Abstract
Clinical hyperthermia (i.e., heating of tumor tissue to 40–43 °C) is used in clinical oncology to enhance the therapeutic effect of chemotherapy and radiotherapy. Most recurrent breast cancer and melanoma lesions reach up to a few cm deep and can be effectively treated with currently available superficial hyperthermia devices using infrared or microwave radiation. Effective heating of more challenging and complex semi-superficial or semi-deep tumor lesions, including intact breast or lesions near silicone implants, requires dedicated treatment protocols. Herein, new treatment protocols are presented, which combine simultaneous and consecutive use of different wIRA, microwave, and radiofrequency hyperthermia devices. Examples are included, showing the clinical setup, applicator choice, and invasive and noninvasive thermometry.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Clinical results of hyperthermia treatment at 40–43 °C for 1 h applied in combination with chemotherapy and/or radiotherapy are excellent. The reported increase in tumor response for various tumor sites is in the order of 15–20%, without significant increase in radiotherapy or chemotherapy-related side effects [1,2,3]. An important application is superficial hyperthermia, as part of the treatment of recurrent breast cancer, chest wall recurrences, or melanoma. Clinical outcome is strongly correlated with the achieved tumor temperatures [4]. Achieving sufficiently high and uniform tumor temperatures of approximately 41–43 °C is therefore important, but at the same time excessive normal tissue temperatures exceeding approximately 43 °C must be avoided to reduce the risk of toxicity [5]. Treatment guidelines for superficial hyperthermia recommend achieving a median tumor temperature exceeding 41 °C and achieving temperatures exceeding 40 °C in 90% of the tumor volume [6].
Dedicated heating devices are available for specific tumor sites, e.g., deep-seated versus superficial tumor locations [7]. Tumor sites located close to the skin surface can be heated adequately with infrared (up to 1.5 cm depth) or microwave radiation (up to 4 cm depth) [8]. However, a different approach is required when the tumor size and shape are such that the entire target region cannot be covered adequately using a single technique. This issue occurs for instance for tumors with large surface areas with partially superficial and partially more deep-seated tumor and semi-deep-seated tumors, which start at the skin and extend deep, beyond 4 cm of depth. This chapter describes the approach used at Amsterdam UMC of treating the complete target region for challenging tumor geometries by sequential or simultaneous application of different hyperthermia devices, each covering another part of the target volume. Our clinical protocols and system combinations are presented, followed by two examples of clinical application of infrared combined with microwave or radiofrequency hyperthermia, including tumor temperatures measured at various depths to monitor and guide treatment quality.
2 Available Equipment for Different Tumor Depths
Superficial chest wall recurrences of breast cancer or melanoma generally extend a few cm down from the skin surface [6] and are treated with different suitable commercially available devices, including water-filtered Infrared-A (wIRA) applicators [9], 915 MHz antennas [10], and 434 MHz microstrip and lucite cone applicators [11]. When treating chest wall recurrences, the skin is always part of the target region, and thus, normal tissue and tumor tissue are heated to similar temperatures. Temperatures beyond 40 °C are required to ensure treatment quality [6], but at the same time temperatures should not exceed 43 °C to avoid thermal toxicity [5]. Therefore, the temperatures in the target volume are limited to a narrow 40–43 °C therapeutic temperature range.
Advantages of using infrared heating include the lack of need for a water bolus between applicator and skin, the ability to heat large and irregularly shaped body surfaces, and high-resolution skin surface temperature monitoring by using infrared cameras. This means that a comparatively fast workflow can be realized, combined with optional surface temperature control. Limitation is that therapeutic heating cannot be guaranteed beyond ≈1.5 cm depth.
More deep-seated lesions up to ca 2–3 or 4 cm depth are generally heated using 915 MHz or 434 MHz antennas, respectively. A water bolus containing temperature-controlled circulating deionized water (typically in the 40–43 °C range) is placed between the antenna and the skin to couple the energy into the skin. A slightly lower water temperature is chosen when a relatively large penetration depth is required. A lower, but still therapeutic, skin temperature permits increasing the amount of applied power to achieve deeper heat penetration up to ≈4 cm [12].
Even more deep-seated tumors, i.e., starting at the skin surface and extending more than 4 cm at depth, require antennas operating at 70–150 MHz to achieve sufficient penetration depth. Dedicated systems have been developed and applied for this category, consisting of either a single or two 70 MHz applicators [13, 14]. A challenge for semi-deep-seated tumors is the need to heat both the surface and at depth, which can create conflicting optimal system settings for each goal. Heating at depth requires the use of more aggressive surface cooling with temperatures below 40 °C to maximize penetration depth [12], thus potentially underdosing the skin surface.
Our department uses three different devices for recurrent breast cancer and melanoma, each representing device categories suitable for one of the aforementioned different tumor depths:
-
1.
The water-filtered infrared-A (wIRA) Hydrosun® 1500 system (Hydrosun Medizintechnik, Müllheim/Baden, Germany) combines a high color temperature halogen lamp (type HPL, Ushio, Tokyo, Japan) with water filtering, resulting in emission of IR-A radiation (wavelength 0.6–1.4 μm). This heating device has the advantage of being contact-free and suitable for more irregularly shaped and very large skin surfaces.
-
2.
Microstrip applicators operating at 434 MHz are available in 5 different sizes with largest aperture size of 20 × 30 cm (Medlogix, Rome, Italy; and Istok, Fryazino, Russia). A rubber water bolus integrated with the applicator is placed between applicator and patient skin to ensure that the emitted electromagnetic energy is coupled effectively into the patient. This water bolus is a plastic bag containing circulating distilled water to provide warming of the skin surface to the therapeutic range. Power is generated by the ALBA 4000 Double-ON system (Medlogix, Rome, Italy), which supports optional simultaneous use of two applicators to cover larger tumor surfaces.
-
3.
An in-house developed water-filled 70 MHz double waveguide system for semi-deep-seated breast tumors. Depending on the target size and location, waveguides with aperture sizes of 34 × 21 cm, 34 x 15 cm, or 34 x 8.5 cm can be selected [14]. A water bolus placed between waveguide and patient skin ensures that the emitted electromagnetic energy is coupled effectively into the patient. This water bolus contains circulating distilled water to provide either warming or cooling of the skin, depending on whether the skin is part of the tumor target or not. The generator system is an 8-channel DDS-based phase and amplitude-controlled RF generator system (SSB Electronic, Iserlohn, Germany) combined with 500 W solid-state 70 MHz amplifiers (Restek, Rome, Italy).
Single applicators or combinations of these three systems are deployed, depending on tumor size and depth. We use wIRA for different subgroups of patients: the first is patients with very widespread cancer en cuirasse type of disease, often spreading to both the ventral and dorsal sides of the patient. Consequently, patients are sometimes heated in seated position with wIRA-radiators on either side of the body to achieve simultaneous heating of the entire target region. A second application of wIRA is for patients with silicone breast prosthesis with tumor recurrence in the narrow strip of skin tissue overlaying the prosthesis. In both aforementioned applications, wIRA may be combined with the other two methods if some tumor sections are too deep-seated to be covered by wIRA. A third application of wIRA involves patients with semi-deep-seated tumors, for instance when an intact breast is the target region, including the skin surface and extending more than 4 cm deep into the breast. In that case, wIRA heating is applied for the skin, and a MW or RF antenna is used for the more deep-seated parts of the tumor. In case of combined use of devices, we select either simultaneous use during a single hyperthermia session, or separate hyperthermia sessions per device/region.
3 Temperature Control and Thermometry
Thermal monitoring during hyperthermia treatments is essential to ensure treatment quality. Amsterdam UMC uses extensive thermometry during hyperthermia treatment of recurrent breast cancer, which includes both noninvasive measurements on the skin surface and invasive intra-tumoral measurements. During RF or MW heating, skin temperatures are monitored using a number of 6–15 thermocouple probes, distributed evenly over the skin surface. Typically, an additional 1–3 probes are placed invasively (if possible) and 2–6 probes on scar tissue. The invasive thermometry catheters are in principle left in situ for the 3- to 4-week duration of the treatment series, unless contraindicated or at signs of inflammation. The Amsterdam UMC uses 7-sensor and 14-sensor copper constantan thermocouple probes (Volenec RD Inc., Hradec Králové, Czech Republic) with an outer diameter of 0.5 mm or 0.9 mm, respectively, and either 5 or 10 mm spacing between consecutive temperature sensors. An in-house developed 196-channel thermometry system is used to measure every 30 sec undisturbed temperatures during a short power-off interval of the RF or MW antenna [15]. During hyperthermia treatments with the wIRA system, skin temperature measurements are performed using an IR camera. The control program provides real-time temperature control by switching off and on the wIRA radiation to maintain the maximum skin temperature in a predefined range of 42–43 °C. When sections of the tumor extend beyond 1 cm depth, catheters are inserted for additional invasive thermometry, prior to the first hyperthermia session. If wIRA is used in conjunction with 434 MHz microstrip applicators, then the thermocouple probes are preferably placed in the direction perpendicular to the dominant field direction of the 434 MHz applicator to minimize the risk of picking up MW energy.
In more simple cases, implantation of thermometry catheters is performed by the staff at the Radiation Oncology Department. Implantation at more challenging locations, e.g., close to blood vessels, is performed under ultrasound guidance by an interventional radiologist. After implantation, the catheter position and insertion depth are determined using a CT scan. Information on the probe positions is important to assist the staff in interpreting temperature data during the hyperthermia sessions and perform adjustments in system settings when needed. A number of relevant thermal dose parameters are derived from the temperature data in accordance with QA guidelines [6]. Target temperatures during treatment are reported as T10, T50, and T90, i.e., the temperature at least achieved in 10%, 50%, and 90% of the target representative measurements during the steady-state period. The thermal dose parameters CEM43 T90 and CEM43 T50 can be derived, which represent the thermal dose given in equivalent number of minutes at 43 °C. This allows inter-comparison of treatment data.
4 Treatment Schedules
Patients with superficial chest wall recurrences of breast cancer are treated with radiotherapy and hyperthermia at Amsterdam UMC. The hyperthermia target volume follows the re-irradiation target volume, and this also applies in case of elective re-irradiation after local tumor resection. Different fractionation schedules are used. The standard schedule consists of 23 daily fractions of 2 Gy for radiotherapy, with hyperthermia given once a week, for 5 weeks, within one hour after radiotherapy that day. The latter is based on preclinical and clinical data, demonstrating better treatment outcome for shorter time intervals between radiotherapy and hyperthermia [16,17,18]. Two hyperthermia sessions are given each week in case parts of the target zone need to be heated in separate sessions, one session using wIRA for the most superficial part and one session using RF/MW antenna(s) for remaining deeper-seated parts of the tumor. Time between those two hyperthermia sessions is at least 48 h to prevent the occurrence of thermotolerance [19].
For frail and very elderly patients an alternative, hypofractionated 8 x 4 Gy schedule is used with radiotherapy twice a week and hyperthermia once a week, again within one hour after radiotherapy. For cancer en cuirasse patients, a hypofractionated 5 x 4 Gy schedule is used, with once a week radiotherapy and once a week hyperthermia. In this case, hyperthermia is given first, followed by radiotherapy aiming at an ultrashort time interval shorter than 5 min [9].
5 Clinical Application of wIRA Combined with Other Hyperthermia Devices
This section covers two clinical applications where wIRA is combined with RF or MW hyperthermia for tumor sections, which are too deep-seated to be covered by wIRA alone. The first case is a patient with a semi-deep breast tumor in an intact breast, and the second case is a patient with silicone breast prosthesis and a laterally extending semi-deep tumor lesion. Selection of RF versus MW depends on the heating depth required.
Case 1
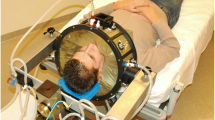
Semi-deep-seated tumor in an intact breast. A typical case for an intact breast is the target region as schematically illustrated in Fig. 7.1. Here, the target region starts at the skin surface and extends more than 4 cm deep into the breast, which requires the use of our 70 MHz system to reach the entire tumor. To heat the skin surface, we used the wIRA system (Fig. 7.1b), and to heat the deeper-seated part of the tumor, we used 70 MHz applicators with water bolus temperatures set to low temperatures (20–30 °C) to provide skin cooling (Fig. 7.1c). The wIRA and RF hyperthermia are applied in separate sessions.
(a) patient with a semi-deep-seated tumor in an intact breast. (b) the breast surface is treated with two wIRA applicators. (c) the more deep-seated tumor section is heated with one ventral (not shown) or a ventral and a dorsal 70 MHz applicator with bags with circulating cool water between antenna and skin. The blue dotted line indicates invasive thermometry present to guide the treatment
In a patient case described in [20] and illustrated in Fig. 7.1, hyperthermia treatment of a semi-deep-seated breast tumor extending 6 cm below the skin was performed using wIRA combined with a single 20x34 cm 70 MHz waveguide in five weekly sessions. The patient received 23 daily radiotherapy fractions of 2 Gy, with two weekly hyperthermia fractions, one for the superficial part and one for the deeper part of the tumor. Invasive thermometry was performed using a catheter placed at maximum tumor depth. Surface thermometry during wIRA sessions was performed using the IR camera. We achieved median tumor temperatures T50 between 40.8 °C and 42.2 °C measured at depth during the 70 MHz sessions and skin temperatures between 42 °C and 43 °C during the wIRA sessions [20]. Thus, therapeutic temperatures were achieved in the entire treatment volume by applying two different hyperthermia devices in two separate sessions, and the treatment was successful and resulted in complete tumor control.
Case 2
Tumor lesion near silicone implant. A 58-year-old patient with a retro-pectoral silicone breast prosthesis directly under the skin had a tumor lesion in the skin, which was extending laterally. The tumor depth was about 1 cm in the skin overlying the prosthesis and up to 4 cm in the lateral section. The patient was treated with 23 × 2 Gy plus hyperthermia. Simultaneous application of combined wIRA and microwave hyperthermia was applied. We heated the skin over the breast with the wIRA system and the lateral section of the tumor with a 434 MHz applicator, as shown in Fig. 7.2.
(a) A patient with tumor lesions in the skin overlying a silicone breast prosthesis and extending laterally. (b) Schematic setup for simultaneous wIRA heating for the breast, combined with a 434 MHz applicator for the more deep-seated lesions. Blue dotted line indicates invasive thermometry present to guide the treatment
Prior to treatment, an interventional radiologist placed three catheters for invasive thermometry close to the prosthesis edge at medial, cranial, and caudal locations with respect to the prosthesis, a fourth catheter was placed lateral to the prosthesis for measurement underneath the 434 MHz applicator as illustrated in Fig. 7.3. In addition, multiple thermocouple probes were placed on the skin just prior to each treatment session.
The catheters are left in situ for the duration of the treatment series, usually 3–5 weeks. After a treatment session, each catheter is covered with patches for hygiene as shown in Fig. 7.4.
The patient received four hyperthermia sessions of one hour each with simultaneous use of the wIRA system and a 434 MHz applicator. The part of the 434 MHz applicator that was in the field of view of the wIRA system was covered with a white towel to prevent IR temperature measurement artifacts: See Figs. 7.5 and 7.3.
Treatment was well-tolerated and recorded temperatures were in the therapeutic range. Median surface and invasive temperatures T50 for the area treated with the wIRA system ranged between 42.5 °C and 43 °C, and between 38.0 °C and 39.9 °C, respectively. Median surface and invasive temperatures T50 under the 434 MHz applicator ranged between 40.4 °C and 41.4 °C, and between 39.9 °C and 40.9 °C, respectively. The treatment was successful and resulted in complete tumor control.
6 Conclusions
Our results demonstrate that a good heating quality can be achieved for heating superficial versus deep subsections of a tumor, when using treatment protocols, which combine the use of wIRA, MW, and RF systems. These combination protocols were successfully applied for different challenging clinical cases, and they permit extending the range of tumor sites and sizes that can be adequately heated with existing hyperthermia devices.
References
Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015;31(6):609–14.
Datta NR, Gomez Ordonez S, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and−/ or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–53.
Peeken JC, Vaupel P, Combs SE. Integrating hyperthermia into modern radiation oncology: what evidence is necessary? Front Oncol. 2017;7:132.
Bakker A, van der Zee J, van Tienhoven G, Kok HP, Rasch CRN, Crezee H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review. Int J Hyperthermia. 2019;36(1):1024–39.
Bakker A, Kolff MW, Holman R, van Leeuwen CM, Korshuize-van Straten L, R. Oldenhof-de kroon et al., "Thermal skin damage during reirradiation and hyperthermia is time temperature dependent". Int J Radiat Oncol Biol Phys. 2017;98(2):392–9.
Trefná HD, Crezee H, Schmidt M, Marder D, Lamprecht U, Ehmann M, et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int J Hyperthermia. 2017;33(4):471–82.
Kok HP, Cressman ENK, Ceelen W, Brace CL, Ivkov R, Grüll H, et al. Heating technology for malignant tumors: a review. Int J Hyperthermia. 2020;37(1):711–41.
Dobšíček Trefná H, Crezee J, Schmidt M, Marder D, Lamprecht U, Ehmann M, et al. Atzelsberg research group quality assurance guidelines for the application of superficial hyperthermia clinical trials: II. Technical requirements for heating devices. Strahlenther Onkol. 2017;193(5):351–66.
Notter M, Piazena H, Vaupel P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: a retrospective study of 73 patients. Int J Hyperthermia. 2017;33(2):227–36.
Johnson JE, Neuman DG, Maccarini PF, Juang T, Stauffer PR, Turner P. Evaluation of a dual-arm Archimedean spiral array for microwave hyperthermia. Int J Hyperthermia. 2004;22(6):475–90.
Lamaitre G, van Dijk JD, Gelvich EA, Wiersma J, Schneider CJ. SAR characteristics of three types of contact flexible microstrip applicators for superficial hyperthermia. Int J Hyperthermia. 1996;12(2):255–69.
Van der Gaag ML, de Bruijne M, Samaras T, van der Zee J, van Rhoon GC. Development of a guideline for the water bolus temperature in superficial hyperthermia. Int J Hyperthermia. 2006;22(8):637–56.
Crezee J, Zweije R, Sijbrands J, Kok HP. Dedicated 70 MHz RF systems for hyperthermia of challenging tumour locations. Int J Microw Wirel Technol. 2020;12:839–47.
van Stam G, Kok HP, Hulshof MC, Kolff MW, van Tienhoven G, Sijbrands J, et al. A flexible 70MHz phase-controlled double waveguide system for hyperthermia treatment of superficial tumours with deep infiltration. Int J Hyperthermia. 2017;33(7):796–809.
De Leeuw AA, Crezee J, Lagendijk JJ. Temperature and SAR measurements in deep-body hyperthermia with thermocouple thermometry. Int J Hyperthermia. 1993;9(5):685–97.
Van Leeuwen CM, Oei AL, Chin KW, Crezee J, Bel A, Franken NA, et al. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat Oncol. 2017;12:75.
Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980;6(11):1507–17.
Crezee J, Oei AL, Franken NAP, Stalpers LJA, Kok HP. Response to: the impact of the time interval between radiation and hyperthermia on clinical outcome in patients with locally advanced cervical cancer. Front Oncol. 2020;10:528.
Overgaard J, Nielsen OS. The importance of thermotolerance for the clinical treatment with hyperthermia. Radiother Oncol. 1983;1(2):167–78.
Crezee J, Zweije R, Bakker A, van Tienhoven G, Kok HP. Combining 70MHz and 434MHz or wIRA hyperthermia applicators for optimal coverage of semi-deep tumour sites. In: 49th European Microwave Conference (EuMC). Paris: IEEE; 2019. p. 164–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Crezee, J. et al. (2022). Combined Use of wIRA and Microwave or Radiofrequency Hyperthermia. In: Vaupel, P. (eds) Water-filtered Infrared A (wIRA) Irradiation. Springer, Cham. https://doi.org/10.1007/978-3-030-92880-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-92880-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92879-7
Online ISBN: 978-3-030-92880-3
eBook Packages: MedicineMedicine (R0)