fiber covered self-expandable metallic stent to prevent granulation tissue formation in a canine urethral model. PLoS One. 2018;13(2):e0192430.
Krane LS, Gorbachinsky I, Sirintrapun J, Yoo JJ, Atala A, Hodges SJ. Halofuginone-coated urethral catheters prevent periurethral spongiofibrosis in a rat model of urethral injury. J Endourol. 2011;25(1):107–12.
Barros AA, Oliveira C, Reis RL, Lima E, Duarte ARC. In vitro and ex vivo permeability studies of paclitaxel and doxorubicin from drug-eluting biodegradable ureteral stents. J Pharm Sci. 2017;106(6):1466–74.
Kram W, Rebl H, Wyrwa R, Laube T, Zimpfer A, Maruschke M, et al. Paclitaxel-coated stents to prevent hyperplastic proliferation of ureteral tissue: from in vitro to in vivo. Urolithiasis. 2018;48(1):47–56.
Liatsikos EN, Karnabatidis D, Kagadis GC, Rokkas K, Constantinides C, Christeas N, et al. Application of paclitaxel-eluting metal mesh stents within the pig ureter: an experimental study. Eur Urol. 2007;51(1):217–23.
Shin JH, Song HY, Choi CG, Yuk SH, Kim JS, Kim YM, et al. Tissue hyperplasia: influence of a paclitaxel-eluting covered stent—preliminary study in a canine urethral model. Radiology. 2005;234(2):438–44.
Wang ZX, Hong BF, Xu Z, Fu WJ, Cui FZ, Kun H. New biodegradable drug-eluting stents for urethral strictures in a rabbit model. J Bioact Compat Polym. 2011;26(1):89–98.
Liourdi D, Kallidonis P, Kyriazis I, Tsamandas A, Karnabatidis D, Kitrou P, et al. Evaluation of the distribution of paclitaxel by immunohistochemistry and nuclear magnetic resonance spectroscopy after the application of a drug-eluting balloon in the porcine ureter. J Endourol. 2015;29(5):580–9.
Virasoro R, DeLong JM, Mann RA, Estrella RE, Pichardo M, Lay RR, et al. A drug-coated balloon treatment for urethral stricture disease: Interim results from the ROBUST I study. Can Urol Assoc J. 2020;14(6):187–91.
Mann RA, Virasoro R, DeLong JM, Estrella RE, Pichardo M, Lay RR, et al. A drug-coated balloon treatment for urethral stricture disease: two-year results from the ROBUST I study. Can Urol Assoc J. 2021;15(2):20–5.
Kallidonis P, Kitrou P, Karnabatidis D, Kyriazis I, Kalogeropoulou C, Tsamandas A, et al. Evaluation of zotarolimus-eluting metal stent in animal ureters. J Endourol. 2011;25(10):1661–7.
Kim KY, Park JH, Kim DH, Tsauo J, Kim MT, Son WC, et al. Sirolimus-eluting biodegradable poly-l-lactic acid stent to suppress granulation tissue formation in the rat urethra. Radiology. 2018;286(1):140–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Kallidonis, P., Vagionis, A., Liourdi, D., Liatsikos, E. (2022). Drug Eluting Devices in the Urinary Tract. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_31
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)