Abstract
In the twenty-first century, it is difficult to understand that a medical device as widely used as urinary stents require a second medical procedure for removal. Research in the development of biocompatible biodegradable urinary stents (BUS) has been one of the most important research areas of innovation in the urology stent technology. The main characteristics of a BUS are related to its ability to degrade into non-obstructive fragments in a predefined time and to be removed through micturition, after providing an appropriate internal scaffold effect and urinary drainage.
One of the barriers slowing down the progress of research are the lack of agreement between in vitro and in vivo degradation rates demonstrated in a large number of experimental studies. Unfortunately, currently the absence of BUSs in clinical practice is mainly due to the complicated degradation rate control, maintenance of mechanical properties and safe urinary excretion of stent fragments. In order to alleviate the weak mechanical properties of degradable biomaterials, research has been started with metallic BUS with very promising results. In this regard, Mg2+ and its alloys have been used in in vitro and in vivo studies.
Another area of current research is the emergence of ureteral BUS to provide a new approach for local drug delivery in upper urinary tract. Drugs may be released while the stent is degrading. Although these innovations are still under research, it is foreseeable that in the near future they could be used to improve the lives of patients.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the twenty-first century, it is difficult to understand that a medical device as widely used as urinary stents require a second medical procedure for removal. When both ureteral and urethral stents are in direct contact with a fluid like urine. These characteristics should be used for their degradation by a simple controlled hydrolysis reaction.
Research in the development of biocompatible biodegradable urinary stents (BUS) has been one of the most important research areas of innovation in the stent technology. The main characteristics of a BUS are related to its ability to degrade into non-obstructive fragments in a predefined time and to be removed through micturition, after providing an appropriate internal scaffold effect and urinary drainage [1]. The main beneficial effect of this type of stent is the avoidance of a second medical procedure for removal, which reduces stress for patients and makes them less reluctant to undergo stenting in the future. It also overcomes the difficulties that in some cases are associated with the removal of a conventional ureteral stent, mainly in those with encrustations [2]. It should also be highlighted that, in the subpopulation of children, where sedation is necessary for stent removal, the risks and associated costs are reduced [1, 3]. Finally, another advantage provided by BUSs is the decrease in indirect healthcare costs. First, by avoiding the classic cystoscopic removal procedure, and second, by lowering the costs associated with the management of complications caused by urinary stents, particularly those related to the treatment of calcified or forgotten stents. The latter can be up to six times more expensive than uncomplicated cystoscopic removal of the ureteral stent [3, 4].
Specific circumstances occur in relation to urethral stents, as they are nowadays generally permanent, so their removal is not the main benefit sought with the development of these devices. The main indications are therefore to allow their temporary use and to reduce the side effects associated with urethral or prostate metallic stents, such as obstructive urothelial hyperplasia [5].
An important desired characteristic of the BUSs is to add to their predictable and controlled degradation capacity other beneficial characteristics, such as the possibility of local drug release or the possibility of becoming the first bio-coated urinary stent designs. In this way, they aim to combine the innovation of a biodegradable stent with the reduction of adverse effects, as well as properties that allow for the extension of stent-associated therapeutics. Biodegradable urethral stents with tissue therapy applications for adjuvant treatment of urethral strictures, as well as the delivery of chemotherapy to the upper urinary tract, are technological developments currently under discussion at [6].
2 Ideal Biodegradable Urinary Stent
The characteristics that a BUS must show are mainly a biodegradable character in relation to its indications. That is, it must show a stable and functional structure, which allows it to maintain its mechanical properties during the degradation time. It should also favour urinary drainage and its important function as a ureteral or urethral tutor as an internal scaffold [1, 7, 8]. It should be highlighted that a BUS is not degradable from the first moment of its placement, and therefore behaves in its first phases as a biostable stent, although once its degradation phase has begun, the biomaterials and their architecture should ensure its stability and functionality.
Obviously, the biomaterials of these stents must be stable and biocompatible in the urinary tract [1, 7]. Both the BUS biomaterials and its metabolites must not lack mutagenic, carcinogenic, antigenic, toxic effects, or present the possibility of being absorbed through the urothelium [1, 7, 8].
Its design and mechanical characteristics must ensure that the BUS remains in place without migration. But these characteristics must be compatible with easy insertion, both at ureteral and urethral lumen, as well as in urinary obstructions [7]. Furthermore, an important consideration for suitable patient follow-up is that an ideal BUS should show good tracing ability under X-ray and ultrasound (Fig. 1).
However, if there is one characteristic of an ideal BUS, it is that it should be completely biodegradable. In this regard, both the biomaterials and the design of the BUS must provide a predictable and controlled degradation in urinary tract. This controlled degradation phenomenon is the cornerstone of an ideal BUS, as its degradation rate must be predictable to fulfil its indication, but it must also be safe. Therefore, the degradation fragments must be easily washed out in the urine and never embedded or retained. Another ideal requirement is that once the degradation phase has started, the urine should be stained, never red, so that the degradation phase can be easily monitored by the patient [1, 7, 8].
Ideal BUSs should allow coating with antibacterial contamination inhibiting agents, as well as allow drug release for topical adjuvant therapy, or allow tissue engineering therapy. The ideal biodegradable stent should also maintain its characteristics unchanged after the required sterilisation process and have a reasonable manufacturing cost [8,9,10] (Table 1).
Unfortunately, none of the technological developments have provided the ideal BUS. Although many advances in design and improvements in mechanical characteristics and biocompatibility have been made in the last decade, one of the main drawbacks for the development of a clinically useful BUS is the lack of control and prediction of device degradation [1]. This is due to the fact that the urinary tract represents a changing environment, depending on each individual and the disease condition, and all these factors affect the degradation process of the BUS [8].
3 Biodegradables Biomaterials
As has been described in depth in previous chapters (Chapter “Biomaterials for ureteral stents: advances and future perspectives”), the main biomaterials used for the manufacture of BUS are summarised, as well as the degradation mechanisms. The main degradation mechanism of biomaterials used in the urinary tract is hydrolysis, whereby hydrogen bonds are broken upon contact with urine [8, 11]. Degradation by hydrolysis is influenced by a multitude of factors, including: changes in urinary pH, urine composition, variations in patients’ fluid intake and the nature of those fluids, and the characteristics of the polymeric biodegradable biomaterial to absorb moisture into its interior, as this last characteristic is responsible for the degradation kinetics [8, 11]. The other main degradation mechanism of BUSs is the biological one that causes the breakdown of biodegradable polymers by enzymatic reactions. No BUS has an exclusive biodegradation mechanism, although there is always one of them that is responsible for the highest percentage of degradation.
So, in summary, biodegradable polymers in the urinary tract first undergo a destabilisation of their bonds causing fragmentation. In this first step, the biomaterial does not lose its biomechanical characteristics. However, further hydrolytic degradation of the chemical bonds causes a loss of molecular weight triggering polymer fragmentation.
The most common biomaterials are natural polymers, synthetics and metals.
3.1 Natural Biomaterials
The use of gelatine, alginate, gellan gum or their combinations has proven to be an attractive material due to its biocompatibility and low immunogenicity [12]. In particular, alginate, a linear polysaccharide from marine algae, due to its high capacity to form hydrogels, is frequently used in the manufacture of medical devices. The most recently described BUS from biomaterials of exclusively natural origin have been developed by the research group of Barros et al. in Portugal [10, 13,14,15].
3.2 Synthetic Biodegradable Biomaterials
Biodegradable polymers of synthetic origin are the most frequently used by BUS research groups. Their main advantages are their evident biocompatibility, as well as the absence of immunogenicity, carcinogenicity, teratogenicity and toxicity [16]. The most frequently used synthetic polymers are: polylactic acid (PLA), polyglycolic acid (PGA), PLGA, PCL and polydioxanone (PDX). PLA and PCL have excessively slow degradation times, while the degradation rates of PGA and PDX are relatively fast, with degradation times from weeks to months [1, 17]. PLA is a polymer that has been widely used for the manufacture of medical devices due to its low toxicity, as it generates lactic acid as a metabolite [18, 19]. In addition, it has good mechanical properties, but a degradation rate of 4–6 months is a limitation for clinical use [20,21,22]. PGA is a slow degrading linear aliphatic polyester that has shown very favourable results with regard to bacterial adhesion and encrustation [12, 22].
Regarding the copolymers, they are more easily degradable than the individual polymers [22]. PLGA is polymerised with glycolic acid and lactic acid in different proportions, thus combining the advantages of both compounds [1]. Due to its excellent properties, PLGA has been evaluated in combination with other polymers for the manufacture of BUS [22]. Recently, the combination of PLGA and PGA has been analysed in vitro, showing that by heat treatment of the polymer cross-linker an ideal crystallisation of PGA is achieved, providing longer degradation times [23]. As well as that the combinations in the ratio between PGA and PLGA allow to control the mechanical properties of the stent [24].
3.3 Metallic Biodegradable Biomaterials
Magnesium (Mg2+) and its alloys have been widely investigated as a material for biodegradable medical devices. However, the fast degradation of Mg-based alloys in a physiological environment has hindered their widespread use [25]. Lock et al. confirmed the use of Mg2+ alloy for BUS development. Their results showed that Mg2+ has suitable mechanical and antimicrobial properties for manufacturing BUS ureteral stents. However, corrosion control of the Mg2+ alloy remains an challenge [26]. Although recently, it has been demonstrated the corrosion rate of magnesium can be tailored by alloying elements, surface treatments and heat treatments [25].
4 Biodegradable Ureteral Stent
The aims for the development of a ureteral BUS are very clear and have been described earlier in this chapter. However, currently the absence of ureteral BUSs in daily clinical practice is mainly due to the complicated degradation rate control, maintenance of mechanical properties and safe urinary excretion of stent fragments [27].
One of the barriers slowing down the progress of research was already described in 2000 and concerns the lack of agreement between in vitro and in vivo degradation rates demonstrated in a large number of experimental studies [21]. Mainly because research in animal models presents changing conditions of urine characteristics, interaction with the urothelium, bacterial contamination and because of the intrinsic hydrodynamic characteristics of the urinary tract that have been poorly investigated in vitro [8]. Fortunately, all biomaterials used in the development of ureteral BUSs have shown adequate biocompatibility, largely because synthetic polymers had already demonstrated their use in medical devices [8].
Nowadays, the research groups focused on this line of research are very well defined and, although they have not achieved a ureteral BUS for clinical use, they have made relevant advances in this area.
Early research (1999–2000) emerged from studies by the research group of Lumiaho et al., developing ureteral BUSs composed of SR-PLA and SR-PLGA polymers [27, 28]. The results showed good biocompatibility of the stents, but also a high tendency to migrate due to weaknesses in the mechanical properties of the stent. They also showed a long-term degradation time of more than 24 weeks with a high risk of hydronephrosis [21, 28]. Subsequently, another group developed a clinical trial with the placement of a ureteral BUS in patients undergoing percutaneous nephrolithotomy [29]. These stents showed proper urine drainage at 48 h and degraded at one month, with only distal migration [29]. However, a subsequent clinical trial did not yield satisfactory results, as 20% of the TUDS® ureteral stents (Boston Scientific Corporation, USA) migrated and 22% failed to maintain adequate urine drainage. Additionally, stent fragments were retained for more than 3 months, which required extracorporeal lithotripsy and ureteroscopy for resolution [30].
Progressing from the first innovations in this area of knowledge, one of the most important research groups should be highlighted. Chew and Lange et al., who have developed the Uriprene® BUS, having improved this stent after three generations of evolution [31,32,33]. The 2008 evaluation of the first generation of Uriprene® in a porcine model showed favourable biocompatibility and degradation without obstructive fragments [31]. Limitations of this first generation Uriprene® were a 16% migration rate, a degradation time of 10 weeks and a slight obstructive activity [31]. In addition, a drawback was that unlike standard stents, Uriprene® had to be inserted through a ureteral access sheath. In later studies, this research group developed two more generations that were evaluated in animal models, technological innovations focused on changing the ratio and composition of the biomaterials to achieve shorter degradation times and to provide sufficient strength for placement of the BUS coaxially to a guidewire [33]. Both the second and third generation Uriprene® had adequate axial and radial strength for successful insertion [32, 33].
Other groups have developed electrospinning ureteral BUSs made from PCL and PLGA [17]. Analysis of safety and degradation in vivo in a porcine model demonstrated that degradation begins at 4 weeks from the distal end of the stent and progresses proximally up to day 10 weeks, causing significantly less hydronephrosis, inflammation and urothelial irritation in a comparative study compared to a conventional ureteral stent [34]. Designs of this nature that allow control over the section of the stent that degrades is an important development in the design of BUSs, as it promotes the maintenance of the internal scaffold while allowing for controlled stent degradation.
One of the most promising groups in the development of ureteral BUSs is Barros et al., from 3B’s Research Group and the company HydruMedical in Portugal. Their ureteral BUS is manufactured with polymers of natural origin, having completed extensive studies in vitro and in a porcine animal model [10, 13,14,15]. The stent showed homogeneous degradation without impairing urinary flow. Studies in a porcine model demonstrated better pathological results compared to a conventional ureteral stent, showing the ideal biocompatibility of these natural materials [14]. However, the problems that currently limit the therapeutic application of this device are its short time, its poor radiopacity and a progressive loss of stability during the degradation process. This same device has been modified by this group to give it the capacity to release ketoprofen, allowing the local application of substances to reduce the morbidity associated with ureteral stents in in vitro studies [35]. Another innovation of this research group is a drug-eluting ureteral BUS for the topical adjuvant treatment of low-grade upper urinary tract urothelial carcinoma, which has also been evaluated in tumoral cell culture studies [15].
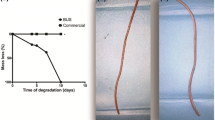
The most widely described ureteral BUS in the scientific literature is the BraidStent® developed by our research group, Soria and de la Cruz. In contrast to other BUSs, our stent is intraureteral, which reduces the morbidity associated with ureteral stents, as it does not cause VUR (vesicoureteral reflux) or LUTS (lower urinary tract symptoms) [36, 37] (Fig. 2). This ureteral BUS is composed of a combination of polymers and copolymers of PGA and Glycomer 631 combined together and arranged in a braided design to provide a different degradation rate between both biomaterials, avoiding obstructive size fragments and the sudden loss of the mechanical properties of the stent [7, 37]. Its main indication is to promote upper urinary tract drainage and to serve as an internal scaffold for healing after ureteral surgery [8]. Early results from the evaluation of BraidStent® in a porcine model demonstrated a predictable and controlled degradation rate between the third and sixth week, with no evidence of obstructive events during the hydrolysis of the biomaterials [7, 38] (Figs. 3 and 4). These good results are due to the cross-linked stent architecture and the combination of two polymers with different degradation rates. A remarkable aspect of this BUS is that it does not affect distal ureteral peristalsis, preserving distal peristalsis in up to 83% of ureters. This is great progress for patients, as it would probably avoid ureteral spasm, which is one of the main causes of pain in patients, as well as decrease the requirement for alpha-blockers or antimuscarinic drugs [7]. These good results contrast with a high rate of migration and asymptomatic bacteriuria, never UTI, shown in early studies. To reduce these undesirable effects, the BraidStent® was coated with heparin, a well-know bacterial anti-adhesive agent. In the three experimental studies evaluating BraidStent®-H in 2021, in a comparative study versus a conventional ureteral stent, in the adjuvant treatment of ureteral perforation and after endourological treatment of ureteral strictures, the coated BUS maintains the positive characteristics previously shown [39,40,41]. However, although heparin coating reduces the early asymptomatic bacteriuria rate, it increases again in the long term. This demonstrates the inability of heparin to reduce bacterial contamination (Fig. 5).
In order to alleviate the weak mechanical properties of degradable biomaterials in recent years, in-depth research has been carried out on metallic BUS. In this respect, Mg2+ and its alloys (Mg–Sr–Ag), polyurethane and Magnesium alloys have been used in in vitro and in vivo studies. These metallic BUS demonstrate good biocompatibility, high strength and homogeneous corrosion rate. With degradation rates ranging from 4 to 14 weeks depending on the alloy [42, 43]. An important advantage of this type of BUS with Mg2+ alloys is that they show a significantly antibacterial activity in upper urinary tract. For these reasons, magnesium alloys have become excellent candidate material for manufacturing BUS [42,43,44,45].
Another area of current research is the emergence of ureteral BUS to provide a new approach for local drug delivery in upper urinary tract. Drugs may be released while the stent is degrading. In this regard, Barros et al. developed a BUS to deliver different anti-tumour agents: paclitaxel, doxorubicin, epirubicin and gemcitabine [15]. Cell culture studies confirmed that these drug-eluted degradable stents could efficiently suppress the growth of T24 urothelial cancer cells. Our group has also developed the biodegradable BraidStent®-MMC, which delivers Mitomycin C for the adjuvant treatment of low grade upper tract urothelial carcinoma (Fig. 6). These innovations, in addition to avoiding a secondary stent removal procedure, allow the release of anti-tumour drugs, preventing their systemic administration and their side effects [46]. Following the same line of innovation on BUS-DES (drug eluting stent), another design that has been evaluated in an animal model is a BUS with mTOR inhibitor-eluting to reduce the progression of fibrosis proteins in ureteral stricture by means of rapamicyn release [47].
5 Biodegradable Urethral Stent
Despite their use to improve drainage of the lower urinary tract, metallic stents have shown significant side effects: migration, obstruction due to urothelial hyperplasia, encrustation and urinary tract infection. As a result, they cause a significant decrease in quality of life, thus reducing their therapeutic use [48].
In order to overcome the limitations of metallic stents, the development of BDG urethral stents was started. The main indications of these BUS devices are:
-
Temporary treatment of urinary retention, pending prostate surgery.
-
Treatment of urethral strictures, as an adjuvant to endoscopic urethrotomy or in cases of recurrence.
-
After BPH treatment, to ensure urinary drainage [6].
-
Scaffold for tissue engineering.
The first clinical efficacy studies of BUS prostatic coil stents were performed with PGA and initially achieved better voiding outcomes compared to a suprapubic catheter. However, the early loss of biomechanical properties inherent to this polymer caused voiding flow to decrease, concurrent with stent degradation [49]. To improve the results, another polymer with a slower degradation rate, PLA, was chosen. Showing good early results that were maintained over the long term, however, the degradation of this polymer was excessively long, 6 months [50]. Subsequently, other research groups demonstrated one of the main weaknesses of this type of urethral BUS, uncontrolled fragmentation of the stent caused infravesical obstruction.
After 10 years of clinical use of the urethral coil stent, a new braided tubular mesh design, similar to vascular stents, was developed to address the complications and weaknesses of early coil stent urethral BUS designs. These were mainly summarised as early migration; sudden collapse of the coil configuration in cases of recurrent stricture treatment, which induced urethral obstruction; fragments embedded in the mucosa after post-urethrotomy placement [51].
Early studies of this tubular stent were performed with PLA or PLGA. Although the new design shows a lower mass of polymeric material and its threads are three times thinner than the spiral stent, it fails to prevent the formation of urothelial hyperplasia [51]. The braided urethral BUS stent did not show any migration in experimental studies. Another improvement is that it does not require the use of a cystoscope, as it is released through an sheath [52].
Despite the advances shown during the first years of development of these stents, they still show weaknesses that preclude their use in patients. The two main problems relate to their limited efficacy in collapsing under pressure and their manifest inability to inhibit the initial fibrosis and polyposis associated with urothelial hyperplasia. One noteworthy fact is that, a biodegradable stent should be designed considering therapeutic force rather than initial force.
One of the suggested options to improve urethral stents is the addition of anti-inflammatory and anti-proliferative drugs. In vitro and animal model studies have been developed to evaluate drug-eluting stents with Dexamethasone, Indomethacin, Simvastatin, Ciprofloxacin and Sirolimus [52]. The latest study of a drug-eluting BUS is the use of Sirolimus to suppress granulation tissue formation after stent placement in a rat urethral model. The animal model study demonstrated suppression of urothelial hyperplasia formation secondary to urethral stent placement [5].
The tissue-engineered repair of urethral strictures with biodegradable stents is a recent research possibility and is perhaps the best approach to reduce complications in the treatment of urethral strictures. Previous studies have shown that cellularised BUS matrices are more effective than acellularised ones [53]. For this reason, Fu et al. developed a PLA-coated bioresorbable stent with autologous urothelial cells for urethral wall regeneration. At the end of the animal model study, 24 weeks, the regenerated urethral mucosa was indistinguishable from the control group and urodynamically there were no differences either [54].
This BUS–DES stent design represents the future in one of the urethral diseases with the worst prognosis, urethral strictures, as it will allow the delivery of cells and modulatory factors that facilitate healthy urethral healing [55].
6 Conclusions
The need to introduce these stents for hospital applications is crucial because of the benefits they provide to patients and the benefits in terms of reduced healthcare costs. Unfortunately, despite the significant progress that has been made recently, there are still limitations that need to be overcome, such as control of degradation rate and mechanical properties. The development of biodegradable metallic stents may be a line of research to overcome the current limitations of these stents. As well as the coating of stents to release drugs as they degrade in the urinary tract. Despite the necessary development that BUSs need, their future is very positive and near.
References
Wang L, Yang G, Xie H, Chen F. Prospects for the research and application of biodegradable ureteral stents: from bench to bedside. J Biomater Sci Polym Ed. 2018;29:1657–66.
Zong X, Ran S, Kim K-S, Fang D, Hsiao BS, Chu B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules. 2003;4:416–23.
De Grazia A, Somani BK, Soria F, Carugo D, Mosayyebi A. Latest advancements in ureteral stent technology. Transl Androl Urol. 2019;8:S436–41.
Sancaktutar AA, Soylemez H, Bozkurt Y, Penbegul N, Atar M. Treatment of forgotten ureteral stents: how much does it really cost? A cost-effectiveness study in 27 patients. Urol Res. 2012;40:317–25.
Kim KY, Park JH, Kim DH, Tsauo J, Kim MT, Son WC, Kang SG, Kim DH, Song HY. Sirolimus-eluting biodegradable poly-l-lactic acid stent to suppress granulation tissue formation in the rat urethra. Radiology. 2018;286(1):140–8.
Isotalo T, Talja M, Hellström P, Perttila I, Välimaa T, Törmälä P, Tammela TLJ. A double-blind, randomized, placebo-controlled pilot study to investigate the effects of finasteride combined with a biodegradable self-reinforced poly l-lactide acid spiral stent in patients with urinary retention caused by bladder outlet obstruction from benign prostatic hyperplasia. BJU Int. 2001;88:30–4.
Soria F, de la Cruz JE, Budía A, Serrano A, Galán-Llopis JA, Sánchez-Margallo FM. Experimental assessment of new generation of ureteral stents: biodegradable and antireflux properties. J Endourol. 2020;34:359–65.
Soria F, Morcillo E, Lopez de Alda A, Pastor T, Sánchez-Margallo FM. Catéteres y stents urinarios biodegradables. ¿Para cuándo? Arch Esp Urol. 2016;69:553–64.
Beysens M, Tailly TO. Ureteral stents in urolithiasis. Asian J Urol. 2018;5:274–86.
Barros AA, Oliveira C, Lima E, Duarte ARC, Healy K, Reis RL. Ureteral stents technology: biodegradable and drug-eluting perspective, vol. 7. Amsterdam: Elsevier; 2017.
Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–20.
Pulieri E, Chiono V, Ciardelli G, Vozzi G, Ahluwalia A, Domenici C, et al. Chitosan/gelatin blends for biomedical applications. J Biomed Mater Res A. 2008;86:311–22.
Barros AA, Rita A, Duarte ARC, Pires RA, Sampaio-Marques B, Ludovico P, et al. Bioresorbable ureteral stents from natural origin polymers. J Biomed Mater Res Part B Appl Biomater. 2015;103:608–17.
Barros AA, Oliveira C, Ribeiro AJ, Autorino R, Reis RL, Duarte ARC, et al. In vivo assessment of a novel biodegradable ureteral stent. World J Urol. 2018;36:277–83.
Barros AA, Browne S, Oliveira C, Lima E, Duarte ARC, Healy KE, et al. Drug-eluting biodegradable ureteral stent: new approach for urothelial tumors of upper urinary tract cancer. Int J Pharm. 2016;513:227–37.
Gunatillake P, Mayadunne R, Adhikari R. Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev. 2006;12:301–47.
Wang X, Zhang L, Chen Q, Hou Y, Hao Y, Wang C, et al. A nanostructured degradable ureteral stent fabricated by electrospinning for upper urinary tract reconstruction. J Nanosci Nanotechnol. 2015;15:9899–904.
Osman Y, Shokeir A, Gabr M, El-Tabey N, Mohsen T, El-Baz M. Canine ureteral replacement with long acellular matrix tube: is it clinically applicable? J Urol. 2004;172:1151–4.
Fu W-J, Xu Y-D, Wang Z-X, Li G, Shi J-G, Cui F-Z, et al. New ureteral scaffold constructed with composite poly(l-lactic acid)-collagen and urothelial cells by new centrifugal seeding system. J Biomed Mater Res A. 2012;100:1725–33.
Li G, Wang Z-X, Fu W-J, Hong B-F, Wang X-X, Cao L, et al. Introduction to biodegradable polylactic acid ureteral stent application for treatment of ureteral war injury. BJU Int. 2011;108:901–6.
Lumiaho J, Heino A, Pietilainen T, Ala-Opas M, Talja M, Valimaa T, et al. The morphological, in situ effects of a self-reinforced bioabsorbable polylactide (SR-PLA 96) ureteric stent; an experimental study. J Urol. 2000;164:1360–3.
Talja M, Valimaa T, Tammela T, Petas A, Tormala P. Bioabsorbable and biodegradable stents in urology. J Endourol. 1997;11:391–7.
Yang G, Xie H, Huang Y, Lv Y, Zhang M, Shang Y, et al. Immersed multilayer biodegradable ureteral stent with reformed biodegradation: an in vitro experiment. J Biomater Appl. 2017;31:1235–44.
Zou T, Wang L, Li W, Wang W, Chen F, King MW. A resorbable bicomponent braided ureteral stent with improved mechanical performance. J Mech Behav Biomed Mater. 2014;38:17–25.
Jana A, Das M, Balla VK. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J Biomed Mater Res A. 2022;110(2):462–87.
Lock JY, Wyatt E, Upadhyayula S, Whall A, Nuñez V, Vullev VI, et al. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J Biomed Mater Res Part A. 2014;102:781–92.
Lumiaho J, Heino A, Kauppinen T, Talja M, Alhava E, Valimaa T, et al. Drainage and antireflux characteristics of a biodegradable self-reinforced, self-expanding X-ray-positive poly-l,d-lactide spiral partial ureteral stent: an experimental study. J Endourol. 2007;21:1559–64.
Lumiaho J, Heino A, Tunninen V, Ala-Opas M, Talja M, Valimaa T, et al. New bioabsorbable polylactide ureteral stent in the treatment of ureteral lesions: an experimental study. J Endourol. 1999;13:107–12.
Lingeman JE, Schulsinger DA, Kuo RL. Phase I trial of a temporary ureteral drainage stent. J Endourol. 2003;17:169–71.
Lingeman JE, Preminger GM, Berger Y, Denstedt JD, Goldstone L, Segura JW, et al. Use of a temporary ureteral drainage stent after uncomplicated ureteroscopy: results from a phase II clinical trial. J Urol. 2003;169:1682–8.
Hadaschik BA, Paterson RF, Fazli L, Clinkscales KW, Shalaby SW, Chew BH. Investigation of a novel degradable ureteral stent in a porcine model. J Urol. 2008;180:1161–6.
Chew BH, Paterson RF, Clinkscales KW, Levine BS, Shalaby SW, Lange D. In vivo evaluation of the third generation biodegradable stent: a novel approach to avoiding the forgotten stent syndrome. J Urol. 2013;189:719–25.
Chew BH, Lange D, Paterson RF, Hendlin K, Monga M, Clinkscales KW, et al. Next generation biodegradable ureteral stent in a yucatan pig model. J Urol. 2010;183:765–71.
Wang X, Shan H, Wang J, Hou Y, Ding J, Chen Q, et al. Characterization of nanostructured ureteral stent with gradient degradation in a porcine model. Int J Nanomed. 2015;10:3055–64.
Barros AA, Oliveira C, Reis RL, Lima E, Duarte ARC. Ketoprofen-eluting biodegradable ureteral stents by CO2 impregnation: in vitro study. Int J Pharm. 2015;495:651–9.
Soria F, Morcillo E, Serrano A, Rioja J, Budía A, Sánchez-Margallo FM. Preliminary assessment of a new antireflux ureteral stent design in swine model. Urology. 2015;86:417–22.
Soria F, Morcillo E, de la Cruz JE, Serrano A, Estébanez J, Sanz JL, et al. Antireflux ureteral stent proof of concept assessment after minimally invasive treatment of obstructive uropathy in animal model. Arch Esp Urol. 2018;71:607–13.
Soria F, Morcillo E, Serrano A, Budía A, Fernandez I, Fernández-Aparicio T, et al. Evaluation of a new design of antireflux-biodegradable ureteral stent in animal model. Urology. 2018;115:59–64.
Soria F, de La Cruz JE, Caballero-Romeu JP, Pamplona M, Pérez-Fentes D, Resel-Folskerma L, Sanchez-Margallo FM. Comparative assessment of biodegradable-antireflux heparine coated ureteral stent: animal model study. BMC Urol. 2021;21(1):32.
Soria F, de La Cruz JE, Budia A, Cepeda M, Álvarez S, Serrano Á, Sanchez-Margallo FM. Iatrogenic ureteral injury treatment with biodegradable antireflux heparin-coated ureteral stent-animal model comparative study. J Endourol. 2021;35(8):1244–9.
Soria F, de La Cruz JE, Fernandez T, Budia A, Serrano Á, Sanchez-Margallo FM. Heparin coating in biodegradable ureteral stents does not decrease bacterial colonization-assessment in ureteral stricture endourological treatment in animal model. Transl Androl Urol. 2021;10(4):1700–10.
Jin L, Yao L, Yuan F, Dai G, Xue B. Evaluation of a novel biodegradable ureteral stent produced from polyurethane and magnesium alloys. J Biomed Mater Res B Appl Biomater. 2021;109(5):665–72.
Tie D, Liu H, Guan R, Holt-Torres P, Liu Y, Wang Y, Hort N. In vivo assessment of biodegradable magnesium alloy ureteral stents in a pig model. Acta Biomater. 2020;15(116):415–25.
Tie D, Hort N, Chen M, Guan R, Ulasevich S, Skorb EV, Zhao D, Liu Y, Holt-Torres P, Liu H. In vivo urinary compatibility of Mg–Sr–Ag alloy in swine model. Bioact Mater. 2021;4(7):254–62.
Shan H, Cao Z, Chi C, Wang J, Wang X, Tian J, Yu B. Advances in drug delivery via biodegradable ureteral stent for the treatment of upper tract urothelial carcinoma. Front Pharmacol. 2020;17(11):224.
Ho DR, Su SH, Chang PJ, Lin WY, Huang YC, Lin JH, Huang KT, Chan WN, Chen CS. Biodegradable stent with mTOR inhibitor-eluting reduces progression of ureteral stricture. Int J Mol Sci. 2021;22(11):5664.
Na HK, Song HY, Yeo HJ, Park JH, Kim JH, Park H, Kim CS. Retrospective comparison of internally and externally covered retrievable stent placement for patients with benign urethral strictures caused by traumatic injury. AJR Am J Roentgenol. 2012;198:55–61.
Petas A, Talja M, Tammela T, et al. A randomized study to compare biodegradable self-reinforced polyglycolic acid spiral stents to suprapubic and indwelling catheters after visual laser ablation of the prostate. J Urol. 1997;157:173–6.
Petas A, Talja M, Tammela TL, Taari K, Valimaa T, Tormala P. The biodegradable self-reinforced poly-l-lactic acid spiral stent compared with a suprapubic catheter in the treatment of post-operative urinary retention after visual laser ablation of the prostate. Br J Urol. 1997;80:439–43.
Isotalo T, Nuutinen JP, Vaajanen A, Martikainen PM, Laurila M, Tormala P, Talja M, Tammela TL. Biocompatibility and implantation properties of two differently braided, biodegradable, self-reinforced polylactic acid urethral stents: an experimental study in the rabbit. J Urol. 2005;174:2401–4.
Isotalo T, Nuutine JP, Vaajanen A, Martikainen PM, Laurila M, Tormala P, Talja M, et al. Biocompatibility properties of a new braided biodegradable urethral stent: a comparison with a biodegradable spiral and braided metallic stent in the rabbit urethra. BJU Int. 2006;97:856–9.
Kotsar A, Nieminen R, Isotalo T, Mikkonen J, Uurto I, Kellomäki M, Talja M, et al. Biocompatibility of new drug-eluting biodegradable urethral stent materials. Urology. 2010;75:229–34.
De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002;168:1789–92.
Fu WJ, Zhang X, Zhang BH, Zhang P, Hong BF, Gao JP, Meng B, et al. Biodegradable urethral stents seeded with autologous urethral epithelial cells in the treatment of post-traumatic urethral stricture: a feasibility study in a rabbit model. BJU Int. 2009;104:263–8.
Rashidbenam Z, Jasman MH, Tan GH, Goh EH, Fam XI, Ho CCK, Zainuddin ZM, Rajan R, Rani RA, Nor FM, Shuhaili MA, Kosai NR, Imran FH, Ng MH. Fabrication of adipose-derived stem cell-based self-assembled scaffold under hypoxia and mechanical stimulation for urethral tissue engineering. Int J Mol Sci. 2021;22(7):3350.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Soria, F., de la Cruz, J.E., Cepeda, M., Serrano, Á., Sánchez-Margallo, F.M. (2022). Biodegradable Urinary Stents. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_29
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)