Abstract
Role of consolidative allogeneic haematopoietic cell transplantation (allo-HCT) for B-cell acute lymphoblastic leukaemia (B-ALL) patients in minimal residual disease-negative (MRD) complete remission (CR) after CD19 CAR-T cell therapy.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Role of consolidative allogeneic haematopoietic cell transplantation (allo-HCT) for B-cell acute lymphoblastic leukaemia (B-ALL) patients in minimal residual disease-negative (MRD) complete remission (CR) after CD19 CAR-T cell therapy.
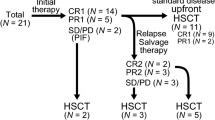
The role of consolidative allo-HCT in B-ALL patients achieving MRD-negative CR after CD19 CAR-T cell therapy is still debated. At the last update of the ELIANA clinical trial, which investigated tisagenlecleucel in children and young adults with relapsed or refractory (R/R) B-ALL, the median duration of remission and overall survival was not reached (median follow-up, 24 months). The 24-month relapse-free survival probability in responders was 62%, with plateauing of the probability curves after 1 year (Grupp et al. 2019). Consolidation with allo-HCT was reported in only 9% of CR patients, suggesting that CD19 CAR-T cell therapy alone with tisagenlecleucel may be curative in a significant proportion of paediatric patients. In contrast, to date, the data do not suggest that CD19 CAR-T cell therapy is a definitive approach in most adults with R/R B-ALL. Across the main academic and industry-sponsored clinical trials of CD19 CAR-T cell therapy for adult B-ALL, the median durations of response ranged from 8 to 19 months, with important variations in the proportion of patients receiving consolidative allo-HCT in CR after treatment (35–75%) (Shah et al. 2019; Frey et al. 2020; Hay et al. 2019; Park et al. 2018). In our experience, we observed favourable outcomes in patients undergoing allo-HCT while in MRD-negative CR after defined-composition CD19 CAR-T cell therapy, with 2-year EFS and OS probabilities of 61% and 72%, respectively. After adjusting for previously identified prognostic factors for event-free survival (EFS; pre-lymphodepletion LDH concentration and platelet count, cyclophosphamide-fludarabine lymphodepletion), the hazard ratio for allo-HCT was 0.39 (95% CI 0.13–1.15, p = 0.09), suggesting a beneficial effect on EFS. Based on these findings, our approach in adult patients is to recommend consolidative allo-HCT in adult patients with R/R B-ALL in MRD-negative CR after CD19 CAR-T cell therapy. Additionally, patient age and preferences, comorbidities, a history of prior transplant, and MRD must be taken into account. Investigators from the NCI/NIH (Lee et al. 2016) and Seattle Children’s Hospital (Summers et al. 2018) reported a survival advantage in children and young adults consolidated with allo-HCT after CD19 CAR-T cell therapy. However, notably, to date, the available data rely on nonrandomized, retrospective analyses, and are potentially subject to important biases (Suissa 2007; Lévesque et al. 2010).
Management of Relapsed B-ALL After CD19 CAR-T Cell Therapy
CD19-Positive Disease After CD19 CAR-T Cell Therapy
If cryopreserved end-manufacturing CAR-T cells are available and the target antigen is still expressed, an attractive approach is to use the “left-over” cells to manufacture a second CAR-T cell product. We have shown that second CD19 CAR-T cell infusions are feasible and well tolerated, but in most cases directed at the murine single chain variable fragment (scFv) CAR domain, antitumour efficacy is limited by anti-CAR immune responses. We observed superior outcomes in patients who received cyclophosphamide and fludarabine lymphodepletion prior to the first CAR-T cell infusion and who received a higher CAR-T cell dose (10 times higher than the first CAR-T cell infusion). However, second CAR-T cell infusions achieved a CR in only 3 of 14 ALL patients (21%) (Gauthier et al. 2020). Efforts are underway to mitigate or circumvent anti-CAR immune responses using a CAR comprising humanized or fully human scFvs (Gauthier et al. 2018; Brudno et al. 2020). Maude et al. evaluated the use of the humanized scFv-bearing CD19 CAR-T cell product CTL119 in 38 children and young adults (Grupp et al. 2015). MRD-negative CR could be achieved in murine CAR-exposed patients (43%), although at a lower rate than in the CAR-naïve population (100%). The 12-month relapse-free survival probabilities in responding patients were 82% and 56% in the CAR-exposed and CAR-naïve cohorts, respectively. In another report, Cao et al. observed CR in 2 of 5 patients previously exposed to murine CD19 CAR-T cells(Cao et al. 2019). Further studies are needed to determine whether immunogenicity, poor CAR-T cell function or disease-related factors underlie the reduced efficacy of CD19 CAR-T cells employing fully human or humanized scFv in the murine CAR-exposed setting.
CD19-Negative Disease After CD19 CAR-T Cell Therapy
Encouraging results have been reported using CD22-targeted CAR-T cells, including in patients with CD19-negative B-ALL blasts after prior CD19 CAR-T cell therapy. Shah et al. reported their experience in 58 children and young adults with R/R B-ALL, including 62% of patients who were previously treated with a CD19 CAR-T cell product (Shah et al. 2020). CD22 CAR-T cells achieved an MRD-negative CR in 61% of cases, with a median duration of response of 6 months (13 of 35 MRD-negative CR patients [37%] went on to receive allo-HCT). The MRD-negative CR rate in patients previously treated with CD19 CAR-T cells was 64%. In another report from Pan et al., CD22 CAR-T cells were administered to 34 children and adult patients. Prior failure of CD19 CAR-T cell therapy was documented in 91% of patients. CD22 CAR-T cells achieved MRD-negative CRs in 76% of patients. Responses were durable, with a 1-year leukaemia-free survival probability of 58% in CR patients (11 of 30 CR patients [37%] went on to receive allo-HCT) (Pan et al. 2019).
Key Points
-
When feasible, allo-HCT should be offered to adult patients with R/R B-ALL in MRD-negative CR after CD19 CAR-T cell therapy.
-
CD22 CAR-T cells are associated with high response rates after CD19 CAR-T cell failure, and the most durable responses are observed after consolidative allo-HCT.
-
CD19 CAR-T cells with fully human or humanized scFv are under investigation to mitigate anti-CAR immune responses, potentially impeding the efficacy of repeat CAR-T cell infusions.
References
Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, et al. Safety and feasibility of anti-CD19 CAR-T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–80.
Cao J, Cheng H, Qi K, Chen W, Shi M, Zheng J, et al. Humanized CD19-specific chimeric antigen receptor T cells for acute lymphoblastic leukemia. Blood. 2019;134(Supplement_1):3872.
Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2020;38(5):415–22.
Gauthier J, Bezerra E, Hirayama AV, Pender BS, Vakil A, Steinmetz RN, et al. Repeat infusions of CD19 CAR-T cells: factors associated with response, CAR-T cell in vivo expansion, and progression-free survival. ASTCT. 2020;26(3):S267–S8.
Gauthier J, Hirayama AV, Hay KA, Sheih A, Pender BS, Hawkins RM, et al. Immunotherapy with T-cells engineered with a chimeric antigen receptor bearing a human CD19-binding single chain variable fragment for relapsed or refractory acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma. Blood. 2018;132(Supplement 1):1415.
Grupp SA, Maude SL, Rives S, Baruchel A, Boyer M, Bittencourt H, et al. Tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia: updated analysis of the ELIANA clinical trial. Biol Blood Marrow Tr. 2019;25(3):S126–S7.
Grupp SA, Maude SL, Shaw PA, Aplenc R, Barrett DM. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019). Blood. 2015;126(23):681.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR-T cell therapy. Blood. 2019;133(15):1652–63.
Lee DW, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B, et al. Long-term outcomes following CD19 CAR-T cell therapy for B-ALL are superior in patients receiving a Fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood. 2016;128(22):218.
Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340(mar12 1):b5087.
Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. CD22 CAR-T cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33(12):2854–66.
Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR-therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
Shah BD, Bishop M, Oluwole OO, Logan A, Baer MR, Donnellan W, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). ASCO. 2019;37(15_suppl):7006.
Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR-T cell trial. J Clin Oncol. 2020;38(17):1938–50.
Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidem Dr S. 2007;16(3):241–9.
Summers C, Annesley C, Bleakley M, Dahlberg A, Jensen MC, Gardner R. Long term follow-up after SCRI-CAR19v1 reveals late recurrences as well as a survival advantage to consolidation with HCT after CAR-T cell induced remission. Blood. 2018;132(Supplement 1):967.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Gauthier, J. (2022). Post-CAR-T Cell Therapy (Consolidation and Relapse): Acute Lymphoblastic Leukaemia. In: Kröger, N., Gribben, J., Chabannon, C., Yakoub-Agha, I., Einsele, H. (eds) The EBMT/EHA CAR-T Cell Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-94353-0_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-94353-0_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94352-3
Online ISBN: 978-3-030-94353-0
eBook Packages: MedicineMedicine (R0)