Abstract
wIRA-transparent small gold nanoparticles (AuNPs) were shown to be shifted to wIRA absorbing when targeted to receptors on tumor cells and aggregated in the tumor cell by enzyme degradation and pH effects. In this way, AuNPs loaded into mouse-grown subcutaneous tumors after both direct intratumoral and intravenous injections cured tumors after either wIRA treatment ablation or wIRA treatment combined with X-ray irradiation. Some GNP constructs, e.g., nanoshells and nanorods, have already progressed to veterinary and human clinical trials. If AuNP/NIR therapy is proven to be useful to treat an appropriately superficial human tumor, the use of the wIRA radiator might make such therapy accessible to large numbers of patients in low- and middle-income countries that lack access to very high-tech expensive therapies.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Gold nanoparticles
- Receptor-mediated endocytosis
- Aggregation
- wIRA radiator
- wIRA heating
- Subcutaneous mouse tumors
1 Introduction
There is intense interest in using hyperthermia (HT) as a therapeutic strategy to directly treat tumors by extreme heating (>43 °C for expanded heating times) or, preferentially, using mild hyperthermia (39–43 °C) to sensitize tumors to other therapies such as X-irradiation and/or chemotherapy. Despite considerable effort in this area, the promise of using hyperthermia to treat human tumors has not yet been fully realized. The holy grail would be an injectable that can access metastatic tumors with a high degree of specificity allowing sufficient heating of the tumor to cause necrosis or sensitization to other therapies while sparing normal tissues. To our knowledge, such an injectable is not yet widely available—despite major efforts to develop such reagents—particularly those efforts using iron nanoparticles and electromagnetic heating. The prospect of using nanoparticles to heat superficial and/or localized tumors in humans seems closer at hand.
This review focuses on the preclinical use of gold nanoparticles and water-filtered infrared A (wIRA) heating using a special wIRA radiator (Model 09.06.00, Hydrosun, Müllheim, Germany). This is discussed in the context of other nanogold constructs (e.g., nanoshells, nanorods, and nanotubes) that have been used for the heating of superficial and/or localized tumors and, with fiber-optic light guides, deeper tumors.
Near-infrared irradiation (NIR and wIRA) has photons of longer wavelength and less energy enabling the irradiation to penetrate deeper into living tissue. Gold nanostructures provide a mechanism for photothermal effect mechanisms whereby wIRA irradiation is converted into heat. A number of investigators have tried to take advantage of the known collective plasmon modes of metal nanoparticles in which the optical properties of gold molecules shift when gold molecules are brought into close apposition to one another [1, 2]. For example, Souza et al. [3] cleverly used phage display methodology to generate networks of gold-filamentous fd (also known as f1 or M13) phage constructs that targeted cells and also functioned as signal reporters for fluorescence, dark-field, and near-infrared (NIR) surface-enhanced Raman scattering (SERS) spectroscopy, since they formed tangled clusters that brought gold molecules close together. Nam et al. [4] designed “smart” 10 nm gold nanoparticles that were designed to clump together under mild acidic intracellular space where electrostatic interactions between nanoparticles form aggregates inside acidic cell organelles, such as lysosomes; once becoming trapped due to their large size, the aggregates of gold nanoparticles exhibited an absorption shift to far-red and near-infrared that could be exploited for therapy since these wavelengths have maximal tissue penetration properties.
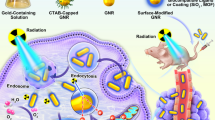
Nanoprobes, Inc., and collaborators developed tumor-cell receptor-targeted spherical gold nanoparticles with net negative charge designed to enter cells by receptor-mediated endocytosis and then aggregate in the acidic and proteolytic environment of the lysosome [5, 6]. Once aggregated by adjusting the pH to 5 in the presence of proteases, they undergo the aforementioned spectral shift, illustrated in Fig. 9.1, in which absorption in the 800 nm range is greatly increased. The extinction amplification factor (extinction after aggregation divided by extinction before aggregation) was ≈20 at 800 nm. This resulted in increased heating. Three different gold nanoparticle constructs were synthesized (Fig. 9.2).
1.1 Construct I
AuNPs had antibodies to epidermal growth factor receptor (EGFR) adsorbed to them; adsorption of antibodies to the AuNPs was via electrostatic interactions. Many tumors overexpress EGFR. After binding to EGFR, the antibody-coated AuNPs were endocytosed. Endocytic vesicles then fused with lysosomes where the proteolytic enzymes of the lysosome degraded the adsorbed antibodies; the low pH of the lysosome resulted in AuNP aggregation. In cell studies, AuNPs were found to accumulate in punctate spots that were distributed in cytoplasmic structures largely around the cell nucleus that likely represented endocytic vesicular structures (endosomes) and lysosomes.
1.2 Construct II
AuNPs were coated with lipoic acid which provided a free carboxyl group and dithio(succinimidyl propionate) (DSP) used to covalently couple anti-EGFR antibodies. Again, after binding to EGFR receptors, endosomal internalization resulted in lysosomal antibody degradation and AuNP aggregation; protonation removed negative charges that kept AuNPs apart. Degradation of antibodies removed steric hindrance to van der Waals-mediated aggregation.
Change in absorption spectra under various conditions. Construct II showed stability in PBS at 37 μC for 24 h (black trace) and also negligible spectral change at pH 5 after 24 h (blue trace). However, at pH 5 and in the presence of pepsin, extensive aggregation was observed (red trace), greatly increasing the absorbance in the near-infrared region (IR-A-region). Reproduced with permission from [5]
Schemes for tumor delivery and aggregation in endosomes/lysosomes. Scheme 1: AuNPs have anti-tumor antibodies adsorbed (a), Construct I) that in the lysosome are degraded, allowing the AuNPs to aggregate (b). Scheme II: AuNPs are coated with lipoic acid and dithio(succinimidyl propionate) (DSP) (a), covalently coupled to anti-EGFr antibodies (b, Construct II), then protonated in the endosome with enzymatic antibody digestion in the lysosome (c), leading to aggregation (d). Scheme III: AuNPs are coated with glutathione (a), reacted with glutaraldehyde (b), and then anti-EGFr antibodies (c, Construct III) to form Schiff’s bases vulnerable to cleavage at endosomal pH (d), resulting in protonation of carboxyl groups and along with enzymatic antibody degradation, causing aggregation (e). Reproduced with permission from [5]
1.3 Construct III
AuNPs were coated with glutathione, then glutaraldehyde, and anti-EGFR. AuNPs with anti-EGFR covalently bound by Schiff’s base were internalized by receptor-mediated endocytosis. Again, the acidic environment of the lysosome resulted in protonation of carboxyl groups, release of antibody, and AuNP aggregation.
All three AuNP constructs remained stable in the blood; the ability to resist aggregation in the blood prior to delivery to the tumor was a critical requirement for success. pH 5 alone caused Construct I but not Construct II and III to aggregate—presumably due to the less-stable electrostatic absorption of anti-EGFR to AuNPs used in Construct I vs the covalent attachments used in Constructs II and III.
2 Treatments and Results
In vivo testing was performed in athymic nude mice with advanced (100–150 mm3) A431 squamous cell carcinomas growing on their flanks subcutaneously and Constructs II and III. The anti-EGFR AuNP conjugates were injected intravenously via the tail vein and showed preferential tumor localization (Fig. 9.3a). Six hours post-i.v. injections, tumor-to-muscle ratios of gold content (determined by atomic absorptions) was 12.9 ± 1.8 to 1; gentle extraction with NaOH and detergent confirmed that all of the tumoral AuNP was in the aggregated state. Water-filtered infrared A irradiation using the Hydrosun wIRA radiator proved very effective treating of tumors that had accumulated anti-EGFR-AuNP constructs that had been injected i.v. or directly into the tumors. Figure 9.3b shows typical before and after wIRA treatment results (1.5 W/cm2, exposed for 1.9 min to the wIRA radiator; short exposure times necessitate higher irradiances) when mice received A, D, No AuNP treatment, B. E, anti-EGFR-AuNP injections directly into the tumors (0.2 g Au/kg in 10 L, and C, F, i.v. injections of anti-EGFR (1.0 g Au/kg). Tumors that received direct injections of Construct II (B, E) or were loaded with Construct II after i.v. injections (C, F) necrosed after wIRA treatment, formed a scab, and healed with no apparent scarring over 2–3 weeks. Untreated tumors progressed. Figure 9.4 shows an experiment utilizing, on average, 9 mice per group. Tumors receiving either direct injection of 0.2 g Construct II (Fig. 9.4b) or i.v. injections of 1.0 g Au/kg Construct II (Fig. 9.4c), or i.v. injection of 1.5 g/kg Construct III (Fig. 9.4d) experienced 89%, 89%, and 100% complete ablation, respectively, whereas untreated tumors (Fig. 9.4a) experienced only 22% ablations after wIRA treatment (1.5 W/cm2, 1.9 min). These results proved the efficacy of Constructs II and III to cure advanced human A431 tumors growing subcutaneously in the flanks of immune-compromised mice when provided either directly to the tumors or intravenously prior to wIRA therapy using the Hydrosun radiator. A follow-up experiment was performed combining gold nanoparticle-mediated wIRA heating and radiation therapy (RT). The synergy between heat and RT has been well known for many years. In this experiment, the radioresistant mouse SCCVII squamous cell carcinoma was implanted subcutaneously in immune-compromised mice and allowed to progress to an advanced stage (100–150 mm3) before initiating therapy. The SCCVII tumor expresses EGFR. The anti-EGFR conjugated to AuNPs in Construct II bind to EGFR on SCCVII cells. The experimental plan was to intratumorally infuse via convection enhanced delivery (CED) Construct II, the lipoic acid AuNPs. After allowing time for adequate diffusion of the AuNPs and cellular uptake (24 hours), tumors receiving heating were treated with wIRA at 48 °C for 5 min. Those tumors slated for RT were irradiated at the indicated doses within minutes after heating. Table 9.1 shows the different experimental groups and how they were treated. The results, shown in Fig. 9.5, are as follows:
-
1.
wIRA-hyperthermia treatment alone after AuNP administration (48 °C, 5 min, Group B) is not effective at tumor control (1 out of 7 or 14% survived (no tumor after 250 days).
-
2.
RT alone (25 Gy) is also not effective therapeutically; 2/6 or 33% survived.
-
3.
RT (25 Gy) plus wIRA (no prior AuNP) was not effective (0/7 survived). Note that wIRA without prior AuNP does not heat the tumor in the experimental set-up chosen.
-
4.
AuNP infusion followed by 25 Gy RT alone (Group D) was also ineffective (1/8 or 12% surviving). The SCCVII is a radiation-resistant tumor requiring 55 Gy for half-maximal control. However, AuNPs + wIRA irradiation followed by X-ray irradiation was very effective. For the radiation doses used, 15 Gy (Group E), 20 Gy (Group F), and 25 Gy (Group G), survival was 5/7 (71%) in all three groups.
Photographs of mice after intravenous injection of AuNPs. (A) Mouse 1.5 h after tail vein injection of 15 nm AuNPs with adsorbed anti-tumor antibody (Construct I) which localized to the tumor on its leg. (B) Mouse before injection of AuNPs. (C) Mouse in (B) 1.5 h after iv injection of non-targeted 15 nm AuNPs coated with 2 k MW PEG. Reproduced with permission from [5]
Mice with Tumors before and after IR/AuNP treatment. Top row before treatment, bottom row 3 weeks after wIRA treatment. (A, D) No AuNPs, but exposed to IR. (B, E) Mouse with 10 μL direct intratumoral injection of 15 nm anti-EGFr lipoic acid AuNP preparation (Construct II) and wIRA. (C, F) Mouse with intravenous 15 nm anti-EGFr lipoic acid AuNP preparation and wIRA. There was no residual normal tissue or body impairment with little to no scarring seen with the AuNP-IR-treated animals. wIRA treatment: 1.5 W/cm2, 1.9-min exposure time. Reproduced with permission from [5]
Plots of tumor volume vs. time after wIRA treatments. (a) wIRA only, 22% survival (2/9); (b) wIRA after direct intra-tumoral injection of 0.2 g Au/kg 15 nm anti-EGFr lipoic acid AuNP preparation (Construct II), 89% tumor ablation (8/9); (c) wIRA after i.v. injection of 1.0 g Au/kg Construct II, 100% tumor ablation (8/8); (d) wIRA after i.v. injection of 1.5 g Au/kg 15 nm anti-EGFr Schiff’s base AuNP preparation (Construct III), 100% tumor ablation (9/9). wIRA treatment: 1.5 W/cm2, 1.9-min exposure time. Reproduced with permission from [5]
Tumor volume vs. time for various treatments corresponding to Table 9.1 (group a–g). Survivors that were tumor-free at 250 days are given at the right of the horizontal axes (e.g., 5/7 meaning 5 out of 7 survived with no measurable tumor). Tumor volumes of individual mice are plotted. Each colored line represents one mouse. Reproduced with permission from [6]
Additional controls (AuNPs only) or wIRA treatment only showed no benefit (0/7 survival). While RT without effective heating slowed tumor growth as would be expected, only the combination of effective heating and RT, even at reduced radiation doses, was effective. At 250 days, there was some loss of muscle mass in treated mice (15–20%), but there was no loss of leg function. Hence, wIRA hyperthermia not only enabled RT to be effective but reduced the dose of RT needed to be effective. This would be very significant therapeutically if translated to the clinic.
Other gold nanostructures (nanoshells, nanorods, nanotubes, nanorings, nanostars, nanocages) have been developed that also convert near-infrared irradiation into heat and are useful for hyperthermia therapy [7, 8]. Gold nanoshells and gold nanorods were nicely described in the introduction to Hainfeld et al. [5] and have been the subject of reviews since then. Gold nanoshells (Nanospectra Biosciences, Inc.) are constructed with a ≈ 110 nm silica core and a ≈ 10-nm-thick gold outer layer. Their absorption properties at 800 nm can be tuned by varying core and shell properties. Efficacy in preclinical studies after both direct and i.v. injections was demonstrated in the early 2000s. Gold-silica nanoshells (GSN) have progressed to clinical trials in which laser excited nanoshells in combination with MRI-ultrasound fusion imaging were used to treat 16 patients with low-to-intermediate grade prostate tumors confined to the prostate, when they are curable [9]. On day 1, patients received 7.5 mg/kg i.v. infusion of GSN (4.8 mg/mL), and on day 2, patients received laser illumination under general anesthesia. Follow-up biopsy showed GSN-mediated focal laser ablation was achieved in 15/16 patients without serious complications or deleterious changes in genitourinary function. A noteworthy feature of this technology is the relatively low amount of GSN infused into patients compared to the gold nanoparticle studies. Presumably, GSNs are more efficient at generating heat after infrared absorption, and numerous avenues are being explored to see how GSNs can be used therapeutically to good advantage.
Gold nanorods (GNRs) (≈50–100 nm in length) also adsorb in the NIR range more efficiently than nanoshells. Preclinical experiments performed over 10 years ago demonstrated subcutaneous tumor control; PEG-coated 13 × 47 nm gold nanorods injected i.v. at 20 mg/kg demonstrated control of small subcutaneous tumors on mice when tumors were irradiated 72 h post-injection with NIR. Once again, the low levels of GNRs required for efficacy was notable. Mammary tumors in dogs and cats were treated with NIR and GNRs directly injected into the tumors [10]. A regimen in which the tumors were injected every 2 weeks for 3 weeks with 7.5 nM, 3.75 nM, and 1.88 nM GNR per 100 mm3 tumor volume used for the first, second, and third injections followed by NIR resulted in complete, durable remissions (>1 year) in all seven of the dogs and cats with spontaneous mammary tumors. A safety study of seven dogs mainly with soft tissue sarcomas with i.v. GNR and NIR treatment was also performed recently [11]. Photothermal therapy with anti EGFR-conjugated GNRs selectively targeted head and neck cancer decreased tumor size with minimal side effects in more recent preclinical experiments. We are not aware of any clinical trial results using the GNRs.
Mouse, cat, and dog trials have shown the gold nanoparticles, gold nanoshells, and the gold nanorods to be safe when administered by direct injection or i.v. They have shown some degree of efficacy for the treatment of diverse collections of tumors including low- and intermediate-risk prostate cancer confined to the prostate, mammary tumors, and soft tissue sarcomas. In most cases, optimized treatment regimens have not been worked out so that it is not known yet how useful this approach can be for superficial tumors of many different origins and some localized deeper tumors. Nor have optimized combination therapy strategies been developed combining heat, radiation, surgery, chemotherapy, and gene-based therapies. As companies raise the millions of dollars needed for the development and testing of these strategies, the clinical potential of nanogold/NIR therapy will become clearer. Hopefully, out of the myriad of nanogold constructs that have been reported on, a small number of most-effective constructs will emerge with the attendant investment and focus needed to have a positive patient impact.
3 Conclusion
While treatment of prostate tumors requires a surgically inserted optical fiber and the use of a laser, treatment of superficial tumors with nanogold and NIR could use the wIRA radiator instead of the laser that is used in a large fraction of preclinical studies. The wIRA radiator is portable and relatively inexpensive. If nanogold/NIR therapy is proven to be useful to treat a readily accessible human tumor, the use of the wIRA radiator used might make such therapy accessible to large numbers of patients in low- and middle-income countries that lack access to very high-tech expensive therapies. Foundations with a strong interest in bringing healthcare to low- and middle-income nations might take note of the potential benefits the wIRA radiator has to offer.
References
Khlebtsov B, Zharov V, Melnikov A, et al. Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology. 2006;17:5167–79.
Su KH, Wei QH, Zhang X, et al. Interparticle coupling effects on plasmon resonances of nanogold particles. Nano Lett. 2003;3:1087–90.
Souza GR, Christianson DR, Staquicini FI, et al. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Natl Acad Sci U S A. 2006;103:1215–20.
Nam J, Won N, Jin H, et al. pH-induced aggregation of gold nanoparticles for photothermal cancer. J Am Chem Soc. 2009;131:13639–45.
Hainfeld JF, O’Connor MJ, Lin P, et al. Infrared-transparent gold nanoparticles converted by tumors to infrared absorbers cure tumors in mice by photothermal therapy. PLoS. 2014;9:e88414.
Hainfeld JF, Lin L, Slatkin DN, et al. Gold nanoparticle hyperthermia reduces radiation dose. Nanomed: Nanotechnol Biol Med. 2014;10:1609–17.
Ahmad T, Sarwar R, Iqbal A, et al. Recent advances in combinatorial cancer therapy via multifunctionalized gold nanoparticles. Nanomedicine (Lond). 2020;15:1221–37.
Wang JI, Wu X, Shen P, et al. Applications of inorganic nanomaterials in Photothermal therapy based on combinational cancer treatment. Int J Nanomedicine. 2020;15:1903–14.
Rastinehad AR, Anastosa H, Wajswola E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A. 2019;116:18590–6.
Ali MRK, Ibrahim IM, Ali HR, et al. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int J Nanomedicine. 2016;11:4849–63.
Schuh EM, Portela R, Gardner HL, et al. Safety and efficacy of targeted hyperthermia treatment utilizing gold nanorod therapy in spontaneous canine neoplasia. BMC Vet Res. 2017;13:294.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Hainfeld, J.F., Smilowitz, H.M. (2022). Gold Nanoparticles and Infrared Heating: Use of wIRA Irradiation. In: Vaupel, P. (eds) Water-filtered Infrared A (wIRA) Irradiation. Springer, Cham. https://doi.org/10.1007/978-3-030-92880-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-92880-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92879-7
Online ISBN: 978-3-030-92880-3
eBook Packages: MedicineMedicine (R0)