Abstract
The aim of this chapter is to clarify certain points which are confusing many if not most of our urologist colleagues on the subject of “stents and stenting the urinary tract”. Another point of confusion in urology is the term of “chronic obstruction” describing an obstruction necessitating long-term stenting. Which stent to use? For how long? Before using a permanent stent along the urinary tract we should think hard about what may happen to a ureter or urethra implanted with a permanent metallic mesh stent. This is especially important when something goes wrong like when the stent lumen becomes obliterated by hyperplastic or malignant tissues, the stent wires fracture, or tissue coverage over the stent wires is incomplete and the resulting stone formation on the wires, ureteral or urethral perforations etc. By adopting the vascular stent technologies in urinary tract design stents, we were hoping that taking a single stent shape, changing its length and caliber they could be used all along the urinary tract. Then asking ourselves, why the results were less than what we were expecting. Here I would like to quote a sentence attributed to Albert Einstein: “Insanity is doing the same thing over and over again and expecting different results”.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In medicine a “stent” is a tube made of a polymer or a metal to be inserted into the lumen of an obstructed tubular organ to keep its lumen open. “Stenting” is the term of inserting such a device into the narrowed or occluded tubular organ due to either benign or malignant obstructive reasons.
The aim of preparing this chapter is to clarify certain points which are confusing many if not most of our urologist colleagues on the subject of “stents and stenting the urinary tract”. Another point of confusion in urology is the term of “chronic obstruction” describing an obstruction necessitating long-term stenting. Which stent to use? For how long? There are clear differences in the occlusion mechanisms of the ureters. These are either intrinsic pathologies causing a benign obstruction or a malignant obstruction. Benign obstructions are either traumatic fibrosis occurring after ureteral manipulations or iatrogenic trauma occurring during abdominal or pelvic surgeries. Such obstructions can be managed by inserting a large caliber JJ or even 2 JJs in tandem and left them in place until regeneration of the injury occurs. Malignant occlusions can be due a primary or infiltrating malignancies or because compression of the ureter by an extra-ureteral mass.
The fact is that the most used stent in the urinary tract is the double-J stent (JJ) or its variations such as the pig-tail stents. To reduce the confusion between the double-J and other urinary tract stents I will use the general term of JJ stents in this chapter.
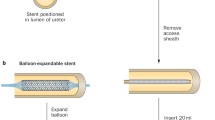
Are there only JJs available to us? No. There are several other but much less used stents which are available for use in the urinary tract, such as large caliber metal coil stents or covered large caliber metal mesh stents. There are also self-expandable or balloon expandable metallic mesh stents originally developed for use in the vascular system.
The question is: Is it logical to insert a mesh stent when malignant tissues are compressing or infiltrating the ureteral lumen? Obviously, not. These are the reasons why there should be a separation between stenting ureters occluded by a benign or a malignant pathology for optimizing the outcome of the stenting.
In 1972, Goodwin described a stent being “a mold made of a compound, for holding some form of a graft in place” [1]. This was much before the wide use of the JJ which was invented by Finney in 1978 [2] and the other intraluminal stents developed since then and are in use in urology today.
One of the common mistakes we do as urologists is to think that any obstruction of the ureter can be managed by inserting a JJ. The truth is that in most cases the JJ provides an immediate but relatively short-term palliation for ureters obstructed by stones or by edema developing after endoscopic procedures. Their use becomes a discussion subject when we confront a chronic obstruction and our aim is to provide long term drainage to the kidneys. Even is such case the ethio-pathology causing the ureteral obstruction plays a role in our decision either using a JJ or another kind of stent. Today we pass a JJ beside a stone, or through the narrowed passage caused by a benign or malignant stricture either causing external compression or infiltrating the ureteral wall, for immediate relieve of the obstruction. The same is done in urethral obstructions. In such cases we pass a urethral catheter for draining the bladder. These are short-term palliative problem solving activities.
In cases of benign narrowing of the urethra like in BPH or external non-malignant ureteral compressions caused by retroperitoneal fibrosis, or in infiltrating or compressing malignant obstructions in the ureter, what is needed is insertion of a large caliber stent into the obstructed segment for long periods or even permanently for re-creating a large ureteral lumen. In benign, intrinsic ureteral stenosis the aim is to create not only long-term drainage but also re-shaping of the ureteric lumen, which can be accepted as curative stenting. The same occur also in urethral obstructions, either due to benign or malignant prostatic obstructions or in distal urethral strictures. All these are for allowing free flow of the urine.
The use of JJs in urology preceded the use of metallic mesh stents developed for the vascular system. However, the vascular stents became much more popular and common treatment option for opening occlusions of the coronary and cerebral arteries, the carotid, iliac and femoral arteries.
In the vascular system stents started to be used for ensuring the patency of compromised arteries to allow blood flow for bringing oxygen and other vital compounds to organs.
When we look to the history of vascular stents we see that it was Julio Palmaz, an interventional vascular radiologist, who invented the balloon-expandable stent in 1985 [3]. This was followed by the Wallstent which is a self-expanding vascular stent, invented by Hans Wallsten who was not a physician. The Wallstent was first used in the vascular system by Ulrich Sigwart in 1986 [4].
The successful use of vascular stents, gradually took the place of certain cardio-vascular surgical procedures, saving the lives of many patients in a minimally invasive way.
This great success induced an idea in urology that said: “If it is good for the vascular system, it is also good for the urinary system”. Based on this idea some urologists thought that such stents may have possible promising implications in urinary tract stenting. This was first described in 1991 by Lugmayr and Pauer who started a new approach for relieving ureteric obstruction [5]. Then several additional reports of successful placement of mesh stents in the urinary tract were published [6, 7].
However, these stents were used predominantly in patients with extrinsic malignant compression of the ureter who had limited life expectancy [8, 9]. Even at that time, Lugmayr and Pauer noted that at the site of the stent implantation they observed transient mucosal edema and tissue hyperplasia causing short term obstruction, and that these obstructions disappeared after the stent wires became fully incorporated into the urothelium. There were also early reports that showed, when used in the ureter the uncovered metal wires of these stents developed encrustations and created obstructions in the stent lumen [5, 10].
Others reported long-term urothelial hyperplasia resulting in obstruction, necessitating insertion of a double-J sent to reestablish lumen patency [11, 12] or removing the encrustations occluding the mesh stent [13]. A problem with bare metallic mesh stents used in the urinary tract is the bare wires remaining uncovered by urothelium, which frequently can happen at the ends of such stents, becoming a starting point for stone formation. A typical case was reported by Smrkolj and Šalinović, in which a patient was, inserted a nitinol mesh stent in the ureteropelvic junction in 2000 after failure of two pyeloplasty procedures. The patient returned 15 years later with a 35 mm stone encrusted on the mesh stent and a hardly functioning kidney [14].
Early experience also showed that balloon-expandable stents developed more urothelial hyperplasia, limiting their use in the ureter [15, 16]. There was even a report on the use of a high-frequency rotablator (a miniature drill capped with an abrasive, diamond-studded burr which is commonly used to pulverize hardened plaque within a coronary artery) for removing the occluding ingrown hyperplastic tissue in a ureteral mesh stent [17]. Despite the experience showing such complications, the enthusiasm around using metal mesh stents in arteries drove some urologists to use similar stents in the urinary tract. Such stents were implanted into the tubular structures of the urinary tract such as the ureter, the prostatic, bulbar and more distal parts of the urethra even in patients who had not a short life expectancy having benign narrowings [12, 18,19,20].
Then came the era of drug eluting vascular stents reducing hyperplastic reactions of the endothelium and resulting vascular re-stenosis. These encouraging results with the drug eluting vascular stents induced a new hope in urologists to use similar stents in the urinary tract [21, 22].
2 Vascular Tract vs. Urinary Tract
It is obvious that not all tubular organs in the body have the same anatomy/histology and physiology, and that the lumen narrowing seen in blood vessels are drastically different than the ones seen in the ureter, urethra, or in the bile duct or the esophagus. Each tubular organ in the body has a different anatomy and also different histological and functional properties. Although both the ureter and the urethra are tubular structures, they are NOT similar to an artery or to any other body tube.
Can we expect a stent designed for the vascular system be as effective in the ureter or the urethra as it is in an artery? It may be, but for only several hours, or until the ureteral or urethral tissues start to react to the geometry and to the material of a metallic mesh stent designed for the vascular system or until the malignant tissue infiltrates through the mesh of the stent.
The pathophysiology of lumen narrowing in arteries is completely different than the narrowing of the ureteral lumen. In arteries the narrowing is mainly caused by “atheromatous plaques” which are made up of cholesterol and calcified material build-up in the luminal wall or by neointimal hyperplasia caused by proliferation and migration of vascular smooth muscle cells that create a thickening of the arterial wall. Where ureteral occlusion or narrowing are entirely different pathologies. A ureter can be occluded by a stone and these occlusions is relieved by passing a JJ beside the stone until definitive treatment. Traumatic fibrosis of the ureter is mainly caused by diagnostic or therapeutic endo-ureteral manipulations. Rarer reasons are iatrogenic injuries during abdominal surgery, retroperitoneal fibrosis encasing the ureter or peri-ureteral tumors compression or infiltrating the ureters. Differing from the vascular system, the urinary tract is an actively functioning system where each part of it has different anatomies and functionalities.
In the urinary tract stents are used to ensure the patency of compromised urine flow. In the ureter they allow to drain urine from the kidneys to the bladder and all along the urethra to allow unobstructed bladder emptying.
Let’s compare an artery with the ureter, which is the most stented part of the urinary tract, and also the urethral segments.
The urinary system has an entirely different anatomy and physiology than the vascular system. Starting from the ureteropelvic junction, down to the intravesical part, the ureter has different segments with varying diameters ever-changing with each passing peristaltic wave for moving forward the urine. Compared with the ureters, arteries with their very gradual decreasing lumen are almost inactive tubes allowing blood pumped by the heart to flow forward. Histologically the layers of the ureter are different than the arterial layers. The cross section of the ureter has a “star-shaped” lumen surrounded by 2–5 cell thick urothelium and 2 layers of smooth muscle for the proximal 2/3 of its length, and 3 layers of muscle for the final 1/3 of its length toward the bladder. These smooth muscles (muscularis propria) contracts in a peristaltic movement to advance the urine from the kidney to bladder. The urothelium covering the lumen of the urinary tract is the most impermeable epithelial barrier in the body. In contrast, the endothelium performs a vital task of providing nutrients to the underlying tissue as well as maintaining tissue oncotic pressure [23]. Although it never happens, in a living organism the lumen of an artery keeps its tubular shape with an open lumen even if no blood flows through it. Hydrodynamically urine acts different than the blood dynamics. Differing from the blood pressure that advances its flow, the pressure in the urine bolus is determined primarily by the pressure created by ureteral peristalsis needed to separate the walls of the collapsed ureter.
The lumen lining the urethra also varies depending on the anatomical section: The prostatic urethra is lined by urothelial cells with patches of stratified columnar epithelium. The membranous urethra which passes through the external sphincter and the penile urethra are lined by stratified columnar and pseudostratified columnar epithelium and the most distal penile urethra is lined with non-keratinized stratified squamous cells.
Can the flow dynamics of continuous arterial blood flow and the changes of intra-arterial pressure be compared with the dynamics of urine during urination which is initiated by bladder contraction and simultaneous relaxation of the urethral sphincters? Where the daily amount of urine transported through the ureters can change in each individual during a single day, depending on the liquid intake affecting the amount of urine excreted from the kidneys, also related to the body and environmental temperature etc. How the urethra through which urine passes once every 2–4 or 6 h can be compared to a blood vessel?
Blood flowing through the arteries has a viscosity and it contains a large collection of living cells that interact with one another. Changes in blood flow occur during systole and diastole. Blood flows quicker at peak-systole because it is physically thinner, and at end-diastole it flows slower because it becomes 2–4 times thicker because of the aggregation of the red cells [24, 25]. This occurs 50–70 times a minute, with blood advancing through the arteries even at diastole. In contrast, without a peristaltic wave moving a bolus of urine, the ureter is empty and in a collapsed state. Since its lumen calibre changes along all its length and during peristaltic movements, the chances of a bare metal stent being completely embedded into the ureteral wall by pushing itself into the wall are low. In most cases parts of the mesh stents remain bare in the ureteral lumen. Because its high lithogenicity, urinary crystals start to depose on the exposed stent wires and form a stone.
Another reason to my opposition for using a permanent stent in the urinary tract is that they become an implant. Even the best foreign body, with time start to cause problems. To believe that it will remain an “innocent implant” for long years is at least a wishful thinking. Even the most solid hip or knee joint implants have to be changed after 10–15 years. If we decide to implant a stent in a segment of the urinary tract, we have to take into account the possibility that in case of an obstructive complication we will have to excise the implanted segment of the ureter or the urethra and do a reconstructive surgery.
Vast experimental and then clinical work showed that the anti-proliferative drug eluting balloons and mesh stents could reduce most arterial re-stenoses. These results created the hope that drugs, like Zotarolimus or Paclitaxel coated stents can be used also in the urinary tract. A study done on porcine and rabbit ureter showed that compared with bare metal stents, these animals developed lower hyperplastic reactions in the ureter [26]. Additional studies were done by the same group to see distribution of Paclitaxel in porcine ureters and rabbit urethras [22, 27].
Both studies indicated that when Paclitaxel coated balloons were inflated in a healthy ureter or urethra, a distribution of the drug was observed in all its layers. The theory behind these experiments was to show the possible use of antiproliferative drugs eluted by high pressure balloons or by stents coated with such drugs in ureteral and urethral strictures, and expect the results obtained in stenosed arteries. Although these findings looked encouraging, they could not prove that the same distribution will occur in the tense fibrous tissues causing the ureteral and urethral strictures. Stent occlusion due to tumor in-growth as well as “overgrowth” was observed with bare metal mesh stents [28]. It is well known that in most recurrent urethral strictures, all urethral layers, and even the peri-urethral spongious tissues become fibrotic. The same deep fibrosis occurs in traumatic/iatrogenic ureteral strictures. These fibrotic scar tissues are entirely different than the connective tissue formation in the smooth muscle layer of an artery that can be managed by drug eluting balloons or stents.
3 Conclusions
In reality, most stents inserted into any place in the body are needed only for relatively short- or long-term. Then they need to be removed. This is true also for the stents used in the vascular tract. But since they are not easily accessible and since they are embedded in the vascular wall, they cannot be removed. In the vascular system, re-entry for stent removal is risky. So, nowadays vascular stents are permanently implanted. If they become occluded their patency are re-created by balloon dilatation with or without implantation of an additional stent (stent-in-stent).
I recommend to be realistic when looking to the facts and not be like typical parents believing that their baby “is the most beautiful and smartest child”.
Before using a permanent stent along the urinary tract we should think hard about what may happen to a ureter or urethra implanted with a permanent metallic mesh stent. This is especially important when something goes wrong like when the stent lumen becomes obliterated by hyperplastic or malignant tissues, the stent wires fracture, or tissue coverage over the stent wires is incomplete and the resulting stone formation on the wires, ureteral or urethral perforations etc.
My almost three decades long experience in using and developing two generations of stents thought me that all stents to be used in the urinary tract needs to be either removable or bioabsorbable/biodegradable. The current technology does not allow replacement of a part of the urinary tract with metal parts like doing a knee or hip joint replacement.
From the beginning of the early 1990s my approach was that, whatever their configuration, since the vascular stents are permanent implants, using them in the urinary tract is an erroneous concept. Even when the drug eluting mesh stents started to be used anywhere in the urinary tract my approach did not change.
Since my involvement with prostatic urethral stents started in the middle 1980s, first using the Prostacath in prostatic obstructions, and then the self-expandable metallic ProstaCoil or UroCoil stents in prostatic or urethral strictures. I defended my thesis that the urinary tract doesn’t need a permanent stent, because the entire collecting system of the urinary tract is accessible, allowing their easy removal after a certain length of time. This become more possible with the introduction of the self-expanding covered Allium stents and the Uventa stent in the early 2010s.
Therapeutically when a large caliber stent is to be used in the urinary tract, the aim is not only allowing urine drainage through the narrowing but also re-shaping the narrowed segment by acting as a cast, when left in place until the ureter or urethra regenerates and its scarring process stabilizes. Those defending the use of permanent stents always mentioned their successful use in blood vessels, but less the complications they created even in them. It is wrong thinking that the various obstructive problems along the ureter can be solved by using a single shape stent, no matter from which material it is done or drug it is coated. Because, differing from the vascular system, in the urinary system ‘one shape, does not fit all’.
My approach is still that, since vascular stents are permanently implanted devices, before using them in the urinary tract, we need to have clear proofs that the urothelium will react the same way as the vascular endothelium reacts to the stent and that the stent will become completely covered. We should take great care in using even a drug coated metal stent before clear and approved studies on its efficacy to the urothelium are proved.
This is not so simple.
Biodegradable stents were tried in the past but they failed clinically. If such stents will be available and will show that they do not collapse and occlude the ureter or the urethra, then a new era in long-term ureteral or urethral stenting will start. So far stent technologists could not develop an effective dissolvable or absorbable vascular stent that will keep its physical properties until they disappear.
By adopting the vascular stent technologies, we were hoping that taking a single stent shape, changing its length and caliber they could be used all along the urinary tract. Then asking ourselves, why the results were less than what we were expecting. Here I would like to requote a sentence attributed to Albert Einstein: “Insanity is doing the same thing over and over again and expecting different results.”
References
Goodwin WE. Splint, stent, stint. Urol Dig. 1972;11:13–4.
Finney RP. Experience with ‘double J’ ureteral catheter stent. J Urol. 1978;120:678–81.
Palmaz JC, Sibbitt RR, Reuter SR, Tio FO, Rice WJ. Expandable intraluminal graft: a preliminary study. Work in progress. Radiology. 1985;156:73–7.
Sigwart U, et al. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–6.
Lugmayr H, Pauer W. Self-expanding metallic stents in malignant ureteral stenosis. Deutsche Medizinische Wochenschrift. 1991;116:573–6.
Flueckiger F, et al. Malignant ureteral obstruction: preliminary results of treatment with metallic self-expandable stents. Radiology. 1993;186:169–73.
Reinberg Y, Ferral H, Gonzalez R, et al. Intraureteral metallic self-expanding endoprosthesis (Wallstent) in the treatment of difficult ureteral strictures. J Urol. 1994;151(6):1619–22.
López-Martínez RA, et al. The use of metallic stents to bypass ureteral strictures secondary to metastatic prostate cancer: experience with 8 patients. J Urol. 1997;158:50–3.
Lang EK, et al. Placement of metallic stents in ureters obstructed by carcinoma of the cervix to maintain renal function in patients undergoing long-term chemo-therapy. Am J Roentgen. 1998;171:1595–9.
Pauer W, Lugmayr H. Metallic Wallstents: a new therapy for extrinsic ureteral obstruction. J Urol. 1992;148:281–4.
Burgos FJ, et al. Self-expanding metallic ureteral stents for treatment of ureteral stenosis after kidney transplantation. Transplant Proc. 2005;37:3828–9.
Daskalopoulos G, et al. Intraureteral metallic endoprosthesis in the treatment of ureteral strictures. Eur J Radiol. 2001;39:194–200.
Pauer W, Kerbl K. Self-expanding permanent endoluminal stents in the ureter: technical considerations. Tech Urol. 1995;1:67–71.
Smrkolj T, Šalinović D, et al. Endoscopic removal of a nitinol mesh stent from the ureteropelvic junction after 15 years. Case Rep Urol. 2015;2015:273614.
Barbalias GA, et al. Metal stents: a new treatment of malignant ureteral obstruction. J Urol. 1997;158:54–8.
Hekimoğlu B, et al. Urothelial hyperplasia complicating use of metal stents in malignant ureteral obstruction. Eur. Radiol. 1996;6:675–81.
Protzel C, et al. High frequency rotablation as a new therapeutic procedure for obstructed metallic ureter stents. J Urol. 2001;166:1399–400.
Barbalias GA, et al. Ureteropelvic junction obstruction: an innovative approach combining metallic stenting and virtual endoscopy. J Urol. 2002;168:2383–6.
Trinchieri A, Montanari E, Ceresoli A, et al. Permanent stenting in the treatment of ureteral strictures. Ann Urol. 1999;33:186–91.
Al-Aown A, et al. Ureteral stents: new ideas, new designs. Ther Adv Urol. 2010;2:85–92.
Liourdi D, et al. Evaluation of the distribution of paclitaxel by immunohisto-chemistry and nuclear magnetic resonance spectroscopy after the application of a drug-eluting balloon in the porcine ureter. J Endourol. 2015;29:580–9.
Barbalias D, et al. Evaluation of the distribution of paclitaxel after application of a paclitaxel-coated balloon in the rabbit urethra. J Endourol. 2018;32:381–6.
Sukriti S, et al. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4:535–51.
Letcher R, et al. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med. 1981;70:1195–202.
Fowkes FGR, et al. The relationship between blood viscosity and blood pressure in a random sample of the population aged 55 to 74 years. Eur Heart J. 1993;14(5):597–601.
Kallidonidis P, et al. Evaluation of zotarolimus-eluting metal stent in animal ureters. J Endourol. 2011;25:1661–7.
Liourdi D, et al. Evaluation of the distribution of paclitaxel by immunohistochemistry and nuclear magnetic resonance spectroscopy after the application of a drug-eluting balloon in the porcine ureter. J Endourol. 2015;29:580–9.
Lang EK, et al. Long-term results of metallic stents for malignant ureteral obstruction in advanced cervical carcinoma. J Endourol. 2013;27:646–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Yachia, D. (2022). Learning from Our Mistakes: Applying Vascular Stent Technologies to the Urinary Tract. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_28
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)