Abstract
Nutritive, but detrimental if at high levels, several nitrogen (N) forms involved in air and soil biogeochemical reactions constitute the N load trees assimilate. Although a large body of literature describes series of tree-ring N isotopes (δ15N) as archival systems for environmental changes, several questions relative to the isotopic integrity and reproducibility of trends still linger in the dendroisotopist community. This chapter reviews the fundamentals of forest N cycling and examines trees as N receptors in their very position, at the interface between the atmosphere and pedosphere. The related scrutiny of intrinsic and extrinsic mechanisms regulating isotopic changes also underlines flaws and forces of tree-ring δ15N series as environmental indicators.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Key nutrient for trees, but forming reactive molecules potentially detrimental to forest ecosystems (e.g., Etzold et al. 2020), N constitutes a central object of research in terrestrial biogeochemistry. After several decades, the substantial body of literature on N in trees reflects the complexity of N cycling through trees, and how some intrinsic and extrinsic processes remain elusive. With anthropogenic emissions of reactive N (Nr) rising globally and driving atmosphere-pedosphere exchanges that can perturb the external terrestrial N cycle, tree-ring δ15N series may record past changes in forest-N cycling.
Studies of long tree-ring δ15N series are rare, largely because ring wood includes very low amounts of N relative to carbon, evidently making tree rings difficult for isotopic determination. Additionally, N translocates between trunk rings, dampening environmental isotopic effects in time series. Nevertheless, several studies report δ15N trends interpreted in relations to changes in soil and air conditions.
How does N assimilation in non N2-fixing trees operate? Do trees react to changes in air and soil Nr contents? Can tree-ring δ15N series help understand environmental changes? The purpose of this review primarily consists in scrutinizing the current understanding of mechanisms responsible for determining δ15N values in tree rings, appraising the type of information δ15N series can provide, and synthesizing the knowledge gaps of this research domain.
2 Sample Preparation and Analytical Procedures
The habitual mechanical separation of tree rings from stem samples using fine blades or microtome at the sought time resolution produces wood sub-samples for δ15N analysis. Treating these sub-samples prior to their isotopic analysis generates a debate regarding the utility of removing their mobile N (resins) to prevent producing false trends. But recent investigations suggest this type of pre-treatment does not modify significantly the final δ15N values (Elhani et al. 2005; Bukata and Kyser 2007; Couto-Vázquez and González-Prieto 2010; Caceres et al. 2011; Doucet et al. 2011; Tomlinson et al. 2014). Another observation arguing against pre-treatment is that samples from several species, for instance Pinus ponderosa, Fagus grandifolia and Picea rubens, show no change of concentrations after resin removal (Hart and Classen 2003; Doucet et al. 2011).
On another note, regardless of pre-treating wood samples or not, several studies have clearly shown trends of higher N concentrations in rings (and coniferous leaves) grown during sampling years, relative to concentrations in previous years. The general pattern forms an increasing trend from the heartwood-sapwood transition to the most recent ring; a physiological effect typical of N translocation. In addition, tree-ring N concentrations show poor inter-tree and inter-species coherence. These observations make N concentrations in tree rings (and dated coniferous leaves) useless in environmental research (Hart and Classen 2003; Saurer et al. 2004; Savard et al. 2009; Gerhart and McLauchlan 2014). However, this inter-ring N mobility does not seem to affect the final tree-ring δ15N values (Doucet et al. 2011).
For isotopic analysis, wood samples wrapped in tin capsules drop automatically from a carousel into an elemental analyser (EA) in continuous flow (CF) with an isotope ratio mass spectrometer (IRMS). The analytical procedure involves combustion in a reaction tube producing N2O, followed by a reduction to N2, which produces the analyses calibrated relative to air N2 (set at 0‰). Tree-ring wood harbors very low concentrations relative to roots or leaves (Scarascia-Mugnozza et al. 2000), and high C/N ratios, making its isotopic analysis difficult. For that reason, the EA-CF IRMS system for δ15N analysis needs close monitoring for performing complete combustion to prevent CO+ derived interferences at masses 28 and 29. A CO trap installed between ovens and GC columns helps for that step. The low N concentrations in wood make internal standards a requirement to avoid poor analytical accuracy from low peak to background ratios (Couto-Vázquez and González-Prieto 2010). Inserting several internal wood standards in sample batches allows monitoring the external precision and accuracy of the complete laboratory procedure. Whole wood materials from three species of trees recently proposed as references may also support this essential task (Qi et al. 2016), although the δ15N range they cover is narrower (−2.4 to +1.8‰) than the natural extent in tree rings (generally between −10 and +5‰).
3 Assimilation, Storage and Fractionation of Nitrogen by Trees
Numerous tree-ring studies dealing with natural δ15N values or 15N-labelled N assume that uptake of soil inorganic N dominates the N assimilated in stems of non N2-fixing trees. However, other means such as soil organic N assimilation and foliar uptake of various atmospheric N forms may significantly contribute to the N loads commonly characterized for δ15N values (Fig. 12.1). This section discusses the knowledge gains from controlled experiments, studies under natural conditions, recent developments in understanding the ultimate source and pathways of N to tree rings, and the role of N remobilization in determining the tree-ring δ15N values.
Representation of the forest nitrogen cycle. Processes influencing the bioavailability of N forms taken up by boreal and temperate trees are included; NO2 loss is significant mostly in wetlands and tropical settings; the tropical cycle would include N2 fixation by trees (not shown). EcM stand for ectomycorrhiza (Sect. 12.3.2)
3.1 Nitrogen through Foliage
Many studies reveal that soil fertilization has direct impacts on foliar N characteristics, however, leaves also assimilate N (e.g., Gebauer and Schulze 1991; Arain et al. 2006; Pardo et al. 2007; Vizoso et al. 2008; Balster et al. 2009; Averill and Finzi 2011). Similarly, articles specifically addressing canopy functions report operational foliar uptake from air for all atmospheric N forms (Garten and Hanson 1990; Rennenberg and Gessler 1999; Krupa 2003; Sparks et al. 2003; Vallano and Sparks 2007; Chaparro-Suarez et al. 2011). In other words, it is widely accepted now that the foliar N loads come from soil as well as from air (e.g., Vallano and Sparks 2013). For its nutritive functions, leaf N plays a crucial role in enhancing activities of Rubisco, the proteins of photosynthesis (Warren et al. 2003; Wright et al. 2004). However, higher atmospheric N availability does not always translate into higher growth rates of stems. The crucial point for the present chapter lies with estimating the atmospheric foliar N contribution to the loads in stems of deciduous and coniferous trees, as atmospheric N transferred from leaves to stems may have a direct impact on the tree-ring δ15N series.
Atmospheric Nr-forms include N in ammonia gases (NH4+, NH3), oxides (NO3−, NO2, NO), nitric acid (HNO3), and organic compounds (amino acids, peroxyacetyl nitratePAN). These N-forms get to ground through wet scavenging or dry deposition upon contact with surfaces such as leaves. The N forms enter leaves either as wet or dry (gaseous) phases through stomata, although the liquid phases appear to pass in the foliar system more readily (Rennenberg and Gessler 1999; Harrison et al. 2000b; Krupa 2003; Choi et al. 2005; Gerhart and McLauchlan 2014). A series of enzymatic reactions transform NH4+ and NO3− into amino acids, which generally enriches the reactants and depletes the products in 15N (e.g., Rennenberg and Gessler 1999). Once incorporated in organic compounds within leaves, N shortly resides in active and non-active parts (Millard and Grelet 2010). Experiments using 15N-labelled N show that the remobilized N can reach down to the root systems (Macklon et al. 1996; Rennenberg and Gessler 1999; Bazot et al. 2016).
Studies rarely quantify stem N originating from foliar uptake. In one known experimental example, the estimated proportions of N from previous-year needles exported to support the growth of shoots vary between 10 and 37% in 5 year-old or younger coniferous trees (Millard and Grelet 2010). Otherwise, in 30-year-old spruce trees, between 8 and 22% of the annual N demands come from leaves, the range depending on the N forms selected for experiments (Harrison et al. 2000a, and references therein). Also, natural abundance of 15N has helped estimating foliar assimilation at 10% in 10- to 20 year-old Norway spruce trees, given that the signal of car exhaust, the single local source of anthropogenic NO2 emissions, was known to strongly deviate from the natural N sources (Ammann et al. 1999). However, in general, the precise quantification of anthropogenic N in the canopy constitutes a complex task because the isotopic signals of N in air can significantly change in space and time, and an array of emitters show overlapping δ15N ranges (e.g., Savard et al. 2017).
In deciduous specimens, the proportion of canopy N uptake used up for annual wood production appears to vary between <5 to >40% (Harrison et al. 2000b). In the case of young poplars exposed to NO2-enriched air with low δ15N values, and grown on high and low N-supplied soils, the calculated foliar contributions were 14 and 18% of the total amount of plant N, respectively, based on δ15N measurements of plant material and the known isotopic signal of NO2 (Siegwolf et al. 2001). In another example, with labelled fertilizers applied at both the foliage and root levels of oak trees, soil N and internal storage contributed 60 and 40%, respectively, to the N of spring leaves (Bazot et al. 2016). Whereas the total autumn root N reserves included 73% from leaves and 27% from soils. At the broad scale, modeling studies reported the canopy to contribute between 3 and 16% of the total N demands for new growth in plants (Vallano and Sparks 2007, and references therein). Thus, on one hand, N in leaves comes partly from soils, and several studies clearly demonstrated partial remobilization of this N. On the other hand, the estimated contributions from leaves to the demands of trees may be more variable than the range covered in the literature because they largely depend on the atmospheric concentrations and the involved N-forms, the studied tree species, and the methodology selected for quantifying the foliar uptake/contribution.
Although trees acquire atmospheric N directly through leaves and without intermediate transformation steps as through soils before root uptake, the overall influence foliar uptake has on the tree-ring N loads is difficult to determine. To our knowledge, research efforts never estimated its contribution to stem N loads of mature trees. Accordingly, the remaining key questions regarding foliar uptake does not relate to its assessment but to the magnitude of its contribution in determining the final tree-ring δ15N values.
3.2 From Soils through Roots to the Stems
3.2.1 Soil Nitrogen Species and Processes
Dinitrogen-fixing microbes and forest organic matter represent the ultimate sources of N in soils of non-disturbed forests (Fig. 12.1). Geological N from rocks and minerals can provide a background influencing the forest N cycling and the overall δ15N values of organic soils, particularly if developed over clay-rich mineral horizons or sedimentary rocks, which generally have high δ15N values (Holloway and Dahlgren 2002; Craine et al. 2015; Houlton et al. 2018). Variability in the distribution of geological N contributes to the heterogeneity of soil N properties. Several studies also report evidence for microbial communities (fungi and bacteria) involved in mineral weathering (e.g., Courty et al. 2010).
Even though the absolute amount of N in soils is large, the dominant proportion of N is immobilized (carbon bound) in organic matter (Knicker 2004; Näsholm et al. 2009), with a small part of this matter available for nutrition (labile N or dissolved organic N—DON). The main N-rich constituents of DON, amino acids, derive from rapid hydrolysis of soil proteins (Näsholm et al. 2009). Furthermore, the soil inorganic (NH4+ and NO3−) parts, largely derived from organic matter constitute only about 1% of the total soil N (Kendall et al. 2007). Forest N demands generally exceed the inputs in bioavailable N forms, limiting the net productivity in most of the boreal and temperate forests. As a consequence, trees compete for soil NH4+, NO3− and DON. Anthropogenic N emissions can add to the regional N loads by wet or dry deposition, and enter the series of transformations leading to the bioavailable N pool mined by tree roots (Fig. 12.1).
The main transformation processes affecting the concentration and δ15N values of inorganic and organic bioavailable N in soils consist in fixation, immobilization, ammonification (mineralization), volatilization, nitrification and denitrification (Hopkins et al. 1998), and the rates of these N transformations vary seasonally and regionally (Handley et al. 1998). Whereas fixation and ammonification generally create minor isotopic N fractionation, volatilization of NH4+, nitrification and denitrification tend to significantly increase the 15N content in the reacting substrates and decrease it in the products (Högberg 1997; Pardo and Nadelhoffer 2010; Hobbie and Högberg 2012). Consequence to the interplaying N transformation processes, nitrate, ammonium and DON generally show δ15N values in increasing order. In addition, in N-limited forests, δ15N values of bulk N tend to increase with sample depth, and its concentration, to decrease (Fig. 12.2a). The general explanations for this pattern are that low nitrification rates and leaching from top horizons depletes the components of the organic horizons in 15N. Shedding of leaves depleted in 15N relative to soil and preferential uptake of 15N by fungi associated to roots (see Sect. 12.3.2.2) may accentuate this pattern (Hobbie and Colpaert 2002; Compton et al. 2007; Högberg et al. 2011). However, in less N-limited forests, the top horizons may show high δ15N values in soil N-species due to increased rates of nitrification in the N- and organic-rich horizons (Fig. 12.2b; Högberg 1997; Mayor et al. 2012; Shi et al. 2014).
Hence, the overall N status of the forest, the proportions of the various soil N forms trees use up, and the depth of root N uptake from the soil all have direct influence on the final δ15N values of tree tissues. A key point to note here is that isotopic studies rarely characterize root (or tree-ring) samples along with individual bioavailable soil N forms, even though this combination would greatly help determining fractionation steps before N uptake by trees.
3.2.2 Direct and Ectomycorrhizal Root Uptake
Trees can use up inorganic N forms and DON directly through their roots (Näsholm et al. 2009; Averill and Finzi 2011). This direct uptake by physical transport shows no evidence of fractionation; N isotopic fractionation occurs during assimilation processes involving enzymatic functions (Handley et al. 1998; Pardo et al. 2013). Alternatively, trees can gain N (and other nutrients) while providing C, through symbiotic associations with fungi (ectomycorrhiza EcM; e.g., Näsholm et al. 2009; Courty et al. 2010; Lilleskov et al. 2019). It is well accepted that EcM generally show higher δ15N values relative to N sources in soils, and to roots and stems of trees. In the process of N uptake, they preferentially incorporate the heavy 15N during the production of their tissues, and provide light N to their hosts (Gebauer and Taylor 1999; Hobbie and Högberg 2012). The extent of this biogenic fractionation and thus the isotopic values of fungi vary widely (Trudell et al. 2004; Mayor et al. 2009; Hobbie and Högberg 2012), inasmuch as different EcM communities may efficiently assimilate specific soil N-compounds. Also, it is established that EcM communities change in structure and abundance under varying soil chemistry (pH), N deposition, N transformation rates, and climatic conditions (Chalot et al. 1995; Wallander et al. 1997; Qian et al. 1998; Schulze et al. 2000; Lilleskov et al. 2002; Averill and Finzi 2011; Högberg et al. 2011; Kjoller et al. 2012; Kluber et al. 2012; van der Linde et al. 2018). The role of EcM in regulating δ15N values in plants during N assimilation in field conditions is illustrated by the measured δ15N patterns in Alaskan trees, EcM and soils (Hobbie et al. 1999). Modeling of these results indicates a net fractionation during the N transfer from EcM to trees.
Another example compares the foliar δ15N values of Acer rubrum seedlings from seven sites distributed along a gradient of atmospheric NO2, with active EcM or manipulated absence of EcM in native soils of New York state (Vallano and Sparks 2013). The foliar δ15N results for seedlings devoid of EcM show no influence of increasing N, but for EcM seedlings, they correlate significantly with ambient NO2 levels, indicating the aid EcM provides to trees for N assimilation. These examples and the above observations make EcM causative agents for changes in tree-ring (and foliar) δ15N series, a key point for understanding the overall δ15N values of N transferred from soils to trees. However, the inventory of responses and functionalities of EcM communities under various environmental conditions, particularly the extent of their isotopic fractionation and implication during N uptake by roots, is not yet comprehensive. Research in that domain could help elucidate the causes of shifts in tree-ring δ15N series.
3.2.3 Preference of Trees for Soil N Species
Most trees absorb NH4+ and NO3−, but experiments conducted using fertilization with 15N-labelled N demonstrated that various species of trees show improved performances if grown with a specific soil N form (Kronzucker et al. 1997; Zhang et al. 2016; Miller and Hawkins 2007). The relative preferences for specific N forms mostly derive from the energy requirement for the production of proteins and the needed level of carboxylates (Arnold and van Diest 1991). Many species of deciduous trees take up NO3− preferentially (e.g., Quercus alba, Fagus grandifolia), whereas it is well established that most coniferous trees lacking the enzyme nitrate reductase assimilate NH4+ more favorably, up to 20 times more than NO3− (Kronzucker et al. 1997; Templer and Dawson 2004; Islam and Macdonald 2009). Other studies have addressed the question of assimilation of DON, and found that coniferous trees such as Chamaecyparis obtusa do not use this form of N-compound (Takebayashi et al. 2010), while Pinus sylvestris and Picea abies assimilate as much DON as NH4+ if the soil contains similar amounts of each N form (Ohlund and Näsholm 2002). To explain long-term deviations between tree-ring δ15N series of various deciduous species, McLauchlan and Craine (2012) linked differences to N-form preferences. Given that soil N compounds undergo different transformation paths and carry distinct δ15N signals, diverse N preferences by trees growing at the same site or under similar conditions ought to generate distinct tree-ring δ15N trends over time.
3.3 N Remobilization, Inter-ring Translocation and Fractionation Within Stems
Many studies explain well the fractionation along the length of trees, from root to stems and leaves (Yoneyama et al. 1998; Gebauer et al. 2000; Evans 2001). Briefly, after assimilation of N-species by trees, enzymatic functions transform NH4+ and NO3− into amino acids (Handley et al. 1998). As mentioned previously, these steps generally enrich the reactants in 15N and deplete the products (Yoneyama et al. 1998; Gebauer et al. 2000). Research efforts also indicated that deciduous trees store N in their bark and wood, whereas coniferous trees predominantly store N as photosynthetic proteins in their youngest needles. The remobilization of these amino acids is seasonal. During spring, deciduous trees transfer non-structural N compounds (arginine and asparagine) from twigs and coarse roots (and stems) to forming leaves (Bazot et al. 2013; Brereton et al. 2014). For instance, N proportion in twigs of oak trees decreases by 55% during that period. During summer, leaves are the dominant storage of N (>50% in June, compared to only 10% in stems; see also Sect. 12.3.1). During autumn, while leaves are shedding, storage begins in stems, coarse roots and twigs. For willow trees, the stems become a major N reserve (Brereton et al. 2014), a pool that new leaves will solicit later on.
During spring, coniferous trees transfer N stored in their youngest needles to support new growth of leaves and stems (Millard and Proe 1993; Bauer et al. 2000; Krupa 2003; Millard and Grelet 2010; Couto-Vázquez and González-Prieto 2014). Translocation generates fractionation and systematically decreases δ15N values in old needles relative to young needles (Gebauer and Schulze 1991; Couto-Vázquez and González-Prieto 2010). In contrast, there is no systematic difference between recent and old tree rings as mentioned in Sect. 12.2. The remobilization steps described above may largely explain why foliage and tree-ring δ15N trends in coniferous trees are different from broad-leave trees (Pardo et al. 2006; Gerhart and McLauchlan 2014; Tomlinson et al. 2015).
For further assessing the impact of N mobility in stems on growth ring δ15N values, various research groups investigated the distribution of 15N after fertilization or misting labelled-N compounds (e.g., Elhani et al. 2005; Tomlinson et al. 2014). In such studies, labelled N detected in rings predating and postdating the marking events, clearly indicate that rings include both C-bound and mobile N (not removable by sample pretreatments). However, in most cases, the 15N maximal contents always peak in rings of the marking years (Schleppi et al. 1999; Elhani et al. 2003, 2005; Tomlinson et al. 2014). These experiments indicate that the inter-ring translocation of N does not erase the record (direction and year of changes) of environmental events, but may minimize the extent of its isotopic impact.
4 Tree-Ring δ15N Responses to Changing Conditions
4.1 Physiological Changes
Some studies suggested that physiological functions, for instance lignification, may modify the δ15N values of rings with age of Spanish Pinus radiata, and proposed further experimentation in order to assess the validity of the hypothesis (Couto-Vázquez and González-Prieto 2010). Acer saccharum and Fagus grandifolia trees investigated for assessing the importance of potentially changing root depth with age on the evolution of δ15N values in leaves (and tree rings by extension) show no significant changes with age, but significant δ15N differences between root, stems and leaves, and averages between the two species (Pardo et al. 2013). These results suggest fractionation during transport and assimilation of N, and physiological differences between species. Such a finding agrees with former studies of temperate trees reporting a general increasing δ15N trend along the height of trees (Kolb and Evans 2002; Couto-Vázquez and González-Prieto 2010), with differences existing between species.
4.2 Regional and Global Climate
Based on the concepts explored in the former sections, in theory climatic conditions can imprint the δ15N values transferred to tree rings. Namely, temperature and precipitation variations may modify the soil bioavailable N pools through changes in organic matter degradation, ammonification and nitrification rates, functions of EcM communities, and depth of drawing available soil water and N species (Savard et al. 2009; Courty et al. 2010; Durán et al. 2016). Such changes modify the overall isotopic signal of bioavailable N, which will reverberate in the δ15N values of trees. Indeed, several studies have linked foliar δ15N results from various species of trees with precipitation, showing either direct or inverse correlations depending on the amounts of precipitation considered (Pardo et al. 2006; and references therein). Likewise, in rain exclusion experiments (simulated droughts) deciduous trees clearly increased their foliar δ15N values due to a relative decrease in soil N availability (Ogaya and Peñuelas 2008). At large scales, plant foliar δ15N trends correlate inversely with mean annual precipitation, but directly with mean annual temperature (MAT) possibly due to higher soil N availability under moist and warm conditions (Craine et al. 2009; Dawes et al. 2017). Instead, inverse correlation of temperature with foliar δ15N values of Populus balsamifera may reflect changing dominance in soil N transformation pathways, from DON leaching (low MAT) to denitrification (high MAT; Elmore et al. 2017). In general, we must keep in mind that soil N availability derives from microbial activity, and thus hinges on temperature and soil water content. Depending on the habitat, microbial activity reaches an optimum at a specific range of soil temperature and water content: too much or too little water reduces or inhibits microbial activity. The same is true for temperature. As such, N availability depends on soil temperature and water content, which ultimately leave their fingerprints on the δ15N values of soil N compounds.
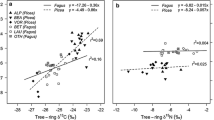
If leaf δ15N values of a given time contain climatic information, tree-ring δ15N series should also record this information. This suggestion is supported by a few studies reporting significant statistical correlations between precipitation or temperature with δ15N series from Fagus grandifolia, Pinus strobus, Pinus massioniama, Fagus sylvatica and Pinus radiata (Savard et al. 2009; Sun et al. 2010; Härdtle et al. 2013; Couto-Vázquez and González-Prieto 2014). Causes for the climate-induced δ15N variations include modified ratios of soil NH4+/NO3−, and change in soil depths of root uptake.
Despite these expressions of climatic triggers for changes in tree-ring δ15N series, one has to consider the potential limitations when evaluating potential climatic effects. High frequency changes in climatic parameters may be impractical to resolve using δ15N values of annually sampled tree rings, as remobilization and translocation of N tend to minimize the isotopic responses (Sect. 12.3.3). Such attempt for quantitative climatic –isotopic correspondence at this resolution may fail. However, tree-ring δ15N series may record low-frequency climate variability. This proposition is supported by a recent investigation of δ15N series in six and ten Picea glauca trees from two Canadian sites (Savard et al. 2020). The results indicate that short-term variations (<7 years) show no inter-tree coherence, whereas middle- (7–15 years) to long-term (>15 years) isotopic changes show strong coherence, encouraging their use as an environmental indicator. One option that may deserve further testing is to pass running averages through long tree-ring δ15N series, and evaluate their correlations with similarly treated climatic series (Savard et al. 2009; similarly treated for global climatic changes, see Sect. 12.4.3—cause number 4). As can be seen, climatic tree-ring δ15N studies require further exploration considering that climatic effects may interplay with anthropogenic impacts, and that improved knowledge on that front may help deciphering intricate δ15N responses of trees to intrinsic and extrinsic triggers.
4.3 Anthropogenic Impacts
There are four main reasons why anthropogenic N emissions are expected to affect the δ15N values in rings of specific species of trees. (1) Shifts in signals in N forms assimilated by trees through addition of large anthropogenic N loads with isotopic ratios markedly different from natural N isotopic abundance. (2) Change in N availability of the forest ecosystem due to high anthropogenic N supply relative to demands, modifying the overall soil N isotopic contents. (3) Modifications of the overall soil microbial structure and related N dynamics having an impact on the signal of N assimilated by stems under moderate anthropogenic N deposition. (4) Global change (climate and pCO2) interplaying with one or a combination of the former causes.
In the first case, much of the N in trees derives from the inorganic soil N pool (NH4+ and NO3−), which forms only a small portion of the total soil N, but that has δ15N signals that may vary with changes in environmental conditions, particularly with enhanced N deposition from anthropogenic emissions. After transition of N contaminants in the soil compartments, trees assimilate anthropogenic N through roots, or root N possibly combines in stems with anthropogenic N transiting through leaves. Key studies have invoked changes in the isotopic signals of assimilated N to account for shifts in tree-ring δ15N values (Saurer et al. 2004; Savard et al. 2009). However, determining the cause of changes in tree-ring δ15N series is possible only when N deposits chronically and in abundance, from a dominant source with δ15N values deviate significantly from soil N, and if the other potential causes for change do not blur this effect.
In the second case, increased N deposition in temperate and boreal forest ecosystems may cause acidification of soils, and nutrient nitrogen imbalances in trees (Aber et al. 1998; Högberg et al. 2007). The soil N status may pass from semi-closed to open, if a high N supply exceeds demands. Such a forest soil would see a high rate of 15N-poor nitrate loss through leaching, increasing the overall δ15N values of the residual pool (Fig. 12.2). The chronic exposure of a forest to such a rate of N input would generate a long-term δ15N increase in tree rings. In contrast, a decrease of anthropogenic N supply would generate a decrease in long-term tree-ring δ15N series in the recovering forests. This interpretation explains the declining δ15N series over 75 years in Picea rubens trees of the central Appalachians (Mathias and Thomas 2018).
In the third case, a combination of modified biogeochemical processes under low to moderate long-term exposure to anthropogenic N inputs alters the overall signal of soil N species prior to their assimilation by trees. On a theoretical basis, one can conceive that microbial communities in forest soils with very low N availability (<1 kg/ha/y) will quickly adapt to enhanced input and chronic exposure to anthropogenic N. As mentioned in Sect. 12.3.2, in such conditions, existing EcM communities may thrive or shift in terms of diversity, and rates of bacterial N transformation may change. A study of four different deciduous species of trees in Indiana (USA), found long-term increasing and decreasing δ15N trends explained by species-specific preferences for inorganic N forms while nitrification increases (McLauchlan and Craine 2012).
In the fourth potential cause for δ15N changes in plant tissue, climatic conditions or rising pCO2 generate long-term changes in soil N processes and N availability. At a continental scale, centennial, standardized, 10 year resolution tree-ring δ15N series of temperate forests seem to evolve independently from anthropogenic N deposition in the USA (McLauchlan et al. 2017). The series instead may reflect changes in N transformation rates and EcM assimilation, and the declining N availability under rising pCO2. Further along this line, at the global scale, rising atmospheric CO2 may have generated decreasing foliage and tree-ring δ15N trends through the last 150 years due to prolonged growing seasons, increased photosynthesis and overall enhanced plant-N demands, ultimately lowering the terrestrial N availability (Craine et al. 2019).
To summarize, tree-ring series may record changes in the forest N cycle or reflect successive N-cycling patterns, but the potential causes for these changes are complex and rigorous interpretations require excellent knowledge of the setting in which trees are growing. Given the attenuated nature of the isotopic changes due to intra-tree N remobilization and intricate enzymatic fractionations along the assimilation path within trees, attempts to quantify anthropogenic impacts on the forest N cycle using tree rings or leaves could be scant. On a more positive note, recognizing and dating perturbations of the forest N cycle using tree-ring δ15N series appears achievable.
4.4 Other Applications
The literature also documents several applications other than the ones presented in Sects. 12.4.1, 12.4.2 and 12.4.3. For tropical and N2-fixing trees, the reader can consult Craine et al. (2015). Boreal wetland and tropical trees emit N2O (Rusch and Rennenberg 1998; Welch et al. 2019), a process significant for understanding the global N cycling, for which δ15N results in stems and emitted gasses may turn useful. Moreover, tree-ring δ15N applications exist on effects of wild fires (Cook 2002; Beghin et al. 2011; Hyodo et al. 2012), clear cutting (Pardo et al. 2002; Bukata and Kyser 2005), and bird nesting (Mizota 2009; Holdaway et al. 2010; Larry et al. 2010). Understandably, researchers should select sites devoid of these perturbations in order to achieve meaningful results and refine our understanding of climatic and anthropogenic influences on tree-ring δ15N series.
5 Knowledge Gaps and Future Directions
Studies dealing with the assimilation of N through either leaves or roots have mostly operated independently, with root assimilation experiments disregarding the potential foliar assimilation, and vice versa. As a consequence, the proportions of N in stems contributed from the foliar and rooting systems still need resolving even if these proportions are highly pertinent for relating tree-ring δ15N values to mechanistic processes and environmental changes.
As with ice cores, lakes sediments and skeletal corals reflecting complex and irrefutable anthropogenic impacts on the atmospheric, aquatic, and marine N cycling, tree-ring series likely represent another natural archive offering potential for unravelling impacts on forest N cycling. Although each archival system potentially offers many applications, in all cases the interplaying mechanisms responsible for changes through time need to be further constrained. With trees, difficulties arise from the requirement to have an excellent understanding of soil conditions to interpret tree-ring trends adequately. Further research should address the knowledge gaps on the steps of fractionation of individual bioavailable N forms in the soil compartments. Similarly, the role of EcM should be explored as it might be effective or non-effective during the transfer of N forms to roots under the broad ranges of existing soil conditions. Tree-ring studies seldom involve the investigation of the full spectrum of N transformations in the air–soil-tree continuum. However, such an interdisciplinary approach may solve several questions regarding the extrinsic controls on tree-ring δ15N changes, perhaps with the combination of running well-adapted models of soil N budgets.
References
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48(11):921–934
Ammann M, Siegwolf R, Pichlmayer F, Suter M, Saurer M, Brunold C (1999) Estimating the uptake of traffic-derived NO2 from 15N abundance in Norway spruce needles. Oecologia 118(2):124–131
Arain MA, Yuan F, Andrew Black T (2006) Soil–plant nitrogen cycling modulated carbon exchanges in a western temperate conifer forest in Canada. Agric For Meteorol 140(1–4):171–192
Arnold G, van Diest A (1991) Nitrogen supply, tree growth and soil acidification. Fertil Res 27:29–38
Averill C, Finzi A (2011) Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15N. Ecology 92(4):883–891
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees 27(4):1023–1034
Bazot S, Fresneau C, Damesin C, Barthes L (2016) Contribution of previous year’s leaf N and soil N uptake to current year’s leaf growth in sessile oak. Biogeosciences 13(11):3475–3484
Beghin R, Cherubini P, Battipaglia G, Siegwolf R, Saurer M, Bovio G (2011) Tree-ring growth and stable isotopes (13C and 15N) detect effects of wildfires on tree physiological processes in Pinus sylvestris L. Trees 25(4):627–636
Brereton NJ, Pitre FE, Shield I, Hanley SJ, Ray MJ, Murphy RJ, Karp A (2014) Insights into nitrogen allocation and recycling from nitrogen elemental analysis and 15N isotope labelling in 14 genotypes of willow. Tree Physiol 34(11):1252–1262
Bukata AR, Kyser KT (2005) Response of the nitrogen isotopic composition of tree-rings following tree-clearing and land-use change. Environ Sci Technol 39:7777–7783
Bukata AR, Kyser TK (2007) Carbon and nitrogen isotope variations in tree-rings as records of perturbations in regional carbon and nitrogen cycles. Environ Sci Technol 41(4):1331–1338
Caceres ML, Mizota C, Yamanaka T, Nobori Y (2011) Effects of pre-treatment on the nitrogen isotope composition of Japanese black pine (Pinus thunbergii) tree-rings as affected by high N input. Rapid Commun Mass Spectrom 25(21):3298–3302
Chalot M, Kytöviita MM, Brun A, Finlay RD, Söderström B (1995) Factors affecting amino acid uptake by the ectomycorrhizal fungus Paxillus involutus. Mycol Res 99(9):1131–1138
Chaparro-Suarez IG, Meixner FX, Kesselmeier J (2011) Nitrogen dioxide (NO2) uptake by vegetation controlled by atmospheric concentrations and plant stomatal aperture. Atmos Environ 45(32):5742–5750
Choi W-J, Lee S-M, Chang SX, Ro H-M (2005) Variations of δ13C and δ15N in Pinus densiflora tree-rings and their relationship to environmental changes in Eastern Korea. Water Air Soil Pollut 164(1):173–187
Compton JE, Hooker TD, Perakis SS (2007) Ecosystem N distribution and δ15N during a century of forest regrowth after agricultural abandonment. Ecosystems 10(7):1197–1208
Cook G (2002) Effects of frequent fires and grazing on stable nitrogen isotope ratios of vegetation in northern Australia. Aust Ecol 26:630–636
Courty P-E, Buée M, Diedhiou AG, Frey-Klett P, Le Tacon F, Rineau F, Turpault M-P, Uroz S, Garbaye J (2010) The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem 42(5):679–698
Couto-Vázquez A, González-Prieto SJ (2010) Effects of climate, tree age, dominance and growth on δ15N in young pinewoods. Trees 24(3):507–514
Couto-Vázquez A, González-Prieto SJ (2014) Effects of biotic and abiotic factors on δ15N in young Pinus radiata. Eur J For Res 133(4):631–637
Craine JM, Elmore AJ, Aidar MP, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Penuelas J, Reich PB, Schuur EA, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183(4):980–992
Craine JM, Elmore AJ, Wang L, Augusto L, Baisden WT, Brookshire EN, Cramer MD, Hasselquist NJ, Hobbie EA, Kahmen A, Koba K, Kranabetter JM, Mack MC, Marin-Spiotta E, Mayor JR, McLauchlan KK, Michelsen A, Nardoto GB, Oliveira RS, Perakis SS, Peri PL, Quesada CA, Richter A, Schipper LA, Stevenson BA, Turner BL, Viani RA, Wanek W, Zeller B (2015) Convergence of soil nitrogen isotopes across global climate gradients. Nature 5:8280
Dawes MA, Schleppi P, Hättenschwiler S, Rixen C, Hagedorn F (2017) Soil warming opens the nitrogen cycle at the alpine treeline. Glob Chang Biol 23(1):421–434
Doucet A, Savard MM, Bégin C, Smirnoff A (2011) Is wood pre-treatment essential for tree-ring nitrogen concentration and isotope analysis? Rapid Commun Mass Spectrom 25(4):469–475
Elhani S, Lema BF, Zeller B, Bréchet C, Guehl J-M, Dupouey J-L (2003) Inter-annual mobility of nitrogen between beech rings: a labelling experiment. Ann For Sci 60(6):503–508
Elhani S, Guehl JM, Nys C, Picard JF, Dupouey J-L (2005) Impact of fertilization on tree-ring delta N-15 and delta C-13 in beech stands: a retrospective analysis. Tree Physiol 25:1437–1446
Elmore AJ, Craine JM, Nelson DM, Guinn SM (2017) Continental scale variability of foliar nitrogen and carbon isotopes in Populus balsamifera and their relationships with climate. Sci Rep 7(1):7759
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6(3):121–126
Garten CT, Hanson PJ (1990) Foliar retention of 15N-nitrate and 15N-ammonium by red maple (Acer rubrum) and white oak (Quercus alba) leaves from simulated rain. Environ Exp Bot 30(3):333–342
Gebauer G, Taylor AFS (1999) 15N natural abundance in fruit bodies of different functional groups of fungi in relation to substrate utilization. New Phytol 142(1):93–101
Gerhart LM, McLauchlan KK (2014) Reconstructing terrestrial nutrient cycling using stable nitrogen isotopes in wood. Biogeochemistry 120(1–3):1–21
Hart SC, Classen AT (2003) Potential for assessing long-term dynamics in soil nitrogen availability from variations in δ15N of tree rings. Isot Environ Health Stud 39(1):15–28
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196(2):367–382
Hobbie EA, Macko SA, Shugart HH (1999) Interpretation of nitrogen isotope signatures using the NIFTE model. Oecologia 120(3):405–415
Högberg MN, Högberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150(4):590–601
Högberg P, Johannisson C, Yarwood S, Callesen I, Nasholm T, Myrold DD, Högberg MN (2011) Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest. New Phytol 189(2):515–525
Houlton BZ, Morford SL, Dahlgren RA (2018) Convergent evidence for widespread rock nitrogen sources in Earth’s surface environment. Science 360(6384):58
Hyodo F, Kusaka S, Wardle DA, Nilsson M-C (2012) Changes in stable nitrogen and carbon isotope ratios of plants and soil across a boreal forest fire chronosequence. Plant Soil 364(1–2):315–323
Islam MA, Macdonald SE (2009) Current uptake of 15N-labeled ammonium and nitrate in flooded and non-flooded black spruce and tamarack seedlings. Ann For Sci 66(1):102–102
Kjoller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012) Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol 194(1):278–286
Knicker H (2004) Stabilization of N-compounds in soil and organic-matter-rich sediments—what is the difference? Mar Chem 92(1–4):167–195
Kolb KJ, Evans RD (2002) Implications of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol 156(1):57–64
Kronzucker HJ, Siddiqi MY, Glass ADM (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385(6611):59–61
Krupa SV (2003) Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ Pollut 124(2):179–221
Larry LCM, Chitoshi M, Toshiro Y, Yoshihiro N (2010) Temporal changes in tree-ring nitrogen of Pinus thunbergii trees exposed to Black-tailed Gull (Larus crassirostris) breeding colonies. Appl Geochem 25(11):1699–1702
Lilleskov E, Hobbie E, Timothy JF (2002) Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. Ecology 154:219–231
Lilleskov EA, Kuyper TW, Bidartondo MI, Hobbie EA (2019) Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environ Pollut 246:148–162
Mayor JR, Schuur EA, Henkel TW (2009) Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol Lett 12(2):171–183
Mayor JR, Schuur EAG, Mack MC, Hollingsworth TN, Bååth E (2012) Nitrogen isotope patterns in alaskan black spruce reflect organic nitrogen sources and the activity of ectomycorrhizal fungi. Ecosystems 15(5):819–831
McLauchlan KK, Craine JM (2012) Species-specific trajectories of nitrogen isotopes in Indiana hardwood forests, USA. Biogeosciences 9(2):867–874
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30(9):1083–1095
Millard P, Proe MF (1993) Nitrogen uptake, partitioning and internal cycling in Picea sitchensis (Bong.) Carr. As influenced by nitrogen supply. New Phytol 125:113–119
Miller BD, Hawkins BJ (2007) Ammonium and nitrate uptake, nitrogen productivity and biomass allocation in interior spruce families with contrasting growth rates and mineral nutrient preconditioning. Tree Physiol 27:901–909
Mizota C (2009) Temporal variations in the concentration and isotopic signature of ammonium- and nitrate–nitrogen in soils under a breeding colony of Black-tailed Gulls (Larus crassirostris) on Kabushima Island, northeastern Japan. Appl Geochem 24(2):328–332
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182(1):31–48
Ogaya R, Peñuelas J (2008) Changes in leaf δ13C and δ15N for three Mediterranean tree species in relation to soil water availability. Acta Oecol 34(3):331–338
Ohlund J, Näsholm T (2002) Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol 21:1319–1326
Pardo LH, Nadelhoffer KJ (2010) Using nitrogen isotope ratios to assess terrestrial ecosystems at regional and global scales. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Isoscapes: understanding movement, pattern, and process on earth through isotope mapping. Springer, Netherlands, Dordrecht, pp 221–249
Pardo LH, Hemond HF, Montoya JP, Fahey TJ, Siccama TG (2002) Response of the natural abundance of 15N in forest soils and foliage to high nitrate loss following clear-cutting. Can J For Res 32(7):1126–1136
Pardo LH, Templer PH, Goodale CL, Duke S, Groffman PM, Adams MB, Boeckx P, Boggs J, Campbell J, Colman B, Compton J, Emmett B, Gundersen P, Kjønaas J, Lovett G, Mack M, Magill A, Mbila M, Mitchell MJ, McGee G, McNulty S, Nadelhoffer K, Ollinger S, Ross D, Rueth H, Rustad L, Schaberg P, Schiff S, Schleppi P, Spoelstra J, Wessel W (2006) Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80(2):143–171
Pardo LH, McNulty SG, Boggs JL, Duke S (2007) Regional patterns in foliar 15N across a gradient of nitrogen deposition in the northeastern US. Environ Pollut 149(3):293–302
Pardo LH, Semaoune P, Schaberg PG, Eagar C, Sebilo M (2013) Patterns in δ15N in roots, stems, and leaves of sugar maple and American beech seedlings, saplings, and mature trees. Biogeochemistry 112(1–3):275–291
Qi H, Coplen TB, Jordan JA (2016) Three whole-wood isotopic reference materials, USGS54, USGS55, and USGS56, for δ2H, δ18O, δ13C, and δ15N measurements. Chem Geol 442:47–53
Rennenberg H, Gessler A (1999) Consequences of N deposition to forest ecosystems—recent results and future research needs. Water Air Soil Pollut 116(1):47–64
Rusch H, Rennenberg H (1998) Black alder (Alnus Glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 201:1–7
Saurer M, Cherubini P, Ammann M, De Cinti B, Siegwolf R (2004) First detection of nitrogen from NOx in tree rings: a 15N/14N study near a motorway. Atmos Environ 38(18):2779–2787
Savard MM, Bégin C, Smirnoff A, Marion J, Rioux-Paquette E (2009) Tree-ring nitrogen isotopes reflect anthropogenic NOX emissions and climatic effects. Environ Sci Technol 43(3):604–609
Savard MM, Cole A, Smirnoff A, Vet R (2017) δ15N values of atmospheric N species simultaneously collected using sector-based samplers distant from sources—isotopic inheritance and fractionation. Atmos Environ 162:11–22
Schleppi P, Bucher-Wallin L, Siegwolf R, Saurer M, Muller N, Bucher JB (1999) Simulation of increased nitrogen deposition to a montane forest ecosystem: partitioning of the added 15N. Water Air Soil Pollut 116(1):129–134
Shi J, Ohte N, Tokuchi N, Imamura N, Nagayama M, Oda T, Suzuki M (2014) Nitrate isotopic composition reveals nitrogen deposition and transformation dynamics along the canopy–soil continuum of a suburban forest in Japan. Rapid Commun Mass Spectrom 28(23):2539–2549
Siegwolf RTW, Matyssek R, Saurer M, Maurer S, Günthardt-Goerg MS, Schmutz P, Bucher JB (2001) Stable isotope analysis reveals differential effects of soil nitrogen and nitrogen dioxide on the water use efficiency in hybrid poplar leaves. New Phytol 149(2):233–246
Takebayashi Y, Koba K, Sasaki Y, Fang Y, Yoh M (2010) The natural abundance of 15N in plant and soil-available N indicates a shift of main plant N resources to NO3− from NH4+ along the N leaching gradient. Rapid Commun Mass Spectrom 24(7):1001–1008
Templer P, Dawson TE (2004) Nitrogen uptake by four tree species of the Catskill Mountains, New York: implications for forest N dynamics. Plant Soil 262:251–261
Tomlinson G, Buchmann N, Siegwolf R, Weber P, Thimonier A, Pannatier EG, Schmitt M, Schaub M, Waldner P (2015) Can tree-ring δ15N be used as a proxy for foliar δ15N in European beech and Norway spruce? Trees 30(3):627–638
Trudell SA, Rygiewicz PT, Edmonds RL (2004) Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytol 164(2):317–335
Vallano DM, Sparks JP (2007) Foliar δ15N values as indicators of foliar uptake of atmospheric nitrogen pollution. Terr Ecol 1:93–109
Vallano DM, Sparks JP (2013) Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 172(1):47–58
Van der Linde S, Suz LM, Orme CDL, Cox F, Andreae H, Asi E, Atkinson B, Benham S, Carroll C, Cools N, De Vos B, Dietrich H-P, Eichhorn J, Gehrmann J, Grebenc T, Gweon HS, Hansen K, Jacob F, Kristöfel F, Lech P, Manninger M, Martin J, Meesenburg H, Merilä P, Nicolas M, Pavlenda P, Rautio P, Schaub M, Schröck H-W, Seidling W, Šrámek V, Thimonier A, Thomsen IM, Titeux H, Vanguelova E, Verstraeten A, Vesterdal L, Waldner P, Wijk S, Zhang Y, Žlindra D, Bidartondo MI (2018) Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 558(7709):243–248
Vizoso S, Gerant D, Marc Guehl J, Joffre R, Chalot M, Gross P, Maillard P (2008) Do elevation of CO2 concentration and nitrogen fertilization alter storage and remobilization of carbon and nitrogen in pedunculate oak saplings? Tree Physiol 28:1729–1739
Warren C, Dreyer E, Adams AM (2003) Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees 17:359–366
Welch B, Gauci V, Sayer EJ (2019) Tree stem bases are sources of CH4 and N2O in a tropical forest on upland soil during the dry to wet season transition. Glob Chang Biol 25(1):361–372
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428(6985):821–827
Zhang C, Meng S, Li Y, Su L, Zhao Z (2016) Nitrogen uptake and allocation in Populus simonii in different seasons supplied with isotopically labeled ammonium or nitrate. Trees 30(6):2011–2018
Balster NJ, Marshall JD, Clayton M (2009) Coupling tree-ring δ13C and δ15N to test the effect of fertilization on mature Douglas-fir (Pseudotsuga menziesii var. glauca) stands across the Interior northwest, USA. Tree Physiol 29(12):1491–1501
Bauer GA, Gebauer G, Harrison AF, Högberg P, Högbom L, Schinkel H, Taylor AFS, Novak M, Buzek F, Harkness D, Persson T, Schulze ED (2000) Biotic and abiotic controls over ecosystem cycling of stable natural nitrogen, carbon and sulphur isotopes. In: Schulze E-D (ed) Carbon and nitrogen cycling in european forest ecosystems. Springer. Berlin, Heidelberg, pp 189–214
Craine JM, Elmore AJ, Wang L, Aranibar J, Bauters M, Boeckx P, Crowley BE, Dawes MA, Delzon S, Fajardo A, Fang Y, Fujiyoshi L, Gray A, Guerrieri R, Gundale MJ, Hawke DJ, Hietz P, Jonard M, Kearsley E, Kenzo T, Makarov M, Maranon-Jimenez S, McGlynn TP, McNeil BE, Mosher SG, Nelson DM, Peri PL, Roggy JC, Sanders-DeMott R, Song M, Szpak P, Templer PH, Van der Colff D, Werner C, Xu X, Yang Y, Yu G, Zmudczynska-Skarbek K (2019) Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat Ecolology Evol 2(11):1735–1744
Durán J, Morse J, Groffman P, Campbell J, Christenson L, Driscoll C, J. Fahey T, Fisk M, E. Likens G, Melillo J, Mitchell M, Templer P, Vadeboncoeur M (2016) Climate change decreases nitrogen pools and mineralization rates in northern hardwood forests. Ecosphere 7
Etzold S, Ferretti M, Reinds GJ, Solberg S, Gessler A, Waldner P, Schaub M, Simpson D, Benham S, Hansen K, Ingerslev M, Jonard M, Karlsson PE, Lindroos A-J, Marchetto A, Manninger M, Meesenburg H, Merilä P, Nöjd P, Rautio P, Sanders TGM, Seidling W, Skudnik M, Thimonier A, Verstraeten A, Vesterdal L, Vejpustkova M, de Vries W (2020) Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. For Ecol Manag 458:117762
Gebauer G, Schulze DE (1991) Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the fichtelgebirge. NE Bavaria 87
Gebauer G, Zeller B, Schmidt G, May C, Buchmann N, Colin-Belgrand M, Dambrine E, Martin F, Schulze E-D, Bottner P (2000) The fate of 15N-labelled nitrogen inputs to coniferous and broadleaf forests. In: Schulze E-D (ed) Carbon and nitrogen cycling in european forest ecosystems. Springer, Berlin, Heidelberg, pp 144–170
Handley LL, Scrimgeour CM, Raven JA (1998) 15N natural abundance levels in terrestrial vascular plants: a précis. In: Griffiths H (ed). Bios Scientific Publishers, Oxford, UK
Härdtle W, Niemeyer T, Assmann T, Baiboks S, Fichtner A, Friedrich U, Lang AC, Neuwirth B, Pfister L, Ries C, Schuldt A, Simon N, von Oheimb G (2013) Long-term trends in tree-ring width and isotope signatures (δ13C, δ15N) of Fagus sylvatica L. on soils with contrasting water supply. Ecosystems 16(8):1413–1428
Harrison AF, Harkness DD, Rowland AP, Garnett JS, Bacon PJ (2000a) Annual carbon and nitrogen fluxes in soils along the european forest transect, determined using 14C-bomb. In: Schulze E-D (ed) Carbon and nitrogen cycling in european forest ecosystems. Springer, Berlin, Heidelberg, pp 237–256
Harrison AF, Schulze ED, Gebauer G, Bruckner G (2000b) Canopy uptake and utilization of atmospheric pollutant nitrogen. In: Schulze E-D (ed) Carbon and nitrogen cycling in European forest ecosystems. Springer, Berlin, Heidelberg, pp 171–188
Hobbie E, Colpaert J (2002) Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol 157
Högberg P (1997) Tansley review no. 95. 15N natural abundance in soil-plant systems. New Phytol 137(2):179–203
Holdaway RN, Hawke DJ, Hyatt OM, Wood GC (2010) Stable isotopic (δ15N, δ13C) analysis of wood in trees growing in past and present colonies of burrow‐nesting seabirds in New Zealand. I. δ15N in two species of conifer (Podocarpaceae) from a mainland colony of Westland petrels (Procellaria westlandica), Punakaiki, South Island. J R Soc N Z 37(2):75–84
Holloway JM, Dahlgren RA (2002) Nitrogen in rock: occurrences and biogeochemical implications. Glob Biogeochem Cycles 16(4):65-61–65-17
Hopkins DW, Wheatley RE, Robinson D (1998) In: Griffiths H (ed) Stable isotope studies of soil nitrogen. Bios Scientific Publishers, Oxford, UK
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing, Hoboken
Kluber LA, Carrino-Kyker SR, Coyle KP, DeForest JL, Hewins CR, Shaw AN, Smemo KA, Burke DJ (2012) Mycorrhizal response to experimental pH and P manipulation in acidic hardwood forests. PLoS ONE 7(11):e48946
Macklon AES, Sheppard LJ, Sim A, Leith ID (1996) Uptake of ammonium and nitrate ions from acid mist applied to Sitka spruce [Picea sitchensis (Bong.) Carr.] grafts over the course of one growing season. Trees 10(4):261–267
Mathias JM, Thomas RB (2018) Disentangling the effects of acidic air pollution, atmospheric CO2 and climate change on recent growth of red spruce trees in the Central Appalachian Mountains. Glob Chang Biol 24(9):3938–3953
McLauchlan KK, Gerhart LM, Battles JJ, Craine JM, Elmore AJ, Higuera PE, Mack MC, McNeil BE, Nelson DM, Pederson N, Perakis SS (2017) Centennial-scale reductions in nitrogen availability in temperate forests of the United States. Sci Rep 7(1)
Qian XM, Kottke I, Oberwinkler F (1998) Influence of liming and acidification on the activity of the mycorrhizal communities in a Picea abies (L.) Karst. stand. Plant Soil 199(1):99–109
Savard MM, Marion J, Bégin C (2020) Nitrogen isotopes of individual tree-ring series—the validity of middle- to long-term trends. Dendrochronologia 62:125726
Scarascia-Mugnozza G, Bauer GA, Persson H, Matteucci G, Masci A (2000) Tree biomass, growth and nutrient pools. In: Caldwell MM, Heldmaier G, Lange OL, Mooney HA, Schulze E-D, Sommer U (eds) Carbon and nitrogen cycling in European forest ecosystems. Springer, Heidelberg, Germany
Schulze E-D, Högberg P, van Oene H, Persson T, Harrison AF, Read D, KjøLler A, Matteucci G (2000) Interactions between the carbon and nitrogen cycles and the role of biodiversity: a synopsis of a study along a North-South transect through Europe. In: Schulze E-D (ed) Carbon and nitrogen cycling in european forest ecosystems. Springer, Berlin, Heidelberg, pp 468–491
Sparks JP, Roberts JM, Monson RK (2003) The uptake of gaseous organic nitrogen by leaves: a significant global nitrogen transfer process. Geophys Res Lett 30(23)
Sun F, Kuang Y, Wen D, Xu Z, Li J, Zuo W, Hou E (2010) Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China. J Soils Sediments 10(8):1453–1465
Tomlinson G, Siegwolf RT, Buchmann N, Schleppi P, Waldner P, Weber P (2014) The mobility of nitrogen across tree-rings of Norway spruce (Picea abies L.) and the effect of extraction method on tree-ring δ15N and δ13C values. Rapid Commun Mass Spectrom 28(11):1258–1264
Wallander H, Arnebrant K, Östrand F, Kårén O (1997) Uptake of 15N-labelled alanine, ammonium and nitrate in Pinus sylvestris L. ectomycorrhiza growing in forest soil treated with nitrogen, sulphur or lime. Plant Soil 195(2):329–338
Yoneyama T, Fujiwara H, Wilson JM (1998) Variations in fractionation of carbon and nitrogen isotopes in higher plants: N metabolism and partitioning of phloem and xylem. In: Griffiths H (ed) Stable isotopes: integration of biological, ecological and geochemical processes. BIOS Scientific Publishers, Oxford, UK
Acknowledgements
The authors are grateful to G. Bordeleau and P. van der Sleen for a pre-submission and official referee reviews of the manuscript. Natural Resources Canada Contribution number: 20190279.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Savard, M.M., Siegwolf, R.T.W. (2022). Nitrogen Isotopes in Tree Rings—Challenges and Prospects. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)