Abstract
Understanding the process of wood formation and its dynamics over the growing season is fundamental to interpret the isotopic signature of tree rings. Indeed, the isotopic signal recorded in wood does not only depend on the conditions influencing carbon, water, and nitrogen uptake in the leaves and roots, but also on how these elements are translocated to the stem and incorporated into the developing xylem. Depending on environmental conditions, tree developmental stage, and physiological status, wood formation dynamics can vary greatly and produce tree-ring structures carrying specific isotopic signatures. In this chapter, we present the physiological processes involved in wood formation, along with their relationships with anatomical, developmental, and environmental factors, to understand when and how photosynthetic assimilates are progressively incorporated into the forming xylem, creating the final isotopic signature of a tree ring. First, we review current knowledge on the structure and functions of wood. Then we describe the xylogenesis process (how and when the new xylem cells produced by the cambium develop through successive differentiation phases), and its relationships with physiological, developmental, and environmental factors. Finally, we explain the kinetics of xylem cell differentiation and show why the knowledge recently acquired in this field allows us to better understand the isotopic signals in tree rings.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Stable isotopes in tree rings represent an important source of information on past environmental conditions and tree functioning (McCarroll and Loader 2004). In a time of global changes, it is crucial to further exploit this valuable natural archive by acquiring a more detailed mechanistic understanding of the processes generating and transferring the isotopic signal into tree rings (Fig. 3.1). Questions regarding the acquisition, transfer, and fixation of the isotopic signal within the ring, in relation with the concurrent environmental factors and tree physiological status, are fundamental to link the source of the isotopic signal to its final destination in wood. Addressing such questions requires a detailed understanding of how tree rings are formed, which and how internal and external factors influence these processes (Fritts 1976; Schweingruber 1996), and how they eventually determine the intra-ring anatomical (Fonti et al. 2010) and isotopic profile (Gessler et al. 2014).
Basic organization of the tree stem and main fluxes affecting stable isotope composition in tree rings. Water absorption by roots and leaf water transpiration (blue arrows) are related to H and O isotope signals; atmospheric C uptake by leaves and reserve storage-mobilization (red arrows) are related to C and O isotope signals; nitrate and ammonium absorption by roots (green arrow) is related to N isotope signal. The transverse micro-section (bottom right) shows the relative position and age (double blue arrow) of developing and mature zones of xylem (left, inwards) and phloem (right, outwards) that are originated from periclinal divisions in the cambial zone (i.e. the cambium and surrounding non-differentiated derivatives)
These issues have been of concern to the scientific community for more than 50 years (Wilson 1970; Denne and Dodd 1981). However, it is only in the last two decades that the number of studies monitoring tree radial growth under both natural or experimental conditions has rapidly increased (De Micco et al. 2019). The approaches developed for the observations of wood growth included the monitoring of stem radial dimension using dendrometers (Kozlowski and Winget 1964; Zweifel 2016), the repeated marking of the cambium via the pinning technique (Gričar et al. 2007b, Yoshimura et al. 1981), and the repeated coring of the stem using a puncher (Rossi et al. 2006a). All these approaches have their strengths and weaknesses, and improvements are still needed. However, thanks to the possibility to assess the developmental stages of xylem cells, only microcoring currently allows to associate the dynamics of xylem cell formation with its anatomical structure and eventually with the tree-ring properties (Rathgeber et al. 2016).
The objective of this chapter is to review the current knowledge on the process of wood formation (i.e. xylogenesis) to better interpret the isotopic signature of tree rings. After reminding the structure and functions of the xylem (Sect. 3.2), we describe the mechanisms and pathways of xylem cell differentiation (Sect. 3.3). Finally, we review the current knowledge on the influence of the internal and environmental factors on the dynamics of tree-ring formation (Sect. 3.4), and the kinetics of xylem cell differentiation (Sect. 3.5), before to conclude on how this knowledge can help to better understand tree-ring isotopic signal (Sect. 3.6).
2 Wood Structure and Functions
Xylem performs three essential functions: (1) conduction of raw sap (i.e. soil water containing diluted phytohormones, mineral elements, and other nutrients) from roots to leaves (Sperry et al. 2008); (2) mechanical support (Fournier et al. 2006); and (3) transport and storage of non-structural carbohydrate and defence compounds (Kozlowski and Pallardy 1996). Xylem mainly consists of tracheids (in gymnosperms), and fibres and vessels (in angiosperms), which are elongated cells that die off at the end of their development to fulfil their functions. In mature xylem, these cells are characterized by a thick, rigid and impermeable wall, which delimits an empty lumen through which water can be transported or stored (Domec and Gartner 2002). Additionally, xylem is also composed of parenchyma cells, which are responsible for the storage and radial transport of reserve and defence compounds. They constitute the living part of mature xylem, displaying an active metabolism in their dense cytoplasm surrounded by a thin wall.
2.1 Xylem Anatomy
2.1.1 The Vascular Cambium
The cambium is composed of a thin layer of meristematic cells located between the xylem (i.e. the wood) and the phloem (i.e. the living bark) (Fig. 3.1), forming a continuous envelope surrounding the stems, branches, and roots of woody plants (Larson 1994; Lachaud et al. 1999; Evert 2006). The cambium produces xylem cells inwards (i.e. towards the pith), and phloem cells outwards (i.e. towards the bark). Cambial initials are surrounded by a thin primary wall (about 0.1–1.0 μm thick) and have a narrow diameter. They contain many small vacuoles (during the dormant period) or a large central vacuole (during the active period) (Prislan et al. 2013a). Two types of cambial initials can be found in the cambium: (1) the radial initials, which are short and isodiametric cells (about 40 μm in length and 5 µm in tangential and radial diameter); and (2) the fusiform initials, which are long, spindle-shaped cells (about 0.4–4.0 mm in length, 30 μm in tangential diameter, and 5 μm in radial diameter). Radial initials produce the radial elements of xylem and phloem (i.e. the elements that are arranged perpendicular to the stem axis in the direction of their length, such as the cells of the ray parenchyma). The fusiform initials are the most numerous (60–90%) and produce the longitudinal elements of xylem and phloem (i.e. the elements that are arranged parallel to the stem axis, e.g. tracheids, fibres, vessels and sieve tube elements; Larson 1994; Evert 2006).
2.1.2 Gymnosperm Xylem Cells
In gymnosperms (i.e. conifers or softwood species), xylem is relatively simple and homogeneous, mainly composed of two types of cells: (1) tracheids, which represent more than 90% of the total number of cells and fulfil both mechanical and water conduction functions; and (2) parenchyma cells, which are in charge of the storage and radial transport of various metabolic and defence compounds. Tracheids are elongated, spindle-shaped cells of 3–6 mm in length and 6–60 µm in diameter (Sperry et al. 2006). Tracheids are interconnected through pits, which facilitate water flow both vertically between overlapping tracheids and horizontally between contiguous tracheids. According to tracheid size, tree rings can be divided into earlywood—composed of large, thin-walled tracheids produced at the beginning of the growing season; and latewood—composed of narrow, thick-walled tracheids produced at the end of the season. In numerous species, resin ducts surrounded by living epithelial cells can appear scattered or arranged in radial or tangential bands. Wider tracheids are more efficient in transporting water but disputably more prone to cavitation (Pratt et al. 2007); whereas narrower thick‐walled tracheids provide most of the mechanical support but are less conductive (Chave et al. 2009; Cochard et al. 2004; Sperry et al. 2006; Tyree and Dixon 1986).
2.1.3 Angiosperm Xylem Cells
The more complex and heterogeneous xylem of angiosperms (i.e. hardwood species) is typically composed of vessels, fibres and parenchyma. Water conduction is mainly carried out by vessels, while fibres provide mechanical support. Parenchyma cells provide transport of metabolites and storage. Vessels are multicellular tubes up to 500 µm in width formed by dead vessel elements connected through perforation plates and assembled to form pipes (Zimmermann 1983; Brodribb et al. 2012). Depending on the arrangement of vessels along the ring, angiosperm wood may be classified as diffuse- or ring-porous. In diffuse-porous wood, all vessels are of similar size and are uniformly distributed along the ring. The ring-porous structure is characterized by large, highly-efficient vessels in the earlywood and smaller latewood vessels. In diffuse-porous trees, vessels range from 1 to 30 cm in length, and from 15 to 150 µm in diameter; in ring-porous trees, vessels range from 1 to 10 m in length, and from 15 to 300 µm in diameter (Zimmermann 1982). On the other hand, fibres are long and narrow tracheid-like cells with scarce pits. Some species can also present epithelial cells and secretory canals. Due to their size, ring-porous earlywood vessels are assumed to be functional for only one year because they are highly vulnerable to freezing- and drought-induced embolism (Cochard and Tyree 1990). On the other hand, small vessels are more resistant to cavitation and can potentially be refilled, and thus can be functional for several years (Sperry and Sullivan 1992). Similarly, diffuse-porous xylem usually remains functional for several years.
2.2 Xylem Cell Wall Structure and Composition
2.2.1 Xylem Cell Wall Composition
The wall of xylem cells consists of three main chemical components: cellulose, hemicelluloses and lignins. Overall, these components represent more than 90% of wood dry matter (Keegstra 2010). Pectins and a wide variety of proteins can also be found as minor components of xylem cell walls. Cellulose is the world’s most abundant organic compound and the key structural component of the cell wall (Mutwil et al. 2008). Cellulose is a long molecule, consisting of a chain of D-glucose units, which presents high tensile strength (Brett 2000). In the cell wall, cellulose is assembled to form long and strong macromolecules, the microfibrils. Hemicelluloses are small and more diverse branched molecules, which interconnect the cell wall components into a coherent whole (Scheller and Ulvskov 2010). Lignins are macromolecules resulting from a complex and heterogeneous assemblage of monolignol units (Freudenberg 1959).
2.2.2 Middle Lamella and Primary Cell Wall Structure and Composition
The first layer to be developed after cell division is called the middle lamella (ML). The ML is found between the wood cells and ensures the adhesion of a cell with its neighbours. The ML is only 0.5 to 1.5 µm thick and is principally made up of pectins (Fromm 2013).
Plant cells are surrounded by a primary wall (P), which regulates cell volume and content, defines and maintains the shape of the cell, stores nutrients and provides a protective barrier (Fagerstedt and Karkonen 2015). The primary cell wall is thin (approximately 1 µm), well-hydrated, flexible and extensible. In the early stages of xylem cell differentiation, P is principally composed of cellulose microfibrils (15–40% of the dry mass) linked via hemicellulosic tethers (20–30% of the dry mass) to form cellulose–hemicellulose networks, which are embedded in a pectin matrix (30–50% of the dry mass) and associated with structural proteins (1–10% of the dry mass) (O'Neill and York 2009).
In contrast, during xylem cell maturation, P and ML become highly lignified and combine to constitute what is conveniently termed the compound middle lamella (CML) (Dickson et al. 2017; Koch and Schmitt 2013). After the completion of secondary cell wall formation, the CML has generally a high lignin concentration, reaching for example 67% in poplar (Fromm 2013).
2.2.3 Secondary Cell Wall Structure and Composition
Unlike the primary wall, the secondary wall (S), which is located between the primary wall and the plasma membrane (PM), only surrounds specific cells, mainly those of woody plants (Evert 2006). Secondary cell walls represent the major constituent of wood and the largest biomass stock in terrestrial plants (Zhong and Ye 2009).
Secondary walls are thick (2–10 µm), poorly hydrated (approximately 30%), rigid and multi-layered structures. They are composed of cellulose (40–60% of dry mass), hemicellulose (10–40%), and lignin (15–35%) (Cosgrove and Jarvis 2012). Cellulose microfibrils, together with hemicellulose, form the main load‐bearing network, in which lignin is impregnated to form another cross-linked matrix ensuring hydrophobicity, rigidity and durability (Zhong and Ye 2009). Hence, with its greater thickness, the lignified secondary wall provides sufficient mechanical support for trees to grow vertically above the ground and cope with the pressures generated in the xylem due to water transport (Speck and Burgert 2011).
Secondary cell walls are commonly composed of three layers: S1 (external), S2 (medium), and S3 (internal). These three layers present similar compositions, but differ in their thickness and orientation of their cellulose microfibrils. Indeed, the thin S1 and S3 layers present transversally oriented microfibril angles (from 60° to 80° concerning the cell axis), while the thick S2 layer presents longitudinally oriented microfibril (5° to 30° concerning the cell axis) (Chaffey 2002; Fromm 2013; Plomion et al. 2001).
2.2.4 Consideration for Isotope Measurements
Isotope measurements of bulk wood could reflect proportional changes (over the growing season, or between years) of the three main components of the wood cell wall (cellulose, hemi-cellulose and lignin), which can carry different isotopic signatures. Because of different metabolic pathways, wood cellulose and lignin, for instance, differ by ca 3–4 ‰ in their carbon isotopes composition (Bowling et al. 2008; Wilson and Grinsted 1977). Differences in oxygen isotopes composition between cellulose and lignin are also expected. Indeed, a large proportion of the oxygen atoms present in lignin, for example, comes directly from molecular oxygen available at the site of synthesis, while the rest comes from the exchange with stem water (Barbour et al. 2002). In contrast, all oxygen atoms in cellulose molecules come from water—either from unenriched source water or from evaporation-enriched leaf water (Treydte et al. 2014).
Moreover, the lignin content of the bulk wood integrates the lignin concentration of the different layers of the cell wall and is therefore affected by the anatomical differences exhibited by tree rings, and in particular, by the thickness of the S2 layer. Knowing that the ratio between the thickness of layer S2 (which has a relatively low lignin content) and that of the middle lamella (which has a relatively high lignin content) varies according to the density of the wood (depending on the proportion of early- to late-wood in the ring, for example), tree rings of different densities will therefore exhibit different lignin contents, even if the lignin concentrations of the different cell wall layers are the same (Siddiqui 1976).
To avoid degrading the environmental signal with changing contributions of the different compounds over time, the use of a single component (generally cellulose) is often preferred for C, O and H isotope measurements (McCarroll and Loader 2004).
3 The Biological Basis of Wood Formation in Relation to Tree Development
Xylogenesis designates the process of xylem formation. During differentiation, the new xylem cells develop into mature and functional wood cells, by undergoing specific morphological and physiological transformations according to their final functions (Wilson 1970). This sequence of processes is common to both angiosperms and gymnosperms, but variations in duration and intensity of the differentiation phases, as well as molecular components, finally result in different cell types and tree-ring structures. Xylogenesis is represented by five successive stages of cell production and differentiation: (1) the division of a cambial cell that creates a new xylem daughter cell; (2) the enlargement of the newly formed xylem cell; (3) the deposition of cellulose and hemicellulose to build the secondary cell wall; (4) the impregnation of the primary and secondary cell walls with lignin; and (5) the programmed cell death (Wilson et al. 1966). Since secondary wall formation and lignification processes occur almost simultaneously, they are frequently grouped in one phase called cell wall thickening or cell maturation (Fig. 3.2). Parenchyma cells also follow this differentiation sequence but remain alive at maturity. After several years, parenchyma cells also die, while in the same area the last functional tracheids and vessels definitively lose their ability to conduct raw sap. This process is called heartwood formation (or duraminisation) and marks the transition from the sapwood—which is composed of alive, functional wood at the periphery of the tree; to the heartwood—which is composed of dead, non-functional wood at the core of the tree (Hillis 1987; Taylor et al. 2002).
The sequence of tracheid differentiation from cambial division to maturation and related internal and external drivers. Each stage represents a differentiating tracheid in the cross-section, indicating cambial division (green arrow), enlargement (pale blue arrows), secondary wall thickening (dark blue arrows), lignification (red arrows), and cell death (hydrolysis of the cell content). Cell wall thickening, wall lignification, and programmed cell death stages are frequently regrouped in one unique phase named wall thickening or cell maturation. The main environmental factors affecting tracheid differentiation (photoperiod, temperature, sugar availability, and water content) are ordered according to their relative importance. This general scheme is also valid for angiosperm vessels and fibres
3.1 The Successive Stages of Xylem Cell Differentiation
3.1.1 Cambial Cell Division
Cambial initials can divide in several directions responding to different needs. Anticlinal divisions occur along the tangential direction and allow the cambium to increase in circumference as the stem grows. Periclinal divisions occur along the radial direction and produce xylem and phloem mother cells (Lachaud et al. 1999). These cells are able to divide again to produce daughter cells that will either retain the characteristics and function of the mother cell (and thus continue to divide) or differentiate into xylem or phloem cells. In general, xylem mother cells divide more often than phloem ones, resulting in a much higher wood than bark production (Evert 2006; Gričar et al. 2009; Grillos and Smith 1959).
Cell division follows a highly ordered sequence of events called the cell cycle (Taiz and Zeiger 2010). A cell generally divides in the direction of its smallest dimension. However, during periclinal division, a cambial cell divides along its length. To facilitate division, fusiform cambial initials contain a large central vacuole filled with water and solutes that reduces the amount of material and energy required for cytoplasm biosynthesis in each mitosis (Lachaud et al. 1999; Cosgrove, 2000a). Nonetheless, the process of cell division is slow in the cambium, with a maximum of one cell being formed per day (Skene 1969; Larson 1994; Mellerowicz and Sundberg 2008; Cuny et al. 2012). To achieve high cell production rates, trees must therefore increase the number of cambial cell layers, which explains the close relationship between the number of cambial cells and xylem production (Cuny et al. 2012; Pérez-de-Lis et al. 2017).
3.1.2 Cell Enlargement
Cell enlargement is the first stage of wood cell differentiation and consists of an irreversible increase in cell volume. For xylem and phloem cells, cell enlargement only occurs radially. Yet, the radial diameter of xylem tracheary elements can be multiplied by 10 (for gymnosperms) to 100 (for angiosperms). As in cell division, this is achieved by filling the cell vacuoles with water and solutes (Cosgrove 2000a).
The solution inside a plant cell is more concentrated than the one outside. Water is hence attracted into the cell and exerts pressure (the osmotic pressure) on the outside of the cell membrane and primary wall. In equilibrium, the primary wall withstands this osmotic pressure, exerting pressure in the opposite direction (the turgor pressure or turgidity) (Schopfer 2006). However, during cell enlargement, the primary cell wall relaxes under the coordinated action of several enzymes, which break the bonds between its compounds, and of the osmotic pressure which exceeds its yield threshold (Cosgrove 2000b). Cell enlargement, as well as cell division, is regulated by phytohormones, primarily auxins but also cytokinins and gibberellins (Taiz and Zeiger, 2010). These hormones would all act by increasing the extensibility of the primary cell wall, but using many different control pathways.
The loosening of the wall leads to a relaxation of the cell wall stress, which in turn causes a decrease in turgor pressure, allowing water to enter the cell by osmosis, restoring the initial equilibrium between osmotic pressure and turgidity. However, the incoming flow of water causes a dilution of solutes and a reduction of the osmotic potential of the cell, which reduces its water absorption capacity and should thus lead to a rapid cessation of its enlargement. To overcome this problem, enlarging cells maintain a constant osmotic pressure by actively transporting and breaking down sucrose in glucose and fructose (Koch 2004). Regular cellular enlargement can thus be maintained for several hours or days (Schopfer 2006). However, this could not be possible without the unique combination of elasticity and rigidity of the primary wall, which can withstand distension while resisting the high mechanical forces imposed by turgor pressure (Cosgrove and Jarvis 2012). The thinning of the primary cell wall due to stretching is prevented by the concomitant deposition of newly synthesized wall material (Cosgrove 2000a). The cessation of wall expansion is generally irreversible and is typically accompanied by a reduction in wall extensibility as a result of the deposition of secondary wall layers (Cosgrove and Jarvis 2012).
3.1.3 Secondary Wall Deposition
The formation of the secondary wall begins with the deposition against the primary wall of a dense matrix of cellulose microfibrils associated with hemicelluloses, forming the S1 layer (Barnett 1981). The synthesis and transport of cellulose and hemicelluloses follow the same processes as in the formation of the primary wall. In the S1 layer, the microfibrils are almost perpendicular to the cell axis (i.e. parallel to the cross section); while in the S2 layer, they are deposited almost parallel to the cell axis (i.e. perpendicular to the cross section). The S3 layer is formed against S2 with a sudden reorientation of the microfibrils along a transverse helix (Fromm 2013). These sequential changes in the orientation of the microfibrils go hand in hand with the reorientation of the cytoskeleton microtubules (Chaffey 2002). This supports the hypothesis that microtubules control the orientation of cellulose microfibrils in the cell wall, which is responsible for secondary wall birefringence under polarized light, used to detect the wall-thickening process under the microscope (Chaffey 2002).
The secondary wall is not deposited along the whole cell wall surface but is absent around the pits. Pits thus form microscopic openings in the secondary wall, where the modified primary wall (the pit membrane) allows the passage of water and solutes from one cell to the next, making possible the upward flow of sap from the roots to the leaves and the lateral exchange of water and solutes between cells (Siau 1984; Zimmermann 1983; Zwieniecki and Holbrook 2000).
3.1.4 Lignification
Cell wall lignification is a complex process occurring exclusively in higher plants to strengthen the plant vascular body. Because of the timing of the lignification process and the composition and structure of the cell walls, the proportion of lignin decreases from the outermost (i.e. the middle lamella and the primary wall) to the innermost (i.e. the secondary wall) layers of the cell wall (Donaldson 1985; Donaldson and Baas 2019). The heavier impregnation of the middle lamella and primary wall allows xylem tracheary elements to stick together and form with fibres (if present) a strong, rigid, and self-supporting network of waterproof “pipes”. Inside the secondary cell wall, lignin proportions also vary greatly. The S2 layer is less lignified (at least in softwood tracheids; Donaldson 1985), whereas the S3 layer is highly lignified, providing tracheids and vessels a more hydrophobic surface lining, which is thought to facilitate water conduction (Donaldson 1987). Cell wall lignification starts at the cell corners, in the ML and the P, at about the same time as the deposition of the S1 layer, and extends along the ML and the P, before progressing inwards into the S following its deposition (Donaldson 2001). Recently, some authors have suggested that living, parenchymatic xylem cells can contribute to tracheary element lignification in a non-cell-autonomous manner, thus enabling the post-mortem lignification of these elements (Pesquet et al. 2013; Smith et al. 2013).
3.1.5 Programmed Cell Death
Programmed cell death, or apoptosis, marks the end of xylem cell differentiation and the advent of mature fully functional wood elements (e.g. vessels, tracheids). In the xylem, only parenchyma cells and, in some species, fibres possess living protoplasts at maturity (Evert 2006). The apoptosis process involves a highly controlled sequence (hence the term “programmed”) of events that induces a cell to kill itself. In xylem elements, the main trigger for programmed cell death is a regulated entry of calcium ions (Ca2+) into the cell, probably through the channels of the plasma membrane (Groover and Jones 1999; Jones 2001). Death then occurs rapidly (in a few minutes) with the cessation of the cytoplasmic streaming, the sudden rupture of the cell vacuole, and the release of hydrolases that degrade the cell organelles and clean up the cell content (Groover and Jones 1999; Bollhöner et al. 2012). After a few days, the cell consists of an empty space (the lumen) surrounded by a thick wall pierced with pits and, in the case of vessel elements, end openings (i.e. perforation plates).
3.2 Heartwood Formation
Wood conductive and storage functions are lost during heartwood formation, which involves the death of living parenchyma cells, the occlusion of the last remaining conductive elements by gums and tyloses, and the impregnation with secondary metabolites commonly referred as extractives (Evert 2006). These transformations increase the durability of heartwood by making it less attractive to decomposing organisms. The formation of heartwood also reduces the energy expenditure related to living cell maintenance by optimizing the volume of sapwood and thus minimizing its metabolic cost (Taylor et al. 2002).
Non-structural biochemical compounds (including polyphenols, tannins, oils, gums and resins) are responsible for heartwood formation. These extractives are synthesized by the living parenchyma cells at the sapwood edge from compounds available locally or brought from the outer sapwood or phloem, then transferred to the heartwood by passing from cell to cell through the pits. The extractives impregnate the cell walls in a way that resembles lignification, i.e. starting with the middle lamella and then continuing with the primary and secondary walls, and block the pits that connect adjacent cells, rendering them non-conductive (Kuroda et al. 2009).
Tyloses (bubble-shaped parenchyma protrusions entering the lumen of the vessels) are also produced during heartwood formation (De Micco et al. 2016a). Tyloses occlude the vessel lumens and prevent pathogens to spread through the vessel network. When the tylosis completes its expansion, wall materials, including cellulose, hemicelluloses, pectins, suberins and lignins, are deposited in the primary wall, rendering it impermeable and providing further defence against pathogens (Bamber 1976; Bamber and Fukazawa 1985).
The strong heterogeneity between heartwood and sapwood, regarding particularly organic compounds of different isotopic signatures, argues against creating any tree-ring stable isotope records based on whole wood when both sapwood and heartwood tree-rings are present. In such cases, cellulose extraction remains necessary (Weigt et al. 2015). Moreover, whereas radial movements of ions and light molecules are observed within sapwood, they are expected to be minimal in the heartwood, due to the absence of living cells (Ohashi et al. 2017). This may be particularly important to consider when studying N stable isotopes, since most of the N compounds in the sapwood are mobile molecules, seasonally fluctuating and eventually withdrawn from the parenchyma cells before their death (see Chap. 12 for more details).
3.3 Influence of Environmental Factors on Wood Formation Processes
Environmental factors affect wood formation processes (Fig. 3.2), either directly or indirectly through their effect on carbohydrate availability or hormone concentration and sensitivity (Buttò et al. 2019a). The role of environment on cambium division and cell enlargement has been the focus of many studies (Denne and Dodd 1981; Deslauriers et al. 2016, Rossi et al. 2016, 2008). However, the influence of environmental factors on the deposition and lignification of the secondary cell wall remains largely unexplored (Balducci et al. 2016; Cuny and Rathgeber 2016; Cuny et al. 2019; Denne and Dodd 1981).
Temperature exerts direct control on cambial cell division, most probably via the polymerization-depolymerization of the cytoskeleton microtubules, which play important roles in cell division and differentiation (Chaffey 2002). Microtubules are sensitive to temperature: while favourable temperatures allow their polymerization, chilling temperatures tend to disassemble them (Begum et al. 2012b, 2013). The increase of spring temperatures also promotes the enzymatic conversion of starch to soluble sugars, which can then supply the energy for the biosynthesis of the new cell walls (Begum et al. 2010, 2013). Besides, temperature plays a role in the expression of genes related to active auxin transport (Schrader et al. 2003) and influences the sensitivity and concentration of division- and enlargement-regulating hormones such as auxins, cytokinins, and gibberellins (Ursache et al. 2013). It also modulates wall extensibility and/or yield threshold, possibly controlling the final radial diameter of xylem cells. The lignification process has also been described as sensitive to temperature (Donaldson 2001), which is coherent with the observation of low lignin content in latewood cell walls formed under cold autumn conditions (Gindl et al. 2000; Piermattei et al. 2015).
Of course, water is needed to sustain cambial cell division and enlargement. Indeed, the cambium has been found to stop dividing under water deficit (most likely through the mediation of abscisic acid), probably to limit the number of cells remaining in differentiation without an adequate amount of water (Deslauriers et al. 2016). Moreover, since osmotic pressure is the ‘engine’ of cell wall expansion, water availability must undoubtedly influence cell growth. When a drought occurs, cell enlargement is physically and physiologically inhibited (Nonami and Boyer 2008), resulting in a rapid reduction of the diameter of the cells produced (Rossi et al. 2009b; Balducci et al. 2016). Cell enlargement is thus often depicted as the plant process the most sensitive to water stress (Hsiao 1973). Hence, decreasing water availability in summer has also been considered as one of the main triggers of the transition from the wide earlywood cells to the narrow latewood cells (Kramer 1964), while transient changes in water availability trigger the formation of intra-annual density fluctuations (Balzano et al. 2018). However, wall deposition and lignification appear to be less sensitive to water stress, so that carbon deposition may continue in forming wood even though radial increment stagnates (Carvalho et al. 2015).
Environmental factors can also trigger anatomical changes in mature wood. For instance, tyloses and gums can be formed in sapwood vessels as early as the second part of the growing season, after they become non-functional due to either frost- or drought-induced cavitation (De Micco et al. 2016a, Pérez-de-Lis et al. 2018).
4 Seasonal Dynamics of Wood Formation in Relation to Tree Phenology
Tree phenology concerns the particular sequence of developmental events occurring in each organ during the tree life span (Fig. 3.3). Since the canopy is easily observable, leaf phenology has been broadly studied, and it has been proved to be crucial in controlling the acquisition of carbon, the loss of water, and the nutrient cycling in trees (Delpierre et al. 2016). In contrast, the phenology of other organs, such as wood or fine roots, has received less attention, even though it also influences overall plant functioning and biochemical cycles. Phenology is a key determinant of tree fitness and species distribution that integrates the effects of genotype and environmental conditions (Chuine 2010; 2016). Over the last decades, plant phenology and its variation have gained particular importance because environmental changes have produced shifts in phenological events (Anderson et al. 2013) or mismatched synchronisms among species (Johnson et al. 2010). Whereas most of the studies concerning leaf phenology deal with deciduous angiosperms, the majority of research efforts on the phenology of wood formation have been conducted in conifers, mainly at high elevations and latitudes of the Northern Hemisphere. There are fewer data on deciduous angiosperms in the recent literature, and among them, the majority relate to diffuse-porous species.
Representation of tree phenology in northern-hemisphere temperate evergreen conifers and their main environmental cues. The annual course of primary growth in the canopy (upper left) includes both vegetative (flowering and seed maturation) and reproductive events (leaf development and bud set). The annual course of secondary growth in the stem (centre right) includes the main periods of xylem enlargement and maturation, cambial activity, and phloem enlargement. Primary growth in the root system (lower left) includes main periods of fine root production and mortality. Circles are divided into twelve sectors indicating the months of the year, while some relevant environmental cues affecting activity-dormancy cycles are located accordingly
Meristem activity underlies several biochemical processes inducing phases of development or maturation of the plant tissues to be renewed. These phases vary according to the organ, ranging from a few days in the primary meristems to several weeks in the secondary meristems (Delpierre et al. 2016; Wilson, 1970). Outside the tropics, the meristems for primary and secondary growth and reproduction follow alternating periods of activity and rest, according to the annual cycle of seasons. This is because most plant physiological processes occur when environmental factors (e.g. temperature, water availability, solar radiation) are favourable for growth and reproduction. The growing season, bounded by the phenological phases of growth and dormancy, represents a trade-off between environmental constraints and resource availability. In ecosystems with cold winters, the period of growth results from an optimization between frost risk and carbon gain (Chuine 2010). All overwintering tree tissues, which include leaves in evergreen species, undergo a process of cold acclimation to cope with winter freezing (Cavender-Bares et al. 2005). Cold acclimation implies several biochemical changes, such as intracellular accumulation of sugars and specialized proteins, allowing cells to prevent ice crystal formation, stabilize membranes, and maintain respiration (Cavender-Bares et al. 2005). The primary and secondary meristems further enter dormancy, a resting state under which no growth or tissue maturation is observed (Arora et al. 2003). Three main phases of cambium and bud dormancy have been identified: para-dormancy, endo-dormancy, and eco-dormancy. During paradormancy (late summer and autumn), growth is repressed by the influence of distant organs via the action of hormones (Horvath et al. 2003). During endodormancy (autumn to mid-winter), growth is inhibited by meristem internal factors, which are suppressed at the end of the period by environmental factors such as low temperatures (hereafter called chilling temperatures) and/or long days (photoperiod) (Little and Bonga 1974). During eco-dormancy (mid-winter to mid-spring), growth is only inhibited by environmental factors, ready to restart as soon as favourable conditions arise (Oribe and Kubo 1997).
4.1 The Phenology of Cambium and Xylem
Cambial activity follows the cycle of the seasons (Denne and Dodd 1981; Ladefoged 1952). In extra-tropical regions, the cambium is dormant during winter and active during summer (Delpierre et al. 2016; Wilson 1970), while in tropical regions it may rest during the dry season and be active during the wet season (Bosio et al. 2016). Annual growth rings and typical tree-ring structures, both result from these periodical changes in cambial activity (Evert 2006). In Mediterranean regions and other seasonally dry areas, one or several pauses in cambial activity can also occur during the growing season, causing intra-annual density fluctuations (IADF), also known as false rings (Balzano et al. 2018).
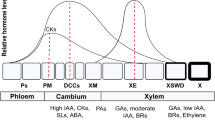
The key phenological events in the process of wood tissue formation are the beginning and cessation of cell division in the cambial zone (bD and cD, respectively), xylem cell enlargement (bE and cE, respectively), and cell-wall thickening (Rathgeber et al. 2011a). Cell-wall thickening starts with deposition of cellulose in the S1 layer (bT) and ends with the lignification of the S3 layer (cT). While bE and cE are proxies commonly used to compute the duration of cambial activity, bE and cT are commonly used to compute the duration of tree-ring formation (Rathgeber et al. 2016). This sequence of phenological events is structurally fixed, and the delays in the beginning and the end of successive wood formation phenophases are highly conserved among temperate and boreal tree species (Lupi et al. 2010; Rossi et al. 2012, 2013). Considering that the xylem cells produced by cambial division must undergo long differentiation processes, an earlier onset or a higher rate of cambial activity, both presumably associated with a higher number of cells produced along the growing season, would result in a later cessation of wood formation (Rathgeber et al. 2011b; Rossi et al. 2012).
4.1.1 Resting Cambium Versus Active Cambium
The inactive cambium is composed of a few layers of dormant cells (ca. 3–6) (Fig. 3.4a), while the active cambium is composed of numerous dividing cells (ca. 6–18) (Fig. 3.4b; Prislan et al. 2013a). During the growing season, new xylem cells, resulting from cambial cell division, are disposed along radial files, and successively undergo the differentiation program related to their identity and their place in the radial file (Fig. 3.4c). At the tissue level, the different stages of differentiation are well coordinated among all the radial files, creating a characteristic spatial pattern composed of strip-like developmental zones that remains rather stable throughout the growing season (Rathgeber et al. 2016).
Cross-sections of dormant a and active b cambial zones and the adjacent xylem and phloem tissues in Silver fir (Abies alba Mill.). c Schematic representation of developing xylem and phloem radial files in conifers, indicating the different zones of cell differentiation. Background colours delimit the different zones of xylem and phloem differentiation zones
4.1.2 Reactivation of Xylem Formation
At the beginning of the growing season, cambium reactivates and then begins to produce new phloem and xylem cells. Generally, a few over-wintering phloem cells also start to enlarge at about the same time, marking the beginning of stem radial growth and phloem formation (Prislan et al. 2013b). Some weeks after the start of cambial cell division, the derivatives begin radial enlargement, marking the onset of xylem radial growth and wood formation (Wilson 1970; Prislan et al. 2013a). At the end of enlargement, these first xylem cells enter the wall thickening phases (also called maturation phase). In ring-porous species, wall thickening finishes earlier in vessels and surrounding cells, while the neighbouring tissue matures later (Čufar et al. 2011). Because secondary walls hold most of the biomass, the appearance of the first thickening cells can be seen as the effective beginning of carbon sequestration into wood (Cuny et al. 2015). Finally, the first-formed xylem cells undergo programmed cell death and reach their final mature and functional state.
4.1.3 Cessation of Xylem Formation
In temperate and boreal forests, cambial activity and xylem radial growth rate generally peak around the summer solstice, when the photoperiod is maximal (Rossi et al. 2006b). This period generally marks the transition between early- and late-wood in both conifer and broadleaf species (Cuny et al. 2014; Pérez-de-Lis et al. 2017; Prislan et al. 2018). Cambial activity then starts to slow down until cessation, being soon followed by the end of cell enlargement (flagging the end of stem radial growth). However, the completion of wood formation (marking the end of carbon sequestration) occurs several weeks or months later, ranging from less than one month in diffuse-porous species as European beech (Prislan et al. 2013b, 2018), to more than two months in ring-porous species as white oaks (Lavrič et al. 2017; Pérez-de-Lis et al. 2017), and even more in some conifer species (Cuny et al. 2014; Rossi et al. 2016). Indeed, lignification is a slow process, so the last xylem cells need up to a couple of months to complete cell wall maturation and reach maturity (Cuny and Rathgeber 2016). In some species, cambial activity and cell differentiation can continue in winter if environmental conditions are favourable enough (Dickson et al. 2017; Vieira et al. 2014).
4.2 The Phenology of Leaves, Roots and Reserves
4.2.1 The Phenology of Leaves
In temperate and boreal regions, the vegetation period manifests strongly in spring with budburst (also called bud break), when leaves or needles emerge from the bud scales and is completed in autumn when deciduous trees avoid winter freezing via the senescence process that leads to leaf abscission (Fig. 3.3). New buds (either vegetative, reproductive, or compound) are formed after the cessation of primary shoot growth, from late summer to early autumn, depending on the species and the prevailing climate (Gyllenstrand et al. 2007; Rohde et al. 2011). Once formed, buds undergo dormancy from late summer to the following spring. While temperatures and photoperiod are the main cues of leaf phenophases (Basler and Körner 2014), drought may delay bud formation and leaf emergence and advance leaf senescence, although this effect seems to be species-specific (Ogaya and Penuelas 2004). Indeed, in regions with dry–wet seasonality, some tree species partially or completely shed their leaves during the dry season to limit canopy transpiration under increased water stress and vapour pressure deficits (Wright and Cornejo 1990).
4.2.2 The Phenology of Roots
The secondary growth of coarse roots has seldom been studied, but it follows the same formation processes as aboveground wood (Fig. 3.3). The timing, however, generally follows a shifted calendar, with later onsets and cessations resulting in shorter durations of the differentiation period compared to stem wood (Thibeault-Martel et al. 2008; Lemay et al. 2017). The phenological cycles of production and mortality of rootlets, the finest and apical parts of the root system, are generally studied for ensembles of root branching orders rather than individually. Although growth and mortality may occur simultaneously, the burst of rootlet growth mostly takes place in spring and early summer while mortality occurs in late summer and autumn (McCormack et al. 2014).
4.2.3 The Seasonal Cycles of Carbon and Nitrogen Reserves
Carbon (C) and nitrogen (N) reserves are key actors of the seasonal variability of tree functioning. In evergreen tree species, starch concentration peaks before budburst, with only slight variations in carbon storage throughout the rest of the growing season (Hoch et al. 2003). On the other hand, the growth of new organs induces a massive, short-distance mobilization of reserves in deciduous trees (Bazot et al. 2013), which typically results in a decrease in carbon reserve concentrations in the majority of tree organs (Barbaroux et al. 2003). However, photosynthesis by the new leaves causes a rapid increase in carbon reserves, which culminates at leaf senescence (El Zein et al. 2011). Leaf senescence and shedding have been observed to be concurrent with a major starch-to-sugar conversion in carbon pools in deciduous oaks, which is probably related to the acquisition of frost resistance before winter (Pérez-de-Lis et al. 2017). The amount of carbon reserves in deciduous species typically decreases during winter as a result of maintenance processes in perennial organs (Barbaroux and Bréda 2002).
In evergreen species, remobilization of nitrogen from older shoots and needles provides the necessary amount for the growth of newly formed tissues (Millard and Proe 1992). Fluctuations of nitrogen reserves for the rest of the year appear to be less variable than those of carbon reserves (Millard and Grelet 2010). In deciduous trees, nitrogen reserves are mobilised in spring to grow leaves, they remain then at low levels throughout the leafy season, until they increase due to the reabsorption of nutrients occurring during the leaf senescence processes in autumn (Millard and Grelet 2010; El Zein et al. 2011; Bazot et al. 2013). During winter, the level of variation in nitrogen reserves seems to be reduced compared to the level of variation in carbon reserves (El Zein et al. 2011).
4.3 Seasonal Dynamics of Wood Formation in Relation to Organ Phenology
The relationships between wood formation and other developmental processes can have a significant effect on tree functioning, including carbon and water fluxes and the allocation of resources (Fig. 3.3). Fine root elongation typically starts before budburst, both in angiosperms and conifers (Lyr and Hoffmann 1967; Konôpka et al. 2005). However, the sequence of wood and leaf phenology differs among tree functional types, affecting their resource-use strategies. For diffuse-porous tree-ring species (e.g. Fagus sylvatica) growth is known to begin just after budburst, while maximal growth rate occurs when the leaves are mature, so non-structural carbohydrate (NSC) content variations are low over the year. Thus, diffuse-porous species radial growth is directly related to leaf photosynthesis. On the other hand, for ring-porous species (e.g. Quercus petraea), earlywood quickly develops before budburst, depleting carbon reserves from April to June (Barbaroux and Bréda 2002). For conifers (e.g. Pinus sylvestris) growth often begins before new needles unfold, but the lack of NSC depletion during the growing season suggests that the substrates for radial growth are provided by previous-year needles (Michelot et al. 2012). Despite being species-specific, the timing of this wood-leaf phenology sequence is not fixed, and delays in the spring phenophases between wood and leaf development are observed from year to year (Takahashi and Koike 2014) and among individuals within species (Perrin et al. 2017).
4.3.1 Relationship Between Wood Formation and Tree Phenology in Gymnosperms
During winter, in temperate regions, xylem and phloem cells can be frozen because of low temperatures. However, xylem cells generally remain functional for several years, while phloem cells are functional for only one or two years maximum. While deciduous conifers overwinter without needles, evergreen conifers still carry several cohorts of needles from previous years, which can photosynthesize when the air temperature is above about −3 °C (Lundmark et al. 1998).
In spring, evergreen conifers start cambial activity early, generally producing new phloem cells first and new xylem cells afterwards. Budburst and new needle unfolding occur a couple of weeks later, at about the same time as shoot elongation (Rossi et al. 2009a; Cuny et al. 2012). Therefore, active cambium and sprouting buds are supplied principally with carbon originating from old needle cohorts. On the other hand, deciduous conifers have been reported to start cambial activity later than cohabiting evergreen conifers (Swidrak et al. 2014). In deciduous conifers, needle unfolding occurs rapidly after the onset of cambial activity, while shoot elongation starts approximately two weeks later, indicating that cambial reactivation relies on carbon reserves stored during the previous year (Rossi et al. 2009a).
At the beginning of the summer, the cambium of conifers produces phloem and xylem cells at a high pace using mainly new assimilates. The transition between the production of early- and late-wood cells occurs at about the same time as the termination of the needle and shoot lengthening (Rossi et al. 2009a; Cuny et al. 2012). Indeed, leaf maturation has long been suggested to be linked to the transition from early- to latewood (Larson 1994).
Cambial activity stops in late-summer or early-autumn, while xylem formation ceases about two months later when latewood is mature (Rossi et al. 2016). Needle abscission in deciduous conifers generally occurs after the end of wood maturation (Swidrak et al. 2014).
4.3.2 Relationships Between Wood Formation and Tree Phenology in Angiosperms
Because their large vulnerable vessels generally become non-functional within one-year, deciduous ring-porous species have to rebuild their earlywood vessel network each spring to supply raw sap to developing leaves (Cochard and Tyree 1990). Therefore, ring-porous angiosperms generally resume cambial activity before budburst (Pérez-De-Lis et al. 2016; Gričar et al. 2017; Lavrič et al. 2017), which appears to be synchronous with the onset of secondary wall deposition in developing vessels (Pérez-De-Lis et al. 2016; Fernández-de-Uña et al. 2018).
In contrast, the more resistant vessel network of diffuse-porous species can be functional for several years. This may explain why species belonging to this functional group resume cambial activity concurrently or shortly after budburst (Michelot et al. 2012; Prislan et al. 2013b, 2018). Therefore, while the earlywood of ring-porous species is formed using principally carbon reserves, diffuse-porous species earlywood is made of a mixture of carbon coming from previous-year reserves and more recent assimilates (Helle and Schleser 2004). A recent study shows that evergreen angiosperms rely nearly exclusively on recent assimilates to form their rings (Vincent-Barbaroux et al. 2019).
In summer, the cambium produces phloem and xylem cells using recent carbon assimilates from fully mature leaves, which are also used to refill the carbon storage pools (Michelot et al. 2012; Prislan et al. 2013b; Pérez-de-Lis et al. 2017). In ring-porous angiosperms, leaf maturation is followed by the onset of latewood formation, which is associated with an increase in wood formation rates (Pérez-de-Lis et al. 2017). A high carbon availability, already evoked for conifers, could account for the early-to-latewood transition, although it could be also attributed to a greater production of gibberellins by mature leaves, which are thought to promote the differentiation of latewood fibres (Buttò et al. 2019a).
In early-autumn, the cambium frequently stops producing new cells, while cessation of wood maturation occurs a few weeks later. Senescent leaves can already be seen in the canopy at that time, although most of the leaves will be shed in late autumn (Gričar et al. 2017; Lavrič et al. 2017).
In areas that experience a dry season, a cessation of cambial activity and yellowing of the leaves can be observed as early as early summer. However, in most of the Mediterranean species, the cambium can then resume activity in the autumn when the environmental conditions are more favourable, producing a false ring (Camarero et al. 2010; Pérez-de-Lis et al. 2017).
4.4 Influence of Environment on Seasonal Dynamics of Wood Formation and Tree Phenology
As described in Sect. 3.3.3, both external and internal factors control xylogenesis and are likely involved in the regulation of its timing. There is strong evidence that temperature has a primary role as an environmental driver for xylogenesis. In natural stands, the onset of cambial activity occurs within a relatively narrow range of daily minimum air temperature (from 2 to 7 °C) (Rossi et al. 2007, 2008, 2013, 2016), resulting in elevation gradients of cambium resumption (Moser et al. 2009; Prislan et al. 2013b; Saderi et al. 2019). Cambial activity can be reactivated during late winter by artificial heating of tree stems (Oribe et al. 2003; Begum et al. 2010). This artificial resumption of cambial activity can nevertheless only be triggered during the ecodormancy phase after the chilling requirement has been fulfilled during endodormancy (Begum et al. 2013). On the other hand, cessation of cambial activity in early autumn occurs at milder temperatures than those for spring resumption (between 5 to 13 °C for conifers) (Rossi et al. 2007, 2008, 2013, 2016), although it can be hastened by artificial cooling (Gričar et al. 2007a; Begum et al. 2012a). This equivocal role of temperature results in the absence of a clear elevation trend in the timing of the cessation of cambial activity (Moser et al. 2009; Prislan et al. 2013b; Saderi et al. 2019). These results demonstrate that cambial division is also driven by other cues such as photoperiod and water stress (Rathgeber et al. 2016). For instance, photoperiod is thought to modulate cambial division rates (Rossi et al. 2006b) and may act as a cue for the onset and cessation of cambial activity (Delpierre et al. 2019; Fernández-de-Uña et al. 2018; Saderi et al. 2019). Likewise, water stress hastens the cessation of cambial activity (Gričar and Čufar 2008; Gruber et al. 2010; Fernández-de-Uña et al. 2017; Saderi et al. 2019).
The close coordination between the phenology of the different organs of a tree suggests that environmental effects on cambial activity could also be indirect via the influence exerted by the environment on other organs, such as buds, leaves and roots. For instance, bud and leaf phenology is mainly under the control of temperature and photoperiod, to prevent frost damage (Chuine 2010). Several stresses, such as drought and mineral deficiencies, can also hasten primary growth cessation and leaf senescence (Delpierre et al. 2016). On the other hand, soil temperature regulates rootlet elongation. A few studies have suggested that rootlet growth can continue at a minimal rate during winter in evergreen trees, although it would not occur below a threshold of 2 to 4 °C (Schenker et al. 2014). Fine root growth may also be reduced or interrupted under drought conditions (Konôpka et al. 2005).
Phenological responses in distant tree organs are coordinated thanks to long-distance signalling mechanisms, which involve the vascular system (Notaguchi and Okamoto 2015). The phytohormones produced in a given organ can regulate the phenology of distant organs, raising the question of the autonomy of an organ phenology. For instance, auxins produced in expanding buds influence the rate of cambial divisions in the stem (Lachaud et al. 1999). However, the presence of auxins in overwintering tissues may decouple the onset of cambial division from the timing of bud elongation, as observed from stem heating experiments (Oribe et al. 2003; Begum et al. 2010). Nonetheless, the auxins produced in elongating buds are likely necessary for sustaining a high division rate in the newly activated cambium. Anyway, temperature remains a major cue for tree phenology because it controls both the break from ecodormancy in buds (hence, the production of auxins), the sensitivity of cambium to auxins, and the level of activity of the enzymes involved in all these different processes.
5 Kinetics of Tracheid Differentiation in Relation with Tree Physiology
A remarkable aspect of xylogenesis is its capacity to generate various wood forms in response to developmental or environmental constraints (Rowe and Speck 2005). Contrasting wood anatomy can thus be observed between different phylogenetic groups, but also between different individuals of the same group, between different organs inside the same individual, or during the ontogenic trajectory of the tree (Lachenbruch et al. 2011). Besides, wood anatomy is also known to change with environmental conditions either in space or in time (Jansen et al. 2004). Variations in wood anatomy may occur within a single tree ring, such as the transition from early- to late-wood in conifers and ring-porous species. Tracheid diameter, for example, is commonly divided by a factor of five from the beginning to the end of a tree ring in conifer species (Schweingruber 2007; Vaganov et al. 2006). This tree‐ring structure reflects structural and physiological trade‐offs that are important for tree functioning and performance, as seen in Sect. 3.3.1. These changes in tracheid and vessel morphology also drive many fundamental wood properties, including the ratio between early- and late-wood and the tree-ring density profile (Fig. 3.5; Rathgeber et al. 2006). Xylogenesis is the key process during which trees balance the aforesaid functional and structural trade-offs and fix them permanently in the wood tissue (Rathgeber et al. 2016). However, which and how climatic factors, in interaction with tree physiological state, and developmental control, influence xylem cell differentiation and the resulting tree-ring structure have still not been fully unravelled yet.
Kinetics of tracheid differentiation and resulting tree-ring structure in conifers. a Timing of cell differentiation phases (division, radial enlargement, and cell-wall thickening) in each successive tracheid along a tree-ring radial file. b Cell longevity and cell wall deposition rate. c Resulting tree-ring structure, with the evolution of the main anatomical parameters along with a scheme of the corresponding mature tree ring
5.1 From Wood Formation Dynamics to the Kinetics of Tracheid Differentiation
The kinetics of wood formation have been predominantly studied in conifer species because of their simpler wood structure, formed by tracheids sequentially arranged in rows perpendicular to the ring boundaries. During xylogenesis, cell enlargement and wall thickening are the two fundamental subprocesses that shape xylem cell dimensions and create the resulting tree-ring structure (Cuny et al. 2014). In his pioneering work, Skene (1969) set the framework for studying the kinetics of conifer tracheid differentiation: the final radial diameter of tracheids is the product of the duration and the rate of cell enlargement; whereas the final amount of secondary cell-wall is the product of the duration and the rate of wall material deposition. Cell wall thickness is thus the result of the total amount of wall material deposited for one cell, relative to its final size. Subsequent studies, however, focused mainly on the duration of the processes (cell enlargement and wall deposition) to explain the observed changes in cell features (cell diameter and wall thickness) along the ring, discounting the rates without assessing their importance (Wodzicki 1971; Skene 1972).
The complex inter-plays between the durations and the rates of xylogenesis subprocesses determine the changes in cell features (e.g. cell and lumen diameter, lumen area, and wall thickness) that, in turn, create the anatomical structure driving tree-ring density profile (Cuny et al. 2014). There is a positive relationship between the cell radial diameter and the duration of the enlargement phase in conifer tracheids (Cuny et al. 2014; Buttò et al. 2019b) and, to a lesser extent, also in angiosperm vessels (Pérez-de-Lis et al. 2016). Indeed, most of the changes in cell radial diameter are attributable to shifts in the duration of cell enlargement along the ring (Cuny et al. 2014; Buttò et al. 2019b). Cell radial diameter itself accounts for most of the changes in wall thickness, thus making the duration of cell enlargement also responsible for a great proportion of the variations in wood density along the ring, together with the variations in the duration and rates of cell-wall deposition, which contribute equally to cell-wall thickness (Cuny et al. 2014).
5.1.1 Relationship between Conifer Tree-Ring Structure and the Kinetics of Tracheid Differentiation
Wood formation monitoring studies reveal that a strong negative relationship links the duration and rate of cell-wall deposition for the majority of the cells of a ring, except for the very last latewood cells (Fig. 3.5; Cuny et al. 2014, 2019; Balducci et al. 2016; Cuny and Rathgeber 2016).
The duration of enlargement decreases progressively across a conifer ring by about two-thirds from the first to the last cells (Wodzicki 1971; Skene 1972; Cuny et al. 2014). This decrease is particularly abrupt in transition wood of species exhibiting contrasted tree-ring structures (e.g. Larix spp., Pinus sylvestris). Enlarging tracheids can take between one to four weeks to reach their final size at the beginning of the growing season, while final latewood cells may only take one week (Wodzicki 1971; Skene 1972; Rathgeber et al. 2011a; Cuny et al. 2014). In comparison, the rate of enlargement shows little variations along tree rings (Wodzicki 1971; Skene 1972; Cuny et al. 2014).
On the other hand, wall-thickening duration tends to increase along tree rings, although some species may show a decrease for the last tracheids (Cuny et al. 2014; Buttò et al. 2019b). Secondary wall formation may take two to three weeks for earlywood cells, and three to seven weeks for latewood cells. Conversely, cell-wall deposition rates tend to decrease in transition- and late-wood cells (Cuny et al. 2014).
5.1.2 Carbon Allocation Along the Ring and the Growing Season
Wall thickness changes have been related to the kinetics of wall deposition (Skene 1969; Wodzicki 1971), and the idea that wall thickness is mainly driven by the duration of wall-thickening is deeply anchored in the scientific literature. However, in opposition to this common belief, the results obtained by Cuny et al. (2014) revealed that the amount of material deposited per cell was almost constant along most of the ring, but decreased dramatically in the last 25% of the ring, reaching minimal values for the last latewood cells, where wood density was maximal. Because of this, changes in the amount of wall material per cell, and the kinetics of wall deposition, only explained 33% of the changes in wall thickness and 25% of the changes in wood density. In other words, more or less constant amounts of wall material are placed into smaller and smaller cell volumes, resulting in increasing wall thickness and wood density. Therefore, increasing cell wall thickness and wood density along tree rings do not reflect higher carbon allocation to woody biomass, but rather a downturn of secondary growth. Thus, the main driver of density changes along the ring is the cell enlargement process.
The same kinetic approach was also used to describe the dynamics of carbon allocation to the xylem (Cuny et al. 2015), showing that woody biomass production lags behind stem-girth increase by over one month in conifers from boreal, temperate, subalpine and Mediterranean regions (Fig. 3.6). In angiosperms, preliminary results show that there is also a time lag between growth in size and in biomass, but it is estimated to be only about two weeks in ring-porous species and probably even less in diffuse-porous species (Andrianantenaina et al. 2019). These time lags question the extension of the equivalence between stem size increase and woody biomass production at intra-annual time scales. They also suggest that these two growth processes exhibit differential sensitivities to local environmental conditions. Indeed, in well-watered sites, the seasonal dynamics of stem-girth increase matched the photoperiod cycle, whereas those of woody biomass production closely followed the seasonal course of temperature (Cuny et al. 2015).
Seasonal dynamics of stem-girth increase, xylem size increase and woody biomass production. a Stem-external radial variations. b Rate of xylem cell production through cambial cell divisions and the number of cambial cells (shading). c Rate of xylem size increase, with isolated contributions of cell production and cell enlargement, together with the number of enlarging cells (shading). d Rate of woody biomass production, which sums the carbon sequestered by wall thickening plus that from cell enlargement and cell production. Shading shows the number of wall thickening cells. Vertical blue and red dashed lines indicate time of maximal rates of xylem size increase and woody biomass production. The curves represent the average data for three sites in Vosges mountains (Northeast of France), including three species (Abies alba, Picea abies and Pinus silvestris) monitored during three years (2007–2009). Figure from Cuny et al. (2015)
5.2 Influence of Environmental Factors on the Kinetics of Wood Formation
In temperate forests, earlywood cells generally develop from mid-April to mid-July; transition-wood, from mid-June to mid-August; and latewood, from the beginning of July to mid-November (Cuny et al. 2014). Each cell in a tree ring experiences particular environmental conditions, potentially uniquely shaping its final dimensions, since each of its differentiation phases occurs within a specific time window. However, the links between tracheid or vessel size and environmental conditions during their formation are still largely unknown.
In conifers, for the majority of the cells along the ring, as the rate of cell wall deposition decreases, the duration of wall deposition increases in the same proportion (Balducci et al. 2016; Cuny and Rathgeber 2016; Cuny et al. 2019). Because of this ‘compensation effect’, the amount of wall material incorporated by the tracheids exhibits little change along a large part of tree rings (Cuny et al. 2014). Only in the second part of the latewood, the compensation effect decreases to become minimal for the very last tracheids (Cuny and Rathgeber 2016). In these last tracheids, variations in the rate of wall deposition result in equivalent variations in the wall cross-area. Because of the compensation effect, the influence of climate on the rate of wall deposition may not be translated into shifts in the amounts of deposited wall material during most of the growing season and thus, could not be recorded in the tree-ring structure, and probably only partially in wood isotopic composition. Indeed, Cuny and Rathgeber (2016) found that for cells presenting a strong compensation, influences of temperature on wall deposition rate were not traced in the wall cross area. Conversely, for cells presenting a weak compensation, highly significant, positive relationships were found between the wall cross area, the daily rate of wall deposition, and the temperature during the period of secondary wall formation (Cuny and Rathgeber 2016; Cuny et al. 2019). Such contrasting sensitivities of early- and late-wood cells to temperature confirm results from dendroclimatology showing that latewood conveys a much stronger climatic signal than earlywood (Wimmer and Grabner 2000). Thus, the lack of compensation for the last latewood cells appears as a clue to explain the supremacy of maximum latewood density as a proxy for past climatic conditions in general, and temperature in particular (Briffa et al. 2002a, b).
On the other hand, Cuny & Rathgeber (2016) observed a strong effect of day-length, but only on the duration of latewood cell enlargement. This absence of a clear climatic determinism of the decreasing tracheid diameter along the ring, a crucial determinant of the whole tree-ring structure, argues for a close control of the kinetics of wood cell differentiation. However, in Mediterranean regions, features such as intra-annual density fluctuations (IADF), show that strong variations of the water regime during the growing season can also break the ‘compensation effect’, and modify tracheid size and tree-ring structure (De Micco et al. 2016b; Zalloni et al. 2016). Indeed, in the first half of the growing season, the enlargement rate of xylem cell can drop to zero during a severe drought, creating a band of latewood-like cells in earlywood (Balducci et al. 2016). Conversely, in the second half of the growing season, when the cambium encounters exceptionally good water conditions, it may produce a band of earlywood-like cells in the latewood (Balzano et al. 2018).
6 How Wood Formation Monitoring Can Help to Better Understand Tree-Ring Isotopic Signal
The isotopic composition of tree rings integrates the specific isotopic signatures of the various compounds used to build xylem cell structures (e.g. cellulose, hemi-celluloses, lignins). The isotopic signature of these compounds is modulated by the physiological processes occurring during the synthesis and delivery of the substrates to the growing xylem (Gessler et al. 2014). We know that the isotopic signature of these substrates is well related to the environmental conditions occurring during their production by photosynthesis (Brandes et al. 2006). However, the relationships between the environmental conditions occurring during carbon assimilation, assimilate transport, xylem formation and the final isotopic signature of a tree ring is rather complex and not completely unravelled yet. For instance, at the beginning of the growing season, the utilization of carbohydrate reserves to build new xylem cells causes a mismatch between the time at which wood is formed and the time at which its carbon was assimilated. Therefore, the isotopic signal recorded in the earlywood could reflect previous-year environmental conditions rather than current spring conditions (Kagawa et al. 2006). Moreover, in this example, the isotopic signal would also be affected by the effect of post-photosynthetic fractionations (see Chap.13). The same considerations apply for the oxygen isotope signal. However, starch remobilization can lead in this case to extended exchange with the reaction water, potentially further diluting the original isotopic signal (Gessler et al. 2014).
High-resolution studies dividing tree rings into a variable number of sectors either manually (Treydte et al. 2014; Zeng et al. 2017) or with automated laser-based systems (Schulze et al. 2004; Schollaen et al. 2014) to provide intra-annual isotopic composition profiles of tree rings are becoming more and more frequent. Such studies not only help to more precisely identify external and endogenous factors driving the isotopic signature of bulk wood or cellulose along the growing season, but can also provide additional insight into the carbohydrate dynamics sustaining xylem cell growth (Helle and Schleser 2004; Ogée et al. 2009; Rinne et al. 2015; Belmecheri et al. 2018).
Intra-ring sectoring in isotope studies is conventionally done by assuming that each ring sector can be somehow attributed to a definite time window according to its position within the ring. This is the case of studies applying both regular (all the sectors exhibit the same width) and irregular (all the sectors do not have the same width, e.g. earlywood and latewood) sectoring. In this chapter, we showed that tree-ring formation is the result of a complex succession of developmental processes, which rates and durations vary along the growing season. Therefore, despite their relatively simple structure, not even conifer tree rings can be seen as a succession of cell rows formed during separate regular time intervals. Rather, the successive cell rows of a tree ring are formed in highly overlapping time intervals of variable durations (Fonti et al. 2018). We showed that, as we move from early- to late-wood, cells spend progressively more time in the wall thickening phase and less in the enlargement phase. A longer period of differentiation, along with higher rates of cell division, increase the amount of forming tissue that can be observed at a given day. Therefore, the amount of tissue in wall thickening is generally higher than that in enlargement zone, increasing along the growing season and with maximum values in summer. This scheme is even more complicated in angiosperms, due to their heterogeneous tree-ring structure and greater variety of cell types with contrasting maturation rates and lifespans. As a result, the association between intra-ring isotopic measurements (taken along several ring sectors) and the time window of formation cannot be accurately inferred by simply using their relative position along the tree ring. The accurate dating of intra-ring sectors requires a good understanding of wood formation processes, which can only be obtained through repeated observation of the developing xylem along the growing season. Therefore, monitoring wood formation is essential for disentangling high-resolution isotope signals stored in the cellulose and bulk wood of tree rings, as well as to assess the extent to which wood formation processes affect the recording of the isotopic signature.
References
Anderson DM, Mauk EM, Wahl ER, Morrill C, Wagner AJ, Easterling D, Rutishauser T (2013) Global warming in an independent record of the past 130 years. Geophys Res Lett 40(1):189–193
Andrianantenaina AN, Rathgeber CBK, Pérez-de-Lis G, Cuny H, Ruelle J (2019) Quantifying intra-annual dynamics of carbon sequestration in the forming wood: a novel histologic approach. Ann for Sci 76:1–12
Arora R, Rowland LJ, Tanino K (2003) Induction and release of bud dormancy in woody perennials: a science comes of age. HortScience 38:911–921
Balducci L, Cuny HE, Rathgeber CBK, Deslauriers A, Giovannelli A, Rossi S (2016) Compensatory mechanisms mitigate the effect of warming and drought on wood formation. Plant, Cell Environ 39:1338–1352
Balzano A, Čufar K, Battipaglia G, Merela M, Prislan P, Aronne G, De Micco V (2018) Xylogenesis reveals the genesis and ecological signal of IADFs in Pinus pinea L. And Arbutus unedo L. And. Annals of Botany 121:1231–1242
Bamber RK (1976) Heartwood, its function and formation. Wood Sci Technol 10:1–8
Bamber RK, Fukazawa K (1985) Sapwood and heartwood: a review. Forestry Abstracts 46:567–580
Barbaroux C, Bréda N (2002) Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 22:1201–1210
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615
Barbour MM, Walcroft AS, Farquhar GD (2002) Seasonal variation in δ13C and δ18O of cellulose from growth rings of Pinus radiata. Plant, Cell Environ 25:1483–1499
Barnett JR (1981) Secondary xylem cell development. In: Barnett JR (ed) Xylem cell development. Castle House Publications LTD, Tunbridge wells, pp 47–95
Basler D, Körner C (2014) Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiol 34:377–388
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees-Struct Funct 27:1023–1034
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010) Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Ann Bot 106:885–895
Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R (2013) Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol Plant 147:46–54
Begum S, Nakaba S, Yamagishi Y, Yamane K, Islam MA, Oribe Y, Ko JH, Jin HO, Funada R (2012) A rapid decrease in temperature induces latewood formation in artificially reactivated cambium of conifer stems. Ann Bot 110:875–885
Begum S, Shibagaki M, Furusawa O, Nakaba S, Yamagishi Y, Yoshimoto J, Jin HO, Sano Y, Funada R (2012) Cold stability of microtubules in wood-forming tissues of conifers during seasons of active and dormant cambium. Planta 235:165–179
Belmecheri S, Wright WE, Szejner P, Morino KA, Monson RK (2018) Carbon and oxygen isotope fractionations in tree rings reveal interactions between cambial phenology and seasonal climate. Plant, Cell Environ 41:2758–2772
Bollhöner B, Prestele J, Tuominen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63:1081–1094
Bosio F, Rossi S, Marcati CR (2016) Periodicity and environmental drivers of apical and lateral growth in a Cerrado woody species. Trees-Struct Funct 30:1495–1505
Bowling DR, Pataki DE, Randerson JT (2008) Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol 178(1):24–40
Brandes E, Kodama N, Whittaker K, Weston C, Rennenberg H, Keitel C, Adams MA, Gessler A (2006) Short-term variation in the isotopic composition of organic matter allocated from the leaves to the stem of Pinus sylvestris: effects of photosynthetic and postphotosynthetic carbon isotope fractionation. Glob Change Biol 12:1922–1939
Brett CT (2000) Cellulose microfibrils in plants: Biosynthesis, deposition, and integration into the cell wall. Int Rev Cytol 199:161–199
Briffa KR, Osborn TJ, Schweingruber FH, Jones PD, Shiyatov SG, Vaganov EA (2002) Tree-ring width and density data around the Northern Hemisphere: Part 1, local and regional climate signals. Holocene 12:737–757
Briffa KR, Osborn TJ, Schweingruber FH, Jones PD, Shiyatov SG, Vaganov EA (2002) Tree-ring width and density data around the Northern Hemisphere: part 2, spatio-temporal variability and associated climate patterns. Holocene 12:759–789
Brodribb TJ, Pittermann J, Coomes DA (2012) Elegance versus speed: examining the competition between conifer and angiosperm trees. Int J Plant Sci 173:673–694
Buttò V, Deslauriers A, Rossi S, Rozenberg P, Shishov V, Morin H (2019a) The role of plant hormones in tree-ring formation. Trees-Struct Funct
Buttò V, Rossi S, Deslauriers A, Morin H (2019) Is size an issue of time? Relationship between the duration of xylem development and cell traits. Ann Bot 123:1257–1265
Camarero JJ, Olano JM, Parras A (2010) Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol 185:471–480
Carvalho A, Nabais C, Vieira J, Rossi S, Campelo F (2015) Plastic response of tracheids in Pinus pinaster in a water-limited environment: adjusting lumen size instead of wall thickness. PLoS ONE 10:1–14
Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A (2005) Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of co-occurring Mediterranean oaks that differ in leaf lifespan. New Phytol 168:597–612
Chaffey NJ (ed) (2002) Wood formation in trees—cell and molecular biology techniques. Taylor and Francis, London, New York, pp 364
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Chuine I (2010) Why does phenology drive species distribution? Philos Trans R Soc B: Biol Sci 365:3149–3160
Cochard H, Froux F, Mayr S, Coutand C (2004) Xylem wall collapse in water-stressed pine needles. Plant Physiol 134:401–408
Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol 6:393–407
Cosgrove DJ (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38:109–124
Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407:321–326
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3(204):201–206
Čufar K, Cherubini M, Gričar J, Prislan P, Spina S, Romagnoli M (2011) Xylem and phloem formation in chestnut (Castanea sativa Mill.) during the 2008 growing season. Dendrochronologia 29:127–134
Cuny HE, Fonti P, Rathgeber CBK, von Arx G, Peters RL, Frank DC (2019) Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant, Cell Environ 42:1222–1232
Cuny HE, Rathgeber CBK (2016) Xylogenesis: coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiol 171:306–317
Cuny HE, Rathgeber CBK, Frank D, Fonti P, Fournier M (2014) Kinetics of tracheid development explain conifer tree-ring structure. New Phytol 203:1231–1241
Cuny HE, Rathgeber CBK, Frank D, Fonti P, Makinen H, Prislan P, Rossi S, Del Castillo EMEM, Campelo F, Vavrčík H et al (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nature Plants 1:1–6
Cuny HE, Rathgeber CBK, Lebourgeois F, Fortin M, Fournier M (2012) Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-east France. Tree Physiol 32:612–625
De Micco V, Balzano A, Wheeler EA, Baas P (2016) Tyloses and gums: a review of structure, function and occurence of vessel occlusions. IAWA J 37:186–205
De Micco V, Campelo F, De Luis M, Bräuning A, Grabner M, Battipaglia G, Cherubini P (2016) Intra-annual density fluctuations in tree rings: how, when, where, and why? IAWA J 37:232–259
De Micco V, Carrer M, Rathgeber CBK, Julio Camarero J, Voltas J, Cherubini P, Battipaglia G (2019) From xylogenesis to tree rings: wood traits to investigate tree response to environmental changes. IAWA J 40:155–182
Delpierre N, Lireux S, Hartig F, Camarero JJ, Cheaib A, Čufar K, Cuny H, Deslauriers A, Fonti P, Gričar J et al (2019) Chilling and forcing temperatures interact to predict the onset of wood formation in Northern Hemisphere conifers. Glob Change Biol 25:1089–1105
Delpierre N, Vitasse Y, Chuine I, Guillemot J, Bazot S, Rutishauser T, Rathgeber CBK (2016) Temperate and boreal forest tree phenology: from organ-scale processes to terrestrial ecosystem models. Ann for Sci 73:5–25
Denne MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Castle House Publications, Tunbridge Wells, pp 236–255
Deslauriers A, Huang JG, Balducci L, Beaulieu M, Rossi S (2016) The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol 170:2072–2084
Dickson A, Nanayakkara B, Sellier D, Meason D, Donaldson L, Brownlie R (2017) Fluorescence imaging of cambial zones to study wood formation in Pinus radiata D. Don. Trees-Struct Funct 31:479–490
Domec JC, Gartner BL (2002) How do water transport and water storage differ in coniferous earlywood and latewood? J Exp Bot 53(379):2369–2379
Donaldson LA (1985) Within- and between-tree variation in lignin concentration in the tracheid cell wall of Pinus radiata. NZ J Forest Sci 15:361–369
Donaldson LA (1987) S3 lignin concentration in radiata pine tracheids. Wood Sci Technol 21:227–234
Donaldson LA (2001) Lignification and lignin topochemistry—an ultrastructural view. Phytochem 859–873
Donaldson LA, Baas P (2019) Wood cell wall ultrastructure the key to understanding wood properties and behaviour. IAWA J 40(4):645–672
El Zein R, Maillard P, Bréda N, Marchand J, Montpied P, Gérant D (2011) Seasonal changes of C and N non-structural compounds in the stem sapwood of adult sessile oak and beech trees. Tree Physiol 31:843–854
Evert RF (2006) Esau's plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. John Wiley and Sons, pp 601
Fagerstedt K, Karkonen A (2015) The plant cell wall. J Integr Plant Biol 57(4):328–329
Fernández-de-Uña L, Aranda I, Rossi S, Fonti P, Canellas I, Gea-Izquierdo G (2018) Divergent phenological and leaf gas exchange strategies of two competing tree species drive contrasting responses to drought at their altitudinal boundary. Tree Physiol 38(8):1152–1165
Fernández-de-Uña L, Rossi S, Aranda I, Fonti P, González-González BD, Cañellas I, Gea-Izquierdo G (2017) Xylem and leaf functional adjustments to drought in Pinus sylvestris and Quercus pyrenaica at their elevational boundary. Front Plant Sci 8(1200):1201–1212
Fonti MV, Vaganov EA, Wirth C, Shashkin AV, Astrakhantseva NV, Schulze E-D (2018) Age-effect on intra-annual δ13C-variability within Scots Pine tree-rings from Central Siberia. For 9(364):1–14
Fonti P, Von Arx G, García-González I, Eilmann B, Sass-Klaassen U, Gärtner H, Eckstein D (2010) Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol 185:42–53
Fournier M, Moulia B, Stokes A, Coutand C, Fourcaud T (2006) Tree biomechanics and growth strategies in the context of forest functional ecology. In: Speck T, Rowe NP (eds) Herrel A. CRC Press, Ecology and biomechanics. a mechanical approach to the ecology of animals and plants. Boca Raton, pp 1–33
Freudenberg K (1959) Biosynthesis and constitution of lignin. Nature 183:1152–1155
Fritts HC (1976) Tree rings and climate. Academic Press, London, pp 582
Fromm J (2013) Xylem development in trees: from cambial division to mature wood cells. In: Fromm J (ed) Cellular aspect of wood formation. Springer-Verlag, Berlin, Heidelberg, New York, Dordrecht, London, pp 3–39
Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK (2014) Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 34:796–818
Gindl W, Grabner M, Wimmer R (2000) The influence of temperature on latewood lignin content in treeline Norway spruce compared with maximum density and ring width. Trees-Struct Funct 14:409–414
Gričar J, Čufar K (2008) Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought. Russ J Plant Physiol 55:538–543
Gričar J, Krže L, Čufar K (2009) Number of cells in xylem, phloem and dormant cambium in silver fir (Abies alba), in trees of different vitality. IAWA J 30:121–133
Gričar J, Lavrič M, Ferlan M, Vodnik D, Eler K (2017) Intra-annual leaf phenology, radial growth and structure of xylem and phloem in different tree parts of Quercus pubescens. Eur J Forest Res 136:625–637
Gričar J, Zupančič M, Čufar K, Oven P (2007) Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci Technol 41:463–475
Gričar J, Zupančič M, Čufar K, Oven P (2007) Wood formation in Norway Spruce (Picea abies) studied by pinning and intact tissue sampling method. Wood Res 52:1–10
Grillos SJ, Smith FH (1959) The secondary phloem of Douglas-fir. For Sci 5(4):377–388
Groover A, Jones AM (1999) Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119:375–384
Gruber A, Strobl S, Veit B, Oberhuber W (2010) Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol 30:490–501
Gyllenstrand N, Clapham D, Källman T, Lagercrantz U (2007) A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol 144:248–257
Helle G, Schleser GH (2004) Beyond CO2-fixation by Rubisco—an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant, Cell Environ 27:367–380
Hillis WE (1987) Heartwood and tree exudates. Springer-Verlag, Berlin, Heidelberg, pp 268
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant, Cell Environ 26:1067–1081
Horvath DP, Anderson JV, Chao WS, Foley ME (2003) Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8:534–540
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Jansen S, Baas P, Gasson P, Lens F, Smets E (2004) Variation in xylem structure from tropics to tundra: evidence from vestured pits. Proc Natl Acad Sci USA 101:8833–8837
Johnson DM, Büntgen U, Frank DC, Kausrud K, Haynes KJ, Liebhold AM, Esper J, Stenseth NC (2010) Climatic warming disrupts recurrent Alpine insect outbreaks. Proc Natl Acad Sci USA 107:20576–20581
Jones AM (2001) Programmed cell death in development and defense. Plant Physiol 125:94–97
Kagawa A, Sugimoto A, Maximov TC (2006) 13CO2 pulse-labelling of photoassimilates reveals carbon allocation within and between tree rings. Plant, Cell Environ 29:1571–1584
Keegstra K (2010) Plant cell walls. Plant Physiol 154:483–486
Koch G, Schmitt U (2013) Topochemical and electron microscopic analyses on the lignification of individual cell wall layers during wood formation and secondary changes. In: Fromm J (ed) Cellular aspect of wood formation. Springer-Verlag, Berlin, Heidelberg, New York, Dordrecht, London, pp 41–69
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Konôpka B, Yuste JC, Janssens IA, Ceulemans R (2005) Comparison of fine root dynamics in Scots pine and Pedunculate oak in sandy soil. Plant Soil 276:33–45
Kozlowski TT, Pallardy SG (1996) The physiological ecology of woody plants. Academic Press, San Diego, London, Boston, New York, Sydney, Tokyo, Toronto, pp 411
Kozlowski TT, Winget CH (1964) Diurnal and seasonal variation in radii of tree stems. Ecol 45(1):149–155
Kramer PJ (1964) The role of water in wood formation. In: Zimmermann MH (ed) The formation of wood in forest trees. Academic Press, New York, London, pp 519–532
Kuroda K, Yamashita K, Fujiwara T (2009) Cellular level observation of water loss and the refilling of tracheids in the xylem of Cryptomeria japonica during heartwood formation. Trees-Struct Funct 23:1163–1172
Lachaud S, Catesson AM, Bonnemain JL (1999) Structure and functions of the vascular cambium. Comptes Rendus de l'Academie des Sciences—Serie III, vol 322, pp 633–650
Lachenbruch B, Moore JR, Evans R (2011) Radial variation in wood structure and function in woody plants, and hypotheses for its occurrence. In: Meinzer FC, Lachenbruch B, Dawson TE (eds) Size- and age-related changes in tree structure and function. Springer, Dordrecht, pp 121–164
Ladefoged K (1952) The periodicity of wood formation. Kobenhavn: Det Kongelige danske videnskabernes selskab, pp 98
Larson PR (1994) The vascular cambium. Development and structure. Springer-Verlag, Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong, Barcelona, Budapest, pp 725
Lavrič M, Eler K, Ferlan M, Vodnik D, Gričar J (2017) Chronological sequence of leaf phenology, xylem and phloem formation and sap flow of Quercus pubescens from abandoned karst grasslands. Front Plant Sci 8:1–11
Lemay A, Krause C, Rossi S, Achim A (2017) Xylogenesis in stems and roots after thinning in the boreal forest of Quebec. Canada. Tree Physiol 37(11):1554–1563
Little CHA, Bonga JM (1974) Rest in cambium of Abies balsamea. Can J Bot 52(7):1723–1730
Lundmark T, Bergh J, Strand M, Koppel A (1998) Seasonal variation of maximum photochemical efficiency in boreal Norway spruce stands. Trees-Struct Funct 13:63–67
Lupi C, Morin H, Deslauriers A, Rossi S (2010) Xylem phenology and wood production: resolving the chicken-or-egg dilemma. Plant, Cell Environ 33:1721–1730
Lyr H, Hoffmann G (1967) Growth rates and growth periodicity of tree roots. Int Rev For Res 181–236
McCarroll D, Loader NJ (2004) Stable isotopes in tree rings. Quatern Sci Rev 23:771–801
McCormack LM, Adams TS, Smithwick EAH, Eissenstat DM (2014) Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecol 95:2224–2235
Mellerowicz EJ, Sundberg B (2008) Wood cell walls: biosynthesis, developmental dynamics and their implications for wood properties. Curr Opin Plant Biol 11:293–300
Michelot A, Simard S, Rathgeber C, Dufrêne E, Damesin C (2012) Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol 32:1033–1045
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30:1083–1095
Millard P, Proe MF (1992) Storage and internal cycling of nitrogen in relation to seasonal growth of Sitka spruce. Tree Physiol 10:45–48
Moser L, Fonti P, Büntgen U, Esper J, Luterbacher J, Franzen J, Frank D (2009) Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol 30:225–233
Mutwil M, Debolt S, Persson S (2008) Cellulose synthesis: a complex complex. Curr Opin Plant Biol 11:252–257
Nonami H, Boyer JS (2008) Turgor and growth at low water potentials. Plant Physiol 89:798–804
Notaguchi M, Okamoto S (2015) Dynamics of long-distance signaling via plant vascular tissues. Front Plant Sci 6:161
O'Neill MA, York WS (2009) The plant cell wall. Annu Plant Rev 1–44
Ogaya R, Penuelas J (2004) Phenological patterns of Quercus ilex, Phillyrea latifolia, and Arbutus unedo growing under a field experimental drought. Ecoscience 11:263–270
Ogée J, Barbour MM, Wingate L, Bert D, Bosc A, Stievenard M, Lambrot C, Pierre M, Bariac T, Loustau D et al (2009) A single-substrate model to interpret intra-annual stable isotope signals in tree-ring cellulose. Plant, Cell Environ 32:1071–1090
Ohashi S, Kuroda K, Takano T, Suzuki Y, Fujiwara T, Abe H, Kagawa A, Sugiyama M, Kubojima Y, Zhang C et al (2017) Temporal trends in 13C concentrations in the bark, sapwood, heartwood, and whole wood of four tree species in Japanese forests from 2011 to 2016. J Environ Radioact 178–179:335–342
Oribe Y, Funada R, Kubo T (2003) Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 17:185–192
Oribe Y, Kubo T (1997) Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol 17(2):81–87
Pérez-de-Lis G, Olano JM, Rozas V, Rossi S, Vázquez-Ruiz RA, García-González I (2017) Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Funct Ecol 31:592–603
Pérez-de-Lis G, Rossi S, Vázquez-Ruiz RA, Rozas V, García-González I (2016) Do changes in spring phenology affect earlywood vessels? Perspective from the xylogenesis monitoring of two sympatric ring-porous oaks. New Phytol 209:521–530
Pérez-de-Lis G, Rozas V, Vázquez-Ruiz RA, García-González I (2018) Do ring-porous oaks prioritize earlywood vessel efficiency over safety? Environmental effects on vessel diameter and tyloses formation. Agric for Meteorol 248:205–214
Perrin M, Rossi S, Isabel N (2017) Synchronisms between bud and cambium phenology in black spruce: Early-flushing provenances exhibit early xylem formation. Tree Physiol 37:593–603
Pesquet E, Zhang B, Gorzsas A, Puhakainen T, Serk H, Escamez S, Barbier O, Gerber L, Courtois-Moreau C, Alatalo E et al (2013) Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25(4):1314–1328
Piermattei A, Crivellaro A, Carrer M, Urbinati C (2015) The “blue ring”: anatomy and formation hypothesis of a new tree-ring anomaly in conifers. Trees-Struct Funct 29:613–620
Plomion C, Leprovost G, Stokes A (2001) Wood formation in trees. Plant Physiol 127(4):1513–1523
Pratt RB, Jacobsen AL, Ewers FW, Davis SD (2007) Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol 174:787–798
Prislan P, Čufar K, Koch G, Schmitt U, Gričar J (2013) Review of cellular and subcellular changes in the cambium. IAWA J 34:391–407
Prislan P, Gričar J, de Luis M, Smith KT, Čufar K (2013) Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric for Meteorol 180:142–151
Prislan P, Mrak P, Žnidaršič N, Štrus J, Humar M, Thaler N, Mrak T, Gričar J (2018) Intra-annual dynamics of phloem formation and ultrastructural changes in sieve tubes in Fagus sylvatica. Tree Physiol 39:262–274
Rathgeber CBK, Cuny HE, Fonti P (2016) Biological basis of tree-ring formation: a crash course. Front Plant Sci 7:734
Rathgeber CBK, Decoux V, Leban JM (2006) Linking intra-tree-ring wood density variations and tracheid anatomical characteristics in Douglas fir (Pseudotsuga menziesii (Mirb.) Franco). Ann for Sci 63:699–706
Rathgeber CBK, Longuetaud F, Mothe F, Cuny H, Le Moguédec G (2011) Phenology of wood formation: Data processing, analysis and visualisation using R (package CAVIAR). Dendrochronologia 29:139–149
Rathgeber CBK, Rossi S, Bontemps JD (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438
Rinne KT, Saurer M, Kirdyanov AV, Loader NJ, Bryukhanova MV, Werner RA, Siegwolf RTW (2015) The relationship between needle sugar carbon isotope ratios and tree rings of larch in Siberia. Tree Physiol 35:1192–1205
Rohde A, Bastien C, Boerjan W (2011) Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiol 31:472–482
Rossi S, Anfodillo T, Čufar K, Cuny HE, Deslauriers A, Fonti P, Frank D, Gričar J, Gruber A, Huang JG et al (2016) Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob Change Biol 22:3804–3813
Rossi S, Anfodillo T, Cufar K, Cuny HE, Deslauriers A, Fonti P, Frank D, Gricar J, Gruber A, King GM et al (2013) A meta-analysis of cambiumphenology and growth: linear and non-linear patterns in conifers of the northern hemisphere. Ann Bot 112:1911–1920
Rossi S, Deslauriers A, Anfodillo T (2006) Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J 27:383–394
Rossi S, Deslauriers A, Anfodillo T, Carraro V (2007) Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152:1–12
Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M (2006) Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol 170:301–310
Rossi S, Deslauriers A, Griçar J, Seo JW, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707
Rossi S, Morin H, Deslauriers A (2012) Causes and correlations in cambium phenology: towards an integrated framework of xylogenesis. J Exp Bot 63:2117–2126
Rossi S, Rathgeber CBK, Deslauriers A (2009) Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann for Sci 66:206
Rossi S, Simard S, Rathgeber CBK, Deslauriers A, De Zan C (2009b) Effects of a 20-day-long dry period on cambial and apical meristem growth in Abies balsamea seedlings. Trees-Struct Funct 23:85–93
Rowe N, Speck T (2005) Plant growth forms: an ecological and evolutionary perspective. New Phytol 166:61–72
Saderi S, Rathgeber CBK, Rozenberg P, Fournier M (2019) Phenology of wood formation in larch (Larix decidua Mill.) trees growing along a 1000-m elevation gradient in the French Southern Alps. Ann for Sci 76:1–17
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Schenker G, Lenz A, Körner C, Hoch G (2014) Physiological minimum temperatures for root growth in seven common European broad-leaved tree species. Tree Physiol 34:302–313
Schollaen K, Heinrich I, Helle G (2014) UV-laser-based microscopic dissection of tree rings—a novel sampling tool for δ13C and δ18O studies. New Phytol 201:1045–1055
Schopfer P (2006) Biomechanics of plant growth. Am J Bot 93:1415–1425
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100:10096–10101
Schulze B, Wirth C, Linke P, Brand WA, Kuhlmann I, Horna V, Schulze ED (2004) Laser ablation-combustion-GC-IRMS—a new method for online analysis of intra-annual variation of δ13C in tree rings. Tree Physiol 24:1193–1201
Schweingruber FH (1996) Tree rings and environment. Dendroecology. Berne, Stuttgart, Vienna: Haupt Paul, Swiss federal institute for forest, snow and landscape research, WSL/FNP, Birmensdorf, pp 609
Schweingruber FH (2007) Wood structure and environment. Springer-Verlag, Berlin, Heidelberg
Siau JF (1984) Transport processes in wood. Springer, Berlin, Heidelberg, New York, Tokyo, pp 245
Siddiqui KM (1976) Relationship between cell wall morphology and chemical composition of earlywood and latewood in two coniferous species. Pak J For 26:21–34
Skene DS (1969) The period of time taken by cambial derivatives to grow and differentiate into Tracheids in Pinus radiata. Ann Bot 33:253–262
Skene DS (1972) The kinetics of tracheid development in Tsuga canadensis Carr. and its relation to tree vigour. Ann Bot 36:179–187
Smith RA, Schuetz M, Roach M, Mansfield SD, Ellis B, Samuels L (2013) Neighboring parenchyma cells contribute to Arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. Plant Cell 25:3988–3999
Speck T, Burgert I (2011) Plant stems: functional design and mechanics. Annu Rev Mater Res 41:169–193
Sperry JS, Hacke UG, Pittermann J (2006) Size and function in conifer tracheids and angiosperm vessels. Am J Bot 93:1490–1500
Sperry JS, Meinzer FC, McCulloh KA (2008) Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant, Cell Environ 31:632–645
Sperry JS, Sullivan JEM (1992) Xylem embolism in response to freeze-thaw cycles and water-stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol 100(2):605–613
Swidrak I, Gruber A, Oberhuber W (2014) Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees-Struct Funct 28:1161–1171
Taiz L, Zeiger E (2010) Plant Physiology. Sinauer Associates Inc., Sunderland, pp 782
Takahashi K, Koike S (2014) Altitudinal differences in bud burst and onset and cessation of cambial activity of four subalpine tree species. Landsc Ecol Eng 10:349–354
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability—a review. Wood Fiber Sci 34:587–611
Thibeault-Martel M, Krause C, Morin H, Rossi S (2008) Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Ann Bot 102:667–674
Treydte K, Boda S, Graf Pannatier E, Fonti P, Frank D, Ullrich B, Saurer M, Siegwolf R, Battipaglia G, Werner W et al (2014) Seasonal transfer of oxygen isotopes from precipitation and soil to the tree ring: source water versus needle water enrichment. New Phytol 202:772–783
Tyree MT, Dixon MA (1986) Water-stress induced cavitation and embolism in some woody-plants. Physiol Plant 66(3):397–405
Ursache R, Nieminen K, Helariutta Y (2013) Genetic and hormonal regulation of cambial development. Physiol Plant 147:36–45
Vaganov EA, Hughes MK, Shashkin AV (2006) Growth dynamics of conifer tree rings: images of past and future environments. Springer-Verlag, Berlin, Heidelberg, pp 351
Vieira J, Rossi S, Campelo F, Freitas H, Nabais C (2014) Xylogenesis of Pinus pinaster under a Mediterranean climate. Ann for Sci 71(1):71–80
Vincent-Barbaroux C, Berveiller D, Lelarge-Trouverie C, Maia R, Máguas C, Pereira J, Chaves MM, Damesin C (2019) Carbon-use strategies in stem radial growth of two oak species, one Temperate deciduous and one Mediterranean evergreen: what can be inferred from seasonal variations in the δ13C of the current year ring? Tree Physiol 39:1329–1341
Weigt RB, Bräunlich S, Zimmermann L, Saurer M, Grams TEE, Dietrich HP, Siegwolf RTW, Nikolova PS (2015) Comparison of δ18O and δ13C values between tree-ring whole wood and cellulose in five species growing under two different site conditions. Rapid Commun Mass Spectrom 29:2233–2244
Wilson AT, Grinsted MJ (1977) 12C/13C in cellulose and lignin as palaeothermometers. Nature 265(5590):133–135
Wilson JW (1970) The growing tree. The University of Massachusetts Press, Amherst, pp 152
Wilson JW, Wodzicki T, Zahner R (1966) Differentiation of cambial derivatives: proposed terminology. For Sci 12:438–440
Wimmer R, Grabner M (2000) A comparison of tree-ring features in Picea abies as correlated with climate. IAWA J 21:403–416
Wodzicki TJ (1971) Mechanism of xylem differentiation in Pinus silvestris L. J Exp Bot 22:670–687
Wright SJ, Cornejo FH (1990) Seasonal drought and leaf fall in a tropical forest. Ecol 71:1165–1175
Yoshimura K, Hayashi S, Takao I, Shimaji K (1981) Studies on the improvement of the pinning method for marking xylem growth I. Minute examination of pin marks in Taeda Pine and other species. Wood Res 67:1–16
Zalloni E, de Luis M, Campelo F, Novak K, De Micco V, Di Filippo A, Vieira J, Nabais C, Rozas V, Battipaglia G (2016) Climatic signals from intra-annual density fluctuation frequency in Mediterranean pines at a regional scale. Front Plant Sci 7:1–11
Zeng X, Liu X, Treydte K, Evans MN, Wang W, An W, Sun W, Xu G, Wu G, Zhang X (2017) Climate signals in tree-ring δ18O and δ13C from southeastern Tibet: insights from observations and forward modelling of intra- to interdecadal variability. New Phytol 216:1104–1118
Zhong R, Ye Z-H (2009) Secondary cell walls. Encycl Life Sci 1–9
Zimmermann MH (1982) Functional xylem anatomy of angiosperm trees. Baas, P. Springer, New perspectives in wood anatomy. Forestry sciences. Dordrecht, pp 59–70
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer, Berlin, Heidelberg, New York, Tokyo, pp 146
Zweifel R (2016) Radial stem variations—a source of tree physiological information not fully exploited yet. Plant Cell & Environ 39(2):231–232
Zwieniecki MA, Holbrook NM (2000) Bordered pit structure and vessel wall surface properties. Implications for embolism repair. Plant Physiol 123 (3):1015–1020
Acknowledgements
The authors would like to thank Henri Cuny for preparing Fig. 3.5 and Daniel Epron for his comments on the final version of the manuscript. Some of the ideas and materials presented in this chapter were developed with the support from the French National Research Agency (through ANR‐11‐LABX‐0002‐01, Lab of Excellence ARBRE), the Swiss National Science Foundation (projects INTEGRAL-121859, CLIMWOOD‐160077, and LOTFOR‐150205), the Canada Foundation for Innovation, the ‘Consortium de Recherche sur la Forêt Boréale Commerciale’, the ‘Fonds de Recherche sur la Nature et les Technologies du Québec’, and the ‘Forêt d’Enseignement et de Recherche de Simoncouche’.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Rathgeber, C.B.K. et al. (2022). Anatomical, Developmental and Physiological Bases of Tree-Ring Formation in Relation to Environmental Factors. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)