Abstract
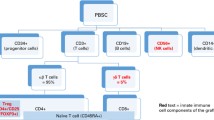
CAR-T cell expansion and persistence are critical parameters for therapeutic efficacy and toxicity (Locke et al. 2020). However, CAR-T cells are patient-specific ‘living drugs’ with an unpredictable ability to expand in vivo. Thus, close postinfusion monitoring should be a major prerequisite to better manage this therapy. Critical parameters include CAR-T cell expansion kinetics and phenotype immune reconstitution and serum biomarkers (Fig. 35.1; Kalos et al. 2011; Hu and Huang 2020). Additionally, prospective collection and storage of patient specimens should be planned for future hypothesis-driven studies at specialized research centres. To date, despite the rapid expansion of CAR-T cell therapy, no standard recommendations exist for CAR monitoring, and harmonization of efforts across multiple centres is urgently needed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
CAR-T cell expansion and persistence are critical parameters for therapeutic efficacy and toxicity (Locke et al. 2020). However, CAR-T cells are patient-specific ‘living drugs’ with an unpredictable ability to expand in vivo. Thus, close postinfusion monitoring should be a major prerequisite to better manage this therapy. Critical parameters include CAR-T cell expansion kinetics and phenotype immune reconstitution and serum biomarkers (Fig. 35.1; Kalos et al. 2011; Hu and Huang 2020). Additionally, prospective collection and storage of patient specimens should be planned for future hypothesis-driven studies at specialized research centres. To date, despite the rapid expansion of CAR-T cell therapy, no standard recommendations exist for CAR monitoring, and harmonization of efforts across multiple centres is urgently needed.
Molecular Monitoring of CAR-T Cells via Digital PCR (dPCR)
Most clinically used CAR-T cell products consist of autologous lymphocytes stably transduced with retro- or lentiviral vectors encoding the respective CAR construct. Integrated CAR vectors are commonly detected at the genomic level using real-time quantitative PCR (qPCR) or dPCR. Surprisingly, outside of clinical trials, CAR-specific diagnostic tools were initially missing, requiring the de novo design of lab-made specific assays to enumerate CAR-T cells in vivo (Badbaran et al. 2020; Fehse et al. 2020; Kunz et al. 2020). Despite technological differences, both qPCR and dPCR assays yield robust and accurate results, with limited requirements regarding sample quality (Table 35.1).
dPCR is extremely sensitive and does not rely on standard curves or multiple repetitions. As a limitation, DNA-directed PCR monitoring provides no information on the expression of the CAR construct. However, the expansion of CAR-T cells strongly depends on the interaction of the CAR with its cognate antigen. In accordance, our data have shown excellent correlation of dPCR with flow cytometry (Badbaran et al. 2020) as well as clinical (Ayuk et al. 2021) results. Because flow cytometry-based assays facilitate phenotypic characterization of CAR-T cells, the two methods complement each other well.
Flow Cytometry Monitoring of CAR-T Cells
Identification of CAR-T cells by flow cytometry (CMF) can be performed by using monoclonal antibodies (mAbs) directly recognizing the CAR (idiotype, linker region) or a specific tag included in the CAR construct. Alternatively, indirect detection can be achieved using antigen-Fc chimeric proteins containing the CAR target antigen fused to a human IgG Fc fragment. A secondary staining step is required for the detection of CAR-expressing cells with an anti-Fc or anti-biotin (if the antigen-Fc is biotinylated) mAb labelled with a fluorochrome (Hu and Huang 2020). In practice, outside of clinical trials, patients receiving commercial CD19 CAR-T cells are monitored with biotinylated CD19-Fc proteins in a two-step staining protocol. The advantage of CMF is the possibility of combining CAR staining with other cell surface markers to characterize CAR-T cells in terms of T cell subtype (CD4 and CD8 expression), differentiation (naïve versus memory), and exhaustion (PD1, TIM3, Lag3). In addition, the results can be provided in real time to physicians. The limitation of the technique is the relatively low sensitivity. Below 0.5% of T cells, the reliability of CMF is weak and justifies pursuing monitoring via PCR. Two important pieces of information can be obtained with sequential CMF analysis of CAR-T cells in the peripheral blood after cell infusion: the expansion peak (Cmax, maximum CAR-T cell rate in percentage or absolute value) and the area under the curve of circulating CAR-T cells between D0 and D28 (AUC0-28). These two parameters have been associated with the response and the risk of complications after treatment in B-lymphoid malignancies (Park et al. 2018; Fraietta et al. 2018; Locke et al. 2020; Ayuk et al. 2021). To determine these parameters, the recommended frequency of CAR-T cell monitoring is two or three times a week for the first 2 weeks after CAR-T cell administration, on days 21 and 28, once a month until 3 months and then every 3 months until 1 year (Rubio et al. 2021).
Monitoring of Additional Immune Parameters (Non-CAR-T, B, and NK Cells and Cytokines)
Patients receiving anti-CD19 CAR-T cells might develop prolonged T CD4 lymphopenia as well as B-cell aplasia with severe hypogammaglobulinaemia, making them particularly susceptible to bacterial and viral infections even after haematopoietic recovery (Logue et al. 2021). Therefore, routine immune surveillance of non-CAR-T CD4 and CD8 T cells, B cells, and NK cells and the levels of serum immunoglobulins is recommended during the first year of follow-up.
Many cytokines are produced in large quantities after CAR-T cell administration as a result of activation of T lymphocytes (IL-6, IFN-γ, sIL2-Rα, sIL-6R, GM-CSF, IL-2, and TNF-α), activation and attraction of mono-macrophages (IFNα, IL-1β, IL-6, IL1Rα, IL10, IL-12, IL-13, IL-15, sIL6-R, TNF-α, CXCL10, CCL2, and IL-8) and in response to tissue damage (IL-6, IL-8, G-CSF, and GM-CSF) (Brudno and Kochenderfer 2019). Confounding factors, such as sepsis, degree of CAR-T cell expansion and tumour burden, also impact cytokine levels. Some cytokine signatures have been described to predict the occurrence of cytokine release syndrome (CRS) (Teachey et al. 2016), immune effector cell-associated neurotoxicity syndrome (ICANS) (Santomasso et al. 2018) or the expansion capacity of CAR-T cells in vivo (Kochenderfer et al. 2017). One major limitation in clinical practice is the absence of a validated fast cytokine quantification test predicting severe complications. Therefore, further studies are required in homogeneous groups of patients to determine whether cytokines can predict the occurrence of complications or treatment efficacy. Participation in prospective studies or collection of serum at each time point of CAR-T cell analysis is recommended.
Key Points
Immune monitoring after CAR-T cell therapy should be carefully performed:
-
Medical CAR-T cell products are complex ‘living drugs’ with unpredictable in vivo performance. Thus, the establishment of accompanying diagnostic and research monitoring programmes is a priority for rational development of this approach.
-
Molecular monitoring, especially dPCR, is an excellent, robust, and sensitive tool for real-time/on-site persistence tracking.
-
Flow cytometry is an easy and rapid tool to monitor early CAR-T cell expansion and characterize CAR-T cell phenotype, both of which have been correlated with the response.
-
Routine monitoring of T, B, and NK cell populations and immunoglobulin levels is recommended to evaluate infection risk.
-
Serum collection is recommended to further explore and identify cytokine signatures that enable prediction of complications or response.
-
Efforts to harmonize patient monitoring across multiple centres following CAR-T cell infusion would be desirable (i.e., reference labs, shared databases, and collaborations with dedicated centres for ‘next generation’ research). Successful implementation of these joint efforts will greatly advance our understanding of the biology involved in transferring CAR-T cells and, most importantly, serve our patients.
References
Ayuk FA, Berger SC, Badbaran A, et al. Axicabtagene ciloleucel in vivo expansion and treatment outcome in aggressive B-cell lymphoma in a real-world setting. Blood Adv. 2021;5(11):2523–7.
Badbaran A, Berger C, Riecken K, et al. Accurate in-vivo quantification of CD19 CAR-T cells after treatment with axicabtagene ciloleucel (Axi-cel) and tisagenlecleucel (Tisa-cel) using digital PCR. Cancers (Basel). 2020;12:1970.
Brudno JN, Kochenderfer JN. Recent advances in CAR-T cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
Campomenosi P, Gini E, Noonan DM, et al. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016;16:60.
Fehse B, Badbaran A, Berger C, et al. Digital PCR assays for precise quantification of CD19-CAR-T cells after treatment with axicabtagene ciloleucel. Mol Ther Methods Clin Dev. 2020;16:172–8.
Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71.
Hu Y, Huang J. The chimeric antigen receptor detection toolkit. Front Immunol. 2020;11:1770.
Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73.
Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35:1803–13.
Kunz A, Gern U, Schmitt A, et al. Optimized assessment of qPCR-based vector copy numbers as a safety parameter for GMP-grade CAR-T cells and monitoring of frequency in patients. Mol Ther Methods Clin Dev. 2020;17:448–54.
Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4:4898–911.
Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978–86.
Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Rubio MT, Varlet P, Allain V, et al. Immunomonitoring of patients treated with CAR-T cells for hematological malignancy: guidelines from the CARTi group and the francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Bull Cancer. 2021;108(12S):S53–64.
Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR-T cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71.
Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Berger, S.C., Fehse, B., Rubio, MT. (2022). Immune Monitoring. In: Kröger, N., Gribben, J., Chabannon, C., Yakoub-Agha, I., Einsele, H. (eds) The EBMT/EHA CAR-T Cell Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-94353-0_35
Download citation
DOI: https://doi.org/10.1007/978-3-030-94353-0_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94352-3
Online ISBN: 978-3-030-94353-0
eBook Packages: MedicineMedicine (R0)