Abstract
The urinary tract is a highly complex, dynamic and variable environment that renders the development of urinary stents extremely challenging. It is mandatory that previous to clinical trials, innovations in urinary medical devices are tested in a preclinical context. The steps of translational research in this regard include in silico, in vitro, ex vivo and in vivo assessments. In this chapter, a critical review of the in vitro stent assessment models is performed, outlining briefly their strengths and weaknesses. Subsequently, a discussion concerning the available animal models for urinary stent evaluation is made with the aim of providing a critical guide for experimental preclinical evaluation of urological devices.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 In-Vitro Encrustation Models: A Critical Review
Implantation of biomaterials into the urinary tract is hampered by crystal formation, bacterial adherence and, ultimately, encrustation through biofilm formation resulting from a multifactorial disturbance of the delicate balance between numerous physico-chemical and biochemical processes. Non-infectious stone formation and encrustation usually result from metabolic imbalances, often on the tubular level. In contrast, infectious stone formation and biofilm-induced encrustation are linked to the enzymatic activity of bacteria. Best known are urease-producing species such as Proteus mirabilis, which increase the pH of the urine. This alkalization, in turn, decreases the solubility of urinary calcium and magnesium salts and thus facilitates encrustation.
Consequently, the use of urinary implants is complicated by several factors stent surface encrustation through deposition of crystal-forming urinary ions, bacterial colonization and biofilm formation despite antibiotic treatment and prophylaxis, mechanical irritation of the urothelium by encrustation, and alterations of urine flow in and around the stent due to encrustation [1].
The development of in vitro models to simulate bacterial infections and biofilm formation started after the initial observation of sessile bacteria and their role in chronic infections in humans. Biofilms form an irregular network matrix. They protect the bacteria from physical, chemical and biological stresses. Shear stress caused by the flow of the fluid medium is hereby one of the main factors impacting on the formation of a stable biofilm.
Early approaches focused on the use of continuous flow systems, such as the chemostat model, which had the advantage of a regular supply of fresh fluid medium whilst maintaining a constant volume [2]. Many in vitro models designed to mimic encrustation on urological devices have been derived from classical microbiological approaches, and often do not reflect important physiological factors such as the complex and variable physico-chemical urinary environment in vivo, or infection with mixed species.
In 1973, Finlayson and Dubois described a dynamic flow in vitro encrustation model which used both, a constant flow of artificial urine and a magnetic stirrer [3]. A number of adaptations to this model have been devised over time to enable the study of urinary encrustations utilizing both, human and artificial urine [4]. Depending on particular research questions, two groups of open systems were designed: The Continuous Flow Stirred Tank Reactor (CFSTR) and the Plug Flow Reactor (PFR). The Modified Robbins Device (MRD) was designed to monitor biofilm formation with different flow speeds in an axial direction, and in a completely mixed reactor using diffusion. This PFR-system consists of a pipe with multiple threaded holes containing coupons. The biofilm reactor of the Center for Disease Control (CDC) is a current, commercially available flow-based CFSTR-system. A vessel with a polyethylene lid bears independent rods housing removable coupons. Inside the reactor, there is a rotating magnetic stirrer exerting a constant high shear force on the coupons. The number of revolutions can be varied and is independent of the feed speed. The system allows for a perfect mixing and operates at a steady state. With this system, structure and physiology of biofilm formation can be monitored by confocal laser scanning microscopy (CLSM) in a non-invasive fashion [5]. The CDC biofilm reactor is indispensable for prototype testing, but less suitable for screening testing. Another disadvantage of the semi-open design of the CDC reactor is its susceptibility to contamination.
This led to the development of high-throughput static biofilm models. Microtiter plate (MTP)-based static systems are the perhaps most commonly used biofilm model systems. They are an important tool to study especially the early stages of biofilm formation. In these systems, biofilms are typically grown on either the bottom or the sidewalls of a MTP. MTP-based systems are closed systems without in- or outflow from the reactor. Consequently, during an experiment the composition of the environment inside the well of an MTP changes. Nutrients are depleted whilst signaling molecules accumulate. It has been suggested that a part of the accumulated biomass may not result from biofilm formation, but rather from cell sedimentation and subsequent entrapment of cell sediments within the matrix of extracellular polymeric substances (EPS).
The Calgary Biofilm Device (CBD) represents a modification of the MTP-based systems, where biofilms are formed on lids with rods that fit into the bacteria-containing wells of the MTP. A newer system to study biofilm formation and encrustation on implants uses this CBD as a commercially available high-throughput screening assay. However, the lid is configured in such a way that materials are held in a matrix. The bottom is a welled plate into which the implant materials to be tested can be inserted. The matrix in combination with the high-throughput capability of the assay allow the study of several encrustation parameters. The use of MTP-based assays offers many of advantages. MTP are cheap and they provide the opportunity for multiplexing, as multiple organisms and treatments can be incorporated in a single experimental run [6].
Both, MTP/CBD-based and flow-based systems share some limitations. One common pitfall in designing in vitro biofilm models is the use of bacterial strains with a low virulence which, in turn, results in a low translation rate from in vitro to in vivo studies.
Most in vitro encrustation models use synthetic urine, based on urease reactions or urease-producing bacteria. However, in real life most urinary tract infections are caused by E. coli. These are acid-producing, and, consequently the urinary pH does not increase. Whilst models using urease-related alkalization are relatively easy to design, the multifactorial physiological conditions in stone- and encrustation formation are not properly represented. In fact, 80% of all urinary stones and probably most urinary implant encrustations consist to a large part of calcium and oxalate. Only 10% of urinary stones contain uric acid crystals, and struvite as a typical infectious stone is clinically found in less than 10% of urinary stones, typically in alkaline urine with a pH > 7. Yet, alkalization models do focus on this group of stones.
In clinical practice, guidelines mandate that urinary catheters and stents with such infectious stone encrustations must always be removed due to the presence of inactive bacteria protected by the biofilm [7]. Using these models seems therefore non-relevant for the development of new stents for a large target population of patients.
The above-mentioned encrustation models could be complimented by in vitro calcium oxalate crystallization methods from urolithiasis research. There are different options to choose from. These vary from simple experiments in defined inorganic solutions to whole human urine experiments replicating urine flow dynamics [8]. Currently, models are being developed that combine the advantages of continuous flow and static models. One such system is the stent-on-chip microfluidic model (SOC). SOC tries to simulate the hydrodynamic areas of a stented ureter under physiological conditions, including drainage holes and the cavity formed by a ureteral obstruction. Encrustation formation over time is monitored and measured by optical microscopy [9].
For the future, examination of the urinary microbiome may provide promising insight into the underlying mechanisms of biofilm formation and encrustation on urinary implants. It has been suggested that the urinary tract is not, contrary to earlier assumptions, a perfectly sterile environment and that commensal bacteria may play a role in patient susceptibility to infection and in the composition of the urinary microbiome associated with stent complications [10].
OMICs (genomics, transcriptomics, proteomics and metabolomics) have improved our understanding of microbial interactions in the urinary tract. It is now possible to identify all microbial species that colonize the urinary tract. Combining results from OMICs studies with in vitro biofilm research has the potential of making a real impact in clinical practice in the future.
2 Preclinical In Vivo Evaluation of Urinary Stents
Experimental in vivo trials represent the final step in the preclinical validation of a medical device. These in vivo evaluations should be preceded by the corresponding in silico simulations, in vitro and ex vivo studies of the newly developed device. The urinary tract constitutes a complex dynamic environment with a high variability, where in vitro and ex vivo models often fail to reflect certain factors that are decisive for the safety and effectiveness of a urinary stent. These factors include urodynamic behavior of the urinary tract, the changing physico-chemical conditions and the multifactorial nature of urinary tract infections, biofilm and encrustations. Besides, ureteral peristalsis and the potential presence of vesicoureteral reflux may play a crucial role in the success of new designs of ureteral stents [1, 11, 12].
Prior to its translation into a clinical setting, the safety and performance of a urinary stent requires to be tested in a whole organism, provided currently by animal models. Animal models overcome the aforementioned limitations of reproducibility in laboratory setting and also allow the evaluation of the systemic effect of a new device on the host, including its potential systemic toxicity [13]. The rational sequence of the preclinical assessment of a new stent design or innovation should follow the order from in silico, in vitro and ex vivo studies, to finally in vivo trials. This thus allows the reduction of the number of animal models used to a minimum that provides adequate statistical power, increasing the likelihood of success of these experimental trials and preserving animal welfare [14, 15].
Concerning animal welfare in experimental studies, ethical evaluation of projects involving animal testing is mandatory in the EU since January 2013, through the Directive 2010/63/EU of the European Parliament and of the Council [16]. Establishing the basic rules applicable to the protection of animals used in experimentation and other scientific purposes [15, 16]. In order to ensure moral standards, scientific validity, and public trust, all projects must be evaluated and approved by an ethical committee prior to development. The use of animals for research should be justified by carefully evaluating each procedure, as to the scientific validity, usefulness and relevance of the expected result of that use. The potential harm to the animal will be balanced against the expected benefits of the project [15, 17].
With regard to the translational perspective of animal research, the choice of the species should be based on the similarity of the conditions studied with those of the human being. Ideally, we should seek for the model that provides anatomic, urodynamic, pathophysiological, histological and biochemical levels as identical as possible to that of humans. Non-human primates represent the closest model in this regard, except for two anatomic variations, they possess unipapillary kidneys and the left kidney lies lower in the abdomen, as opposed to human kidneys [18]. Nevertheless, the scientific literature has not reported the assessment of urinary stents in primates, which may be due to ethical, legal, economic and logistical considerations [16, 19].
2.1 Porcine Model
The porcine species are the animal models most frequently used for the assessment of urinary stent designs. The anatomy of the human and porcine urinary tracts are highly similar, rendering this model ideal for analyzing the behavior of the urinary tract in the presence of new devices [20] (Fig. 1). Pigs have multipapillary kidneys, with 8–12 papillae compared to humans, which usually have 4–18 [21]. Porcine ureters tend to be longer and more tortuous than those of humans [20, 22, 23]. Moreover, porcine renal physiology parallels that of humans with respect to maximal urine concentration, glomerular filtration rate and total renal blood flow [24]. Since the male porcine urethra prevents retrograde approach due to its sigmoid morphology, research involving endourologic procedures is performed on females. Ideally, interventions should be carried out on 35–40 kg models, as the dimensions of their urinary tract at that weight are comparable to a human adult [25, 26].
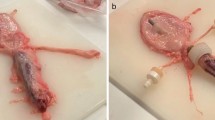
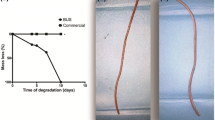
The devices assessed in the porcine model are mainly ureteral stents, including polymeric stents, antireflux, biodegradable, drug-eluting and metallic stents [24,25,26,27,28,29]. This animal enables the transurethral retrograde insertion of the devices, although antegrade and cystostomy approaches have also been described [24, 29,30,31,32]. The evaluation of the performance in vivo of the urinary devices involves blood and urinalysis, urine culture and imaging tests that include the ultrasonographic assessment of the hydronephrosis degrees [33] (Fig. 2). Radiologic tests comprising excretory urography and retrograde ureteropyelography, provide valuable information on urinary patency, stent migration, radiopacity and fashion of degradation of biodegradable devices [12, 34, 35] (Fig. 3). As a limitation, this animal model prevents the assessment of vesicoureteral reflux by means of a voiding cystourethrography; which can be examined via a simulated voiding cystourethrography [27, 36] (Fig. 4). Histological analysis may be performed for the analysis of biocompatibility, tissue damage and more specifically, of the ureteral healing provided with the stents [34, 36, 37]. In addition, intravesical and renal pressures in stented ureters have also been measured, as well as ureteral peristalsis and contractility [29, 38, 39]. Research on urinary stents in the porcine species is generally performed on healthy intact models. However, pigs may undergo the surgical and pharmacological induction of pathologic features such as ureteral strictures and urolithiasis [31, 35, 40].
2.2 Canine Model
The validation of urethral and prostatic stents is generally not performed on pigs, given the particularities of male porcine urethra and the anatomical differences of the accessory sex glands [22]. The dog has proven to be an adequate model for the study of prostate diseases, as it develops benign prostatic hyperplasia (BPH) and prostate cancer both spontaneously and experimentally induced [41, 42]. Metallic, covered, drug-eluting and biodegradable urethral stents have been assessed in healthy and in BPH induced canine models, via transurethral insertion [43,44,45]. Urethral diameter is measured by means of a retrograde urethrography, which enables the monitoring of position, expansion, patency and migration of the stents [44,45,46]. Besides, histological evaluation is also included for the follow-up of stent-related urethral damage and urothelial hyperplasia [44, 47]. Nevertheless, the use of urodynamic studies for testing the therapeutic response in BPH canine models does not seem reliable as, unlike humans, canine hyperplastic prostate produces rectal obstruction rather than lower urinary tract symptoms [42].
The canine model has occasionally been chosen for the evaluation of biodegradable ureteral stents [48,49,50]. Noteworthy, the group of Lumiaho et al., tested their first prototypes of their biodegradable ureteral stent in dogs, placing them with an open surgical approach [49, 50]. The analysis of renal function, ureteral patency and the presence of vesicoureteral reflux are carried out similarly to the methodology in pigs, in addition to renograms [48,49,50].
2.3 Rat Model
Smaller laboratory animals, such as rabbits and rats, provide the advantages of easier handling, are more cost effective and require less infrastructure and logistics [40]. Unlike porcine and canine models, whose dimensions and anatomy allow the evaluation of the urinary stents that will be tested in future clinical trials, the devices inserted on rabbits and rats may differ from the definitive prototype under development. Small laboratory animals are therefore of great use for the assessment of stent upgrades including biomaterials, coatings and the release of substances [51, 52].
As for the rat model, it enables the analysis of the antimicrobial and anti-encrustation potential of new stents, since urolithiasis and urinary tract infection (UTI) can be experimentally induced in a controlled manner [40, 52]. UTI models are performed by the intravesical instillation of bacterial suspensions, being the most common S. aureus, E. faecalis and P. aeruginosa [52,53,54]. The induction of urolithiasis in rats to promote stent encrustation is carried out with dietary manipulations, gastrointestinal resections and the administration of lithogenic agents [40]. These animals are often chosen for the validation of both urethral and ureteral stents. Ureteral stents are inserted through a cystotomy in either the bladder or the ureter [51, 55, 56]. Besides the evaluation of the device’s performance, when placed in the ureter, uretero-ureteral anastomosis may also be performed for the histological analysis of ureteral healing and scarring processes [13, 55, 56]. Urethral stents are tested in the bladder and the urethra, and depending on stent size and characteristics, transurethral placement may be feasible [57,58,59]. The rat’s urethra allows the detection, as well as the histological analysis, of injuries during stent placement and the development of urethral strictures secondary to fibrotic and hyperplastic tissue formation [59].
2.4 Rabbit Model
The rabbit has been used for biocompatibility studies of stent materials. To this end, stent samples can be inserted in the muscle by blunt dissection, preferably the dorsal muscle to prevent the animal from self-mutilation [60]. The scientific literature regarding urinary stent validation in this animal model is scarce, probably due to the significant differences between rabbit’s and human’s urine composition [61]. The potential of biomaterials and drug-release against stent-related urinary tract infections has been assessed by transurethral intravesical placement of ureteral stent samples, for the performance of microbiological cultures and histological analysis [62, 63]. The rabbit’s urethra enables the evaluation of urethral and prostatic stents, including placement, degradation of materials, therapeutic success and histology in both healthy and urethral stricture models [64, 65].
3 Guidelines for Animal Research
Finally, for reporting animal research, it is recommended to follow the ARRIVE guidelines [66]. These guidelines have been developed to ensure that studies involving live animals follow methodological rigour, are reported in enough detail and enable reproducibility. This tool is primarily aimed for the writing and revision of scientific publications. However, they are also valuable for study planning and conducting, as they help researchers to design rigorous and reliable in vivo experiments, minimize bias and to record important information about study methods. Besides, ethical review boards, funders, institutions and learned societies may rely on them to help promote best practice and ensure rigorous design and transparent reporting of in vivo preclinical research [66].
References
Kram W, Buchholz N, Hakenberg OW. Ureteral stent encrustation. Pathophysiology. Arch Esp Urol. 2016;69(8):485–93.
Novick A, Szilard L. Description of the chemostat. Science. 1950;112(2920):715–6.
Finlayson B, Dubois L. Kinetics of calcium oxalate deposition in vitro. Investig Urol. 1973;10(6):429–33.
Gilmore BF, et al. Models for the assessment of biofilm and encrustation formation on urological materials. In Biomaterials and tissue engineering in urology. Sawston: Woodhead Publishing; 2009. p. 59–81.
Gilmore BF, et al. Validation of the CDC biofilm reactor as a dynamic model for assessment of encrustation formation on urological device materials. J Biomed Mater Res Part B Appl Biomater. 2010;93(1):128–40.
Azeredo J, et al. Critical review on biofilm methods. Crit Rev Microbiol. 2017;43(3):313–51.
Hesse A, et al. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44(6):709–13.
Chow K, et al. A stone farm: development of a method for simultaneous production of multiple calcium oxalate stones in vitro. Urol Res. 2004;32(1):55–60.
Mosayyebi A, et al. Reducing deposition of encrustation in ureteric stents by changing the stent architecture: a microfluidic-based investigation. Biomicrofluidics. 2019;13(1):014101.
Buhmann MT, et al. Encrustations on ureteral stents from patients without urinary tract infection reveal distinct urotypes and a low bacterial load. Microbiome. 2019;7(1):60.
Lumiaho J, Heino A, Pietilainen T, Ala-Opas M, Talja M, Valimaa T, et al. The morphological, in situ effects of a self-reinforced bioabsorbable polylactide (SR-PLA 96) ureteric stent; an experimental study. J Urol. 2000;164:1360–3.
Barros AA, Oliveira C, Ribeiro AJ, Autorino R, Reis RL, Duarte ARC, et al. In vivo assessment of a novel biodegradable ureteral stent. World J Urol. 2018;36:277–83.
Kram W, Rebl H, Wyrwa R, Laube T, Zimpfer A, Maruschke M, et al. Paclitaxel-coated stents to prevent hyperplastic proliferation of ureteral tissue: from in vitro to in vivo. Urolithiasis. 2020;48(1):47–56.
De Angelis I, Ricceri L, Vitale A. The 3R principle: 60 years taken well. Preface. Annali dell’Istituto superiore di sanita. 2019;55:398–9.
Tjärnström E, Weber EM, Hultgren J, Röcklinsberg H. Emotions and ethical decision-making in animal ethics committees. Animals. 2018;8(10):1–19.
European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Brussels: European Union; 2010.
Hansen LA, Goodman JR, Chandna A. Analysis of animal research ethics committee membership at American institutions. Animals. 2012;2(1):68–75.
Roberts J. The urinary system. In: Fiennes RN, editor. Pathology of simian primates part I: general pathology. London: Karger; 1972. p. 821–40.
Crisóstomo Ayala V, Maynar Moliner M, Sun F, Usón Gargallo J, Sánchez Margallo FM. Ultrasonographic histological study on the evolution of a canine model of hormone-induced benign prostatic hyperplasia. Actas Urol Esp. 2009;33(8):895–901.
Pereira-Sampaio MA, Favorito LA, Sampaio FJB. Pig kidney: anatomical relationships between the intrarenal arteries and the kidney collecting system. Applied study for urological research and surgical training. J Urol. 2004;172:2077–81.
Cullen-McEwen L, Sutherland MR, Black MJ. The human kidney: parallels in structure, spatial development, and timing of nephrogenesis. In Kidney development, disease, repair and regeneration. Amsterdam: Elsevier Inc.; 2015. p. 27–40.
Swindle M, Smith AC. Comparative anatomy and physiology of the pig. Scand J Lab Anim Sci. 1998;23:1–10.
Sampaio FJ, Pereira-Sampaio MA, Favorito LA. The pig kidney as an endourologic model: anatomic contribution. J Endourol. 1998;12:45–50.
Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43(1–3):185–91.
Tunc L, Resorlu B, Unsal A, Oguz U, Diri A, Gozen AS, et al. In vivo porcine model for practicing retrograde intrarenal surgery. Urol Int. 2014;92(1):64–7.
Soria F, Rioja LA, Blas M, Durán E, Usón J. Evaluation of the duration of ureteral stenting following endopyelotomy: animal study. Int J Urol. 2006;13:1333–8.
Lumiaho J, Heino A, Aaltomaa S, Vålimaa T, Talja M. A short biodegradable helical spiral ureteric stent provides better antireflux and drainage properties than a double-J stent. Scand J Urol Nephrol. 2011;45:129–33.
Liatsikos EN, Karnabatidis D, Kagadis GC, Katsakiori PF, Stolzenburg J-U, Nikiforidis GC, et al. Metal stents in the urinary tract. EAU-EBU Update Ser. 2007;5(2):77–88. https://doi.org/10.1016/j.eeus.2006.11.003.
Venkatesh R, Landman J, Minor SD, Lee DI, Rehman J, Vanlangendonck R, et al. Impact of a double-pigtail stent on ureteral peristalsis in the porcine model: initial studies using a novel implantable magnetic sensor. J Endourol. 2005;19:170–6.
Seitz C, Liatsikos E, Porpiglia F, Tiselius H-G, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455–71.
Soria F, Morcillo E, Serrano A, Budía A, Fernandez I, Fernández-Aparicio T, et al. Evaluation of a new design of antireflux-biodegradable ureteral stent in animal model. Urology. 2018;115:59–64.
Soria F, Morcillo E, Pamplona M, Uson J, Sanchez-Margallo FM. Evaluation in an animal model of a hybrid covered metallic ureteral stent: a new design. Urology. 2013;81:458–63.
Soria F, de La Cruz JE, Fernandez T, Budia A, Serrano Á, Sanchez-Margallo FM. Heparin coating in biodegradable ureteral stents does not decrease bacterial colonization-assessment in ureteral stricture endourological treatment in animal model. Transl Androl Urol. 2021;10(4):1700–10.
Chew BH, Paterson RF, Clinkscales KW, Levine BS, Shalaby SW, Lange D. In vivo evaluation of the third generation biodegradable stent: a novel approach to avoiding the forgotten stent syndrome. J Urol. 2013;189:719–25.
Soria F, de La Cruz JE, Budia A, Cepeda M, Álvarez S, Serrano Á, et al. Iatrogenic ureteral injury treatment with biodegradable antireflux heparin-coated ureteral stent—animal model comparative study. J Endourol. 2021;35(8):1244–9.
Soria F, de la Cruz JE, Budía A, Serrano A, Galán-Llopis JA, Sánchez-Margallo FM. Experimental assessment of new generation of ureteral stents: biodegradable and antireflux properties. J Endourol. 2020;34:359–65.
Soria F, Delgado MI, Rioja LA, Blas M, Pamplona M, Durán E, et al. Ureteral double-J wire stent effectiveness after endopyelotomy: an animal model study. Urol Int. 2010;85:314–9.
Janssen C, Buttyan R, Seow CY, Jäger W, Solomon D, Fazli L, et al. A role for the hedgehog effector Gli1 in mediating stent-induced ureteral smooth muscle dysfunction and aperistalsis. Urology. 2017;104:242.e1–8.
Johnson LJ, Davenport D, Venkatesh R. Effects of alpha-blockade on ureteral peristalsis and intrapelvic pressure in an in vivo stented porcine model. J Endourol. 2016;30:417–21.
Tzou DT, Taguchi K, Chi T, Stoller ML. Animal models of urinary stone disease. Int J Surg. 2016;36:596–606. https://doi.org/10.1016/j.ijsu.2016.11.018.
Sun F, Báez-Díaz C, Sánchez-Margallo FM. Canine prostate models in preclinical studies of minimally invasive interventions: Part I, canine prostate anatomy and prostate cancer models. Transl Androl Urol. 2017;6(3):538–46.
Sun F, Báez-Díaz C, Sánchez-Margallo FM. Canine prostate models in preclinical studies of minimally invasive interventions: Part II, benign prostatic hyperplasia models. Transl Androl Urol. 2017;6(3):547–55.
Yoon CJ, Song H-Y, Kim JH, Park HG, Kang HS, Ro J-Y, et al. Temporary placement of a covered, retrievable, barbed stent for the treatment of hormone-induced benign prostatic hyperplasia: technical feasibility and histologic changes in canine prostates. J Vasc Interv Radiol. 2010;21(9):1429–35.
Han K, Park J-H, Yang S-G, Lee DH, Tsauo J, Kim KY, et al. EW-7197 eluting nano-fiber covered self-expandable metallic stent to prevent granulation tissue formation in a canine urethral model. PLoS One. 2018;13(2):e0192430.
Park J-H, Song H-Y, Shin JH, Kim JH, Jun EJ, Cho YC, et al. Polydioxanone biodegradable stent placement in a canine urethral model: analysis of inflammatory reaction and biodegradation. J Vasc Interv Radiol. 2014;25(8):1257–64.
Lee JH, Kim SW, Il YB, Ha U-S, Sohn DW, Cho Y-H. Factors that affect nosocomial catheter-associated urinary tract infection in intensive care units: 2-year experience at a single center. Korean J Urol. 2013;54(1):59–65.
Wang C-J, Huang S-W, Chang C-H. Effects of specific alpha-1A/1D blocker on lower urinary tract symptoms due to double-J stent: a prospectively randomized study. Urol Res. 2009;37:147–52.
Li G, Wang Z-X, Fu W-J, Hong B-F, Wang X-X, Cao L, et al. Introduction to biodegradable polylactic acid ureteral stent application for treatment of ureteral war injury. BJU Int. 2011;108:901–6.
Lumiaho J, Heino A, Tunninen V, Ala-Opas M, Talja M, Valimaa T, et al. New bioabsorbable polylactide ureteral utent in the treatment of ureteral lesions: an experimental study. J Endourol. 1999;13:107–12.
Lumiaho J, Heino A, Kauppinen T, Talja M, Alhava E, Valimaa T, et al. Drainage and antireflux characteristics of a biodegradable self-reinforced, self-expanding X-ray-positive poly-l,d-lactide spiral partial ureteral stent: an experimental study. J Endourol. 2007;21:1559–64.
Hildebrandt P, Sayyad M, Rzany A, Schaldach M, Seiter H. Prevention of surface encrustation of urological implants by coating with inhibitors. Biomaterials. 2001;22(5):503–7.
Wang X, Cai Y, Xing H, Wu W, Wang G, Li L, et al. Increased therapeutic efficacy of combination of azithromycin and ceftazidime on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. BMC Microbiol. 2016;16(1):124. https://doi.org/10.1186/s12866-016-0744-1.
Cirioni O, Silvestri C, Ghiselli R, Kamysz W, Minardi D, Castelli P, et al. In vitro and in vivo effects of sub-MICs of pexiganan and imipenem on Pseudomonas aeruginosa adhesion and biofilm development. Le Infez Med. 2013;21(4):287–95.
Minardi D, Cirioni O, Ghiselli R, Silvestri C, Mocchegiani F, Gabrielli E, et al. Efficacy of tigecycline and rifampin alone and in combination against enterococcus faecalis biofilm infection in a rat model of ureteral stent. J Surg Res. 2012;176:1–6.
Maruschke M, Kram W, Nebe JB, Vollmar B, Zimpfer A, Hakenberg OW. Development of a rat model for investigation of experimental splinted uretero-ureterostomy, ureteral stenting and stenosis. In Vivo. 2013;27(2):245–9.
Wang T, Yu Z, Chen C, Song Y, Zeng X, Su Y, et al. Ureteral anastomosis with a polyimide stent in rat kidney transplantation. Ren Fail. 2020;42:193–9.
Lim KS, Jeong MH, Bae IH, Park JK, Park DS, Kim JM, et al. Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model. J Cardiol. 2014;64(5):409–18. https://doi.org/10.1016/j.jjcc.2014.02.015.
Park J-H, Kim T-H, Cho YC, Bakheet N, Lee SO, Kim S-H, et al. Balloon-expandable biodegradable stents versus self-expandable metallic stents: a comparison study of stent-induced tissue hyperplasia in the rat urethra. Cardiovasc Interv Radiol. 2019;42(9):1343–51.
Kim KY, Park J-H, Kim DH, Tsauo J, Kim MT, Son W-C, et al. Sirolimus-eluting biodegradable poly-l-lactic acid stent to suppress granulation tissue formation in the rat urethra. Radiology. 2018;286(1):140–8.
Wang X, Zhang L, Chen Q, Hou Y, Hao Y, Wang C, et al. A nanostructured degradable ureteral stent fabricated by electrospinning for upper urinary tract reconstruction. J Nanosci Nanotechnol. 2015;15:9899–904.
Block WD, Hubbard RW. Amino acid content of rabbit urine and plasma. Arch Biochem Biophys. 1962;96:557–61.
Fung LCT, Mittelman MW, Thorner PS, Khoury AE. A novel rabbit model for the evaluation of biomaterial associated urinary tract infection. Can J Urol. 2003;10(5):2007–12.
Cadieux PA, Chew BH, Knudsen BE, DeJong K, Rowe E, Reid G, et al. Triclosan loaded ureteral stents decrease Proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175:2331–5.
Fu W-J, Zhang B-H, Gao J-P, Hong B-F, Zhang L, Yang Y, et al. Biodegradable urethral stent in the treatment of post-traumatic urethral strictures in a war wound rabbit urethral model. Biomed Mater. 2007;2(4):263–8. https://doi.org/10.1088/1748-6041/2/4/009.
Kotsar A, Isotalo T, Mikkonen J, Juuti H, Martikainen PM, Talja M, et al. A new biodegradable braided self-expandable PLGA prostatic stent: an experimental study in the rabbit. J Endourol. 2008;22(5):1065–9.
du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):1–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Kram, W., de la Cruz, J.E., Humphreys, O., Buchholz, N., Soria, F. (2022). Methodology for the Development and Validation of New Stent Designs: In Vitro and In Vivo Models. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)