Abstract
Chimeric antigen receptor (CAR) T cells have emerged as breakthrough therapies in patients with refractory haematologic malignancies, and the highly encouraging clinical results have fuelled expectations of implementing these strategies in other cancer types. However, a similar success of CAR-T cell treatment has not yet been observed in solid tumours. Various factors, including the immunosuppressive nature of the tumour microenvironment, hinder CAR-T cell trafficking and infiltration into scarcely accessible tumour sites, and difficulties in identifying targetable antigens with optimal expression and a good toxicity profile, limiting CAR-T dose escalation, must be overcome to achieve success in the treatment of solid cancers (Comoli et al. 2019).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Chimeric antigen receptor (CAR) T cells have emerged as breakthrough therapies in patients with refractory haematologic malignancies, and the highly encouraging clinical results have fuelled expectations of implementing these strategies in other cancer types. However, a similar success of CAR-T cell treatment has not yet been observed in solid tumours. Various factors, including the immunosuppressive nature of the tumour microenvironment, hinder of CAR-T cell trafficking and infiltration into scarcely accessible tumour sites, and difficulties in identifying targetable antigens with optimal expression and a good toxicity profile, and limiting CAR-T dose escalation, must be overcome to achieve success in the treatment of solid cancers (Comoli et al. 2019).
Several clinical trials have tested the efficacy of CAR-T cells in solid tumours. To date, clinical results have not been encouraging, with a general lack of therapeutic response and the presence of on-target off-tumour toxicity. However, some studies have achieved promising outcomes that justify further exploration of this approach in solid tumours, as is happening in many areas of the world (Comoli et al. 2019).
Early experiences with GD2-specific CAR-T cells showed objective responses in paediatric patients with neuroblastoma (Pule et al. 2008). Since then, lymphocytes have been engineered via insertion of third-generation CARs and, more recently, by delivery of a GD2-CAR-IL-15 construct to NK cells (Heczey et al. 2020).
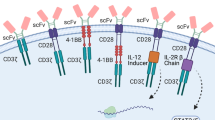
Similarly, the safety and antitumour activity of CAR-T cells targeting a variety of antigens, such as IL-13Rα2, epidermal growth factor receptor-vIII, and human epidermal growth factor receptor-2 (HER2), have been assessed in patients with glioblastoma multiforme (GBM). Infusion of second-generation CD28ζ HER2-specific CAR-modified virus-specific T cells was well tolerated, with no dose-limiting toxic effects, and led to clinical benefit; 1 patient showed a partial response (PR) lasting more than 9 months, and 7 patients had stable disease (SD) for several months (Ahmed et al. 2017). Other clinical trials have demonstrated the feasibility, safety, and clinical efficacy of second-generation EGFRvIII-specific and IL13BBζ–specific CAR-T cells (Brown et al. 2016) in patients with refractory GBM.
In gastrointestinal neoplasms, a clinical trial utilizing CEA CAR-T therapy in ten patients with metastatic colorectal cancer (CRC) resulted in SD in seven patients, without severe adverse events related to CAR-T therapy (Zhang et al. 2017). A previous case report of HER2-specific cell therapy for CRC using third generation CAR-T cells caused fatal acute respiratory distress syndrome due to recognition of lung epithelial cells expressing low levels of HER2 (Morgan et al. 2010). A phase I study of second-generation CAR-T cells targeting HER2 was conducted in 11 patients with advanced biliary tract cancer or pancreatic cancer. A 4.5-month partial response was observed, and 5 subjects achieved stable disease. Toxicity was manageable, with grade 3 fever and one patient showing elevation of liver enzymes as CAR-T-related adverse events; one episode of reversible severe upper gastrointestinal haemorrhage occurred in a patient with gastric involvement 11 days after the HER2 CAR-T- cell infusion, and 2 cases of grade 1–2 delayed fever accompanied by increases in C-reactive protein and interleukin-6 were observed (Feng et al. 2018). Epidermal growth factor receptor (EGFR) and CD133-specific CAR-T sequential immunotherapy were employed by the same group in a patient with advanced unresectable/metastatic cholangiocarcinoma (CCA), resulting in a PR lasting more than 12 months; however, slight liver toxicity secondary to EGFR CAR-T therapy and epidermal and endothelial damage due to CD133-specific CAR-T immunotherapy was observed.
Similar to these experiences, second-generation HER2-specific CAR-T cells, used in a phase I clinical trial conducted on 19 patients with refractory HER2-positive sarcoma, induced SD lasting from 12 weeks to 14 months in 4 of the evaluable patients (Ahmed et al. 2015).
Investigators at the University of Pennsylvania explored an approach based on mRNA-transduced CAR-T cells that target mesothelin (meso CAR-T) in patients with advanced malignant pleural mesothelioma (MPM) or advanced pancreatic cancer. In the first two patients reported, meso CAR-T cells showed some antitumour activity in vivo in the absence of distinct toxicities (Beatty et al. 2014). Second-generation CAR-T cells specific for EGFR were employed in a phase I study to treat 11 patients with advanced non-small-cell lung cancer (NSCLC), resulting in 2 PR cases and 5 SD cases, lasting from 2 to 8 months, with limited adverse events, including skin toxicity, nausea, vomiting, dyspnoea, and hypotension (Feng et al. 2016).
A few phase I studies and case series have reported CAR-T cell treatment for other solid tumours, such as melanoma, breast cancer, renal cell carcinoma, prostate cancer, and ovarian and seminal vesicle cancer (reviewed Fucà et al. 2020).
Various toxicities were observed after CAR-T cell infusion for treatment of solid tumours. In the setting of haematologic malignancies, cytokine release syndrome (CRS) is a frequent, potentially severe adverse event following CAR-T cell therapy. However, CRS, as well as the neurological toxicity (ICAN) sometimes observed in the haematologic setting, has not yet become a common event after CAR-T cell therapy for solid tumours, perhaps because of the lower tumour load. Conversely, CAR-T cells trials conducted in solid tumour cohorts showed critical, unexpected on-target, off-tumour toxicities resulting from the recognition by CAR-T cells of tumour antigens expressed on healthy tissues (Morgan et al. 2010; Lamers et al. 2013). Targeting tumour-specific antigens appears to result in fewer off-tumour effects, but whether these CAR-T cells have promising clinical efficacy remains to be seen, and many trials are still ongoing. Strategies to increase tumour selectivity while sparing healthy tissues are being evaluated to control on-target off-tumour toxicity.
Despite novel genetic engineering techniques and combinatorial approaches to counteract biological barriers, tumour heterogeneity, and the immunosuppressive properties of the tumour microenvironment, targeting solid tumours with CAR-T cells in the clinical setting remains challenging. Therefore, T cell therapy, alone or in combination with immune checkpoint inhibitors or other agents targeting either the cancer cell or the tumour environment, will likely play a role in improving cancer treatment outcomes (Apetoh et al. 2015). Designing and selecting the most appropriate clinical trials or settings to rapidly identify combinatorial approaches that are efficacious in different patient populations and identifying patients who will best benefit from immune checkpoint inhibitors alone (Chalabi et al. 2020) or from the addition of other targeted immunotherapies will be the most pressing need for the future success of CAR immunotherapy in solid cancer.
-
CAR-T therapy is under development for many solid cancer types, but important breakthroughs have not yet been achieved.
-
Tumour heterogeneity, the immunosuppressive tumour microenvironment, and other barriers are hurdles that must be overcome before CAR-T cells can be effective against solid cancers.
-
The field may benefit from network models for CAR-T cell production in academic centres.
References
Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;20(33):1688–96.
Ahmed N, Brawley V, Hegde M, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3:1094–101.
Apetoh L, Ladoire S, Coukos G, et al. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26:1813–23.
Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–20.
Brown C, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–9.
Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–76.
Comoli P, Chabannon C, Koehl U, Lanza F, Urbano-Ispizua A, Hudecek M, Ruggeri A, Secondino S, Bonini C, Pedrazzoli P. Development of adaptive immune effector therapies in solid tumors. Ann Oncol. 2019;30:1740–50.
Feng K, Guo Y, Dai H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59:468–79.
Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9:838–47.
Fucà G, Reppel L, Landoni E, Savoldo B, Dotti G. Enhancing chimeric antigen receptor T-cell efficacy in solid tumors. Clin Cancer Res. 2020;26:2444–51.
Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, Ghatwai N, Dakhova O, Liu B, Raveh-Sadka T, Chauvin-Fleurence CN, Xu X, Ngai H, Di Pierro EJ, Savoldo B, Dotti G, Metelitsa LS. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–90.
Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51.
Pule M, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70.
Zhang C, Wang Z, Yang Z, et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol Ther. 2017;25:1248–58.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Pedrazzoli, P., Haanen, J.B.A.G. (2022). Developments in Solid Tumours. In: Kröger, N., Gribben, J., Chabannon, C., Yakoub-Agha, I., Einsele, H. (eds) The EBMT/EHA CAR-T Cell Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-94353-0_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-94353-0_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94352-3
Online ISBN: 978-3-030-94353-0
eBook Packages: MedicineMedicine (R0)