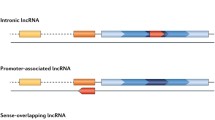
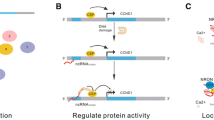
Abstract
The last decade has seen an enormous increase in long non-coding RNA (lncRNA) research within rheumatology. LncRNAs are arbitrarily classed as non-protein encoding RNA transcripts that exceed 200 nucleotides in length. These transcripts have tissue and cell specific patterns of expression and are implicated in a variety of biological processes. Unsurprisingly, numerous lncRNAs are dysregulated in rheumatoid conditions, correlating with disease activity and cited as potential biomarkers and targets for therapeutic intervention. In this chapter, following an introduction into each condition, we discuss the lncRNAs involved in rheumatoid arthritis, osteoarthritis and systemic lupus erythematosus. These inflammatory joint conditions share several inflammatory signalling pathways and therefore not surprisingly many commonly dysregulated lncRNAs are shared across these conditions. In the interest of translational research only those lncRNAs which are strongly conserved have been addressed. The lncRNAs discussed here have diverse roles in regulating inflammation, proliferation, migration, invasion and apoptosis. Understanding the molecular basis of lncRNA function in rheumatology will be crucial in fully determining the inflammatory mechanisms that drive these conditions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:15. https://doi.org/10.1038/s41413-018-0016-9
Crowson CS, Matteson EL, Myasoedova E et al (2011) The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 63(3):633–639. https://doi.org/10.1002/art.30155
Xu B, Lin J (2017) Characteristics and risk factors of rheumatoid arthritis in the United States: an NHANES analysis. Peer J 5:e4035. https://doi.org/10.7717/peerj.4035
Fukui S, Iwamoto N, Takatani A et al (2017) M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to Osteoclastogenesis. Front Immunol 8:1958. https://doi.org/10.3389/fimmu.2017.01958
Kung CC, Dai SP, Chiang H, Huang HS, Sun WH (2020) Temporal expression patterns of distinct cytokines and M1/M2 macrophage polarization regulate rheumatoid arthritis progression. Mol Biol Rep 47(5):3423–3437. https://doi.org/10.1007/s11033-020-05422-6
Pap T, Korb-Pap A (2015) Cartilage damage in osteoarthritis and rheumatoid arthritis--two unequal siblings. Nat Rev Rheumatol 11(10):606–615. https://doi.org/10.1038/nrrheum.2015.95
Ostrowska M, Maśliński W, Prochorec-Sobieszek M, Nieciecki M, Sudoł-Szopińska I (2018) Cartilage and bone damage in rheumatoid arthritis. Reumatologia 56(2):111–120. https://doi.org/10.5114/reum.2018.75523
Sudoł-Szopińska I, Kontny E, Maśliński W, Prochorec-Sobieszek M, Warczyńska A, Kwiatkowska B (2013) Significance of bone marrow edema in pathogenesis of rheumatoid arthritis. Pol J Radiol 78(1):57–63. https://doi.org/10.12659/PJR.883768
Malemud CJ (2013) Intracellular signaling pathways in rheumatoid arthritis. J Clin Cell Immunol 4:160. https://doi.org/10.4172/2155-9899.1000160
Madhok R, Crilly A, Watson J, Capell HA (1993) Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis 52(3):232–234. https://doi.org/10.1136/ard.52.3.232
Noack M, Miossec P (2017) Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol 39(4):365–383. https://doi.org/10.1007/s00281-017-0619-z
Arleevskaya MI, Larionova RV, Brooks WH, Bettacchioli E, Renaudineau Y (2020) Toll-like receptors, infections, and rheumatoid arthritis. Clin Rev Allergy Immunol 58(2):172–181. https://doi.org/10.1007/s12016-019-08742-z
Kloppenburg M, Berenbaum F (2020) Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil 28(3):242–248. https://doi.org/10.1016/j.joca.2020.01.002
Hootman JM, Helmick CG, Brady TJ (2012) A public health approach to addressing arthritis in older adults: the most common cause of disability. Am J Public Health 102(3):426–433. https://doi.org/10.2105/AJPH.2011.300423
Philp AM, Collier RL, Grover LM, Davis ET, Jones SW (2017) Resistin promotes the abnormal type I collagen phenotype of subchondral bone in obese patients with end stage hip osteoarthritis. Sci Rep 7(1):4042. https://doi.org/10.1038/s41598-017-04119-4
Pearson MJ, Herndler-Brandstetter D, Tariq MA et al (2017) IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep 7(1):3451. https://doi.org/10.1038/s41598-017-03759-w
Safiri S, Kolahi AA, Smith E et al (2020) Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis 79(6):819–828. https://doi.org/10.1136/annrheumdis-2019-216515
Palazzo C, Nguyen C, Lefevre-Colau M-M, Rannou F, Poiraudeau S (2016) Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 59(3):134–138. https://doi.org/10.1016/j.rehab.2016.01.006
Nanus DE, Wijesinghe SN, Pearson MJ et al (2020) Regulation of the inflammatory synovial fibroblast phenotype by metastasis-associated lung adenocarcinoma transcript 1 long noncoding RNA in obese patients with osteoarthritis. Arthritis Rheumatol 72(4):609–619. https://doi.org/10.1002/art.41158
Tonge DP, Pearson MJ, Jones SW (2014) The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthr Cartil 22(5):609–621. https://doi.org/10.1016/j.joca.2014.03.004
Jones SW, Brockbank SM, Clements KM et al (2009) Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr Cartil 17(1):124–131. https://doi.org/10.1016/j.joca.2008.05.001
Cooke ME, Lawless BM, Jones SW, Grover LM (2018) Matrix degradation in osteoarthritis primes the superficial region of cartilage for mechanical damage. Acta Biomater 78:320–328. https://doi.org/10.1016/j.actbio.2018.07.037
Chang J, Jackson SG, Wardale J, Jones SW (2014) Hypoxia modulates the phenotype of osteoblasts isolated from knee osteoarthritis patients, leading to undermineralized bone nodule formation. Arthritis Rheumatol 66(7):1789–1799. https://doi.org/10.1002/art.38403
Hügle T, Geurts J (2017) What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford) 56(9):1461–1471. https://doi.org/10.1093/rheumatology/kew389
Philp AM, Davis ET, Jones SW (2017) Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford) 56(6):869–881. https://doi.org/10.1093/rheumatology/kew278
Nanus DE, Badoume A, Wijesinghe SN et al (2020) Identification of synovial fibroblasts subsets associated with pain and progression of knee osteoarthritis by single cell sequencing. Osteoarthr Cartil 28:S133. https://doi.org/10.1016/j.joca.2020.02.220
Wijesinghe SN, Badoume A, Nanus DE, Davis ET, Lindsay MA, Jones SW (2020) Obese synovial fibroblasts exhibit single cell subsets with specific pathological inflammatory functions in osteoarthritis patients. Osteoarthr Cartil 28:S84. https://doi.org/10.1016/j.joca.2020.02.128
Pearson MJ, Jones SW (2016) Review: long noncoding RNAs in the regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis. Arthritis Rheumatol 68(11):2575–2583. https://doi.org/10.1002/art.39759
Saito T, Tanaka S (2017) Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res Ther 19(1):94. https://doi.org/10.1186/s13075-017-1296-y
Olivotto E, Otero M, Marcu KB, Goldring MB (2015) Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open 1(Suppl 1):e000061. https://doi.org/10.1136/rmdopen-2015-000061
Ahmed AS, Gedin P, Hugo A et al (2018) Activation of NF-κB in synovium versus cartilage from patients with advanced knee osteoarthritis: a potential contributor to inflammatory aspects of disease progression. J Immunol 201(7):1918. https://doi.org/10.4049/jimmunol.1800486
Barreto G, Manninen M, Eklund K (2020) Osteoarthritis and Toll-Like receptors: when innate immunity meets chondrocyte apoptosis. Biology (Basel) 9(4). https://doi.org/10.3390/biology9040065
Bar-Or D, Rael LT, Thomas GW, Brody EN (2015) Inflammatory pathways in knee osteoarthritis: potential targets for treatment. Curr Rheumatol Rev 11(1):50–58. https://doi.org/10.2174/1573397111666150522094131
Hsu CC, Lin CL, Jou IM, Wang PH, Lee JS (2017) The protective role of nitric oxide-dependent innate immunosuppression in the early stage of cartilage damage in rats: role of nitric oxide in cartilage damage. Bone Joint Res 6(4):253–258. https://doi.org/10.1302/2046-3758.64.BJJ-2016-0161.R1
Huang H, Zheng J, Shen N et al (2018) Identification of pathways and genes associated with synovitis in osteoarthritis using bioinformatics analyses. Sci Rep 8(1):10050. https://doi.org/10.1038/s41598-018-28280-6
Azamar-Llamas D, Hernández-Molina G, Ramos-Ávalos B, Furuzawa-Carballeda J (2017) Adipokine contribution to the pathogenesis of osteoarthritis. Mediat Inflamm 2017:5468023. https://doi.org/10.1155/2017/5468023
Ji P, Diederichs S, Wang W et al (2003) MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22(39):8031–8041. https://doi.org/10.1038/sj.onc.1206928
Tripathi V, Ellis JD, Shen Z et al (2010) The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 39(6):925–938. https://doi.org/10.1016/j.molcel.2010.08.011
Li ZX, Zhu QN, Zhang HB, Hu Y, Wang G, Zhu YS (2018) MALAT1: a potential biomarker in cancer. Cancer Manag Res 10:6757–6768. https://doi.org/10.2147/CMAR.S169406
Pan L, Liu D, Zhao L, Wang L, Xin M, Li X (2018) Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced inflammatory injury by upregulating microRNA-19b in murine chondrogenic ATDC5 cells. J Cell Biochem 119(12):10165–10175. https://doi.org/10.1002/jcb.27357
Gao GC, Cheng XG, Wei QQ, Chen WC, Huang WZ (2019) Long noncoding RNA MALAT-1 inhibits apoptosis and matrix metabolism disorder in interleukin-1β-induced inflammation in articular chondrocytes via the JNK signaling pathway. J Cell Biochem 120(10):17167–17179. https://doi.org/10.1002/jcb.28977
Arun G, Aggarwal D, Spector DL (2020) Long non-coding RNA: functional implications. Noncoding RNA 6(2). https://doi.org/10.3390/ncrna6020022
Liang J, Xu L, Zhou F et al (2018) MALAT1/miR-127-5p regulates Osteopontin (OPN)-mediated proliferation of human chondrocytes through PI3K/Akt pathway. J Cell Biochem 119(1):431–439. https://doi.org/10.1002/jcb.26200
Zhang Y, Wang F, Chen G, He R, Yang L (2019) LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci 9:54. https://doi.org/10.1186/s13578-019-0302-2
Liu C, Ren S, Zhao S, Wang Y (2019) LncRNA MALAT1/MiR-145 adjusts IL-1β-induced chondrocytes viability and cartilage matrix degradation by regulating ADAMTS5 in human osteoarthritis. Yonsei Med J 60(11):1081–1092. https://doi.org/10.3349/ymj.2019.60.11.1081
Li H, Xie S, Zhang R, Zhang H (2020) LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci 254:116801. https://doi.org/10.1016/j.lfs.2019.116801
Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ (2015) PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 15(1):121–126. https://doi.org/10.1007/s10238-013-0271-4
Li GQ, Fang YX, Liu Y et al (2019) MALAT1-driven inhibition of Wnt signal impedes proliferation and inflammation in fibroblast-like Synoviocytes through CTNNB1 promoter methylation in rheumatoid arthritis. Hum Gene Ther 30(8):1008–1022. https://doi.org/10.1089/hum.2018.212
Wan L, Liu J, Huang C et al (2020) Decreased long-chain non-coding RNA MALAT1 expression and increased hsa-miR155-3p expression involved in Notch signaling pathway regulation in rheumatoid arthritis patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 36(6):535–541
Pan F, Zhu L, Lv H, Pei C (2016) Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med 38(5):1507–1514. https://doi.org/10.3892/ijmm.2016.2755
Rinn JL, Kertesz M, Wang JK et al (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129(7):1311–1323. https://doi.org/10.1016/j.cell.2007.05.022
Rinn JL, Kertesz M, Wang JK et al (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by Noncoding RNAs. Cell 129(7):1311–1323. https://doi.org/10.1016/j.cell.2007.05.022
Bond CS, Fox AH (2009) Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol 186(5):637–644. https://doi.org/10.1083/jcb.200906113
Imamura K, Imamachi N, Akizuki G et al (2014) Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell 53(3):393–406. https://doi.org/10.1016/j.molcel.2014.01.009
Chen H, Qi J, Bi Q, Zhang S (2017) Expression profile of long noncoding RNA (HOTAIR) and its predicted target miR-17-3p in LPS-induced inflammatory injury in human articular chondrocyte C28/I2 cells. Int J Clin Exp Pathol 10(9):9146–9157
Hu J, Wang Z, Shan Y, Pan Y, Ma J, Jia L (2018) Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis 9(7):711. https://doi.org/10.1038/s41419-018-0746-z
Yang Y, Xing D, Wang Y, Jia H, Li B, Li JJ (2020) A long non-coding RNA, HOTAIR, promotes cartilage degradation in osteoarthritis by inhibiting WIF-1 expression and activating Wnt pathway. BMC Mol Cell Biol 21(1):53. https://doi.org/10.1186/s12860-020-00299-6
He B, Jiang D (2020) HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int 44(2):524–535. https://doi.org/10.1002/cbin.11253
Chen Y, Zhang L, Li E et al (2020) Long-chain non-coding RNA HOTAIR promotes the progression of osteoarthritis via sponging miR-20b/PTEN axis. Life Sci 253:117685. https://doi.org/10.1016/j.lfs.2020.117685
Dou P, Hu R, Zhu W et al (2017) Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie 72(2):113–117. https://doi.org/10.1691/ph.2017.6649
Zhang C, Wang P, Jiang P et al (2016) Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene 586(2):248–253. https://doi.org/10.1016/j.gene.2016.04.016
Mao T, He C, Wu H, Yang B, Li X (2019) Silencing lncRNA HOTAIR declines synovial inflammation and synoviocyte proliferation and promotes synoviocyte apoptosis in osteoarthritis rats by inhibiting Wnt/β-catenin signaling pathway. Cell Cycle 18(22):3189–3205. https://doi.org/10.1080/15384101.2019.1671716
Zhang HJ, Wei QF, Wang SJ et al (2017) LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol 50:283–290. https://doi.org/10.1016/j.intimp.2017.06.021
Ji J, Dai X, Yeung SJ, He X (2019) The role of long non-coding RNA GAS5 in cancers. Cancer Manag Res 11:2729–2737. https://doi.org/10.2147/CMAR.S189052
Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP (2010) Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 3(107):ra8. https://doi.org/10.1126/scisignal.2000568
Xing D, Liang JQ, Li Y et al (2014) Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop Surg 6(4):288–293. https://doi.org/10.1111/os.12147
Song J, Ahn C, Chun CH, Jin EJ (2014) A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res 32(12):1628–1635. https://doi.org/10.1002/jor.22718
Ji Q, Qiao X, Liu Y, Wang D, Yan J (2020) Silencing of long-chain non-coding RNA GAS5 in osteoarthritic chondrocytes is mediated by targeting the miR-34a/Bcl-2 axis. Mol Med Rep 21(3):1310–1319. https://doi.org/10.3892/mmr.2019.10900
Li F, Sun J, Huang S, Su G, Pi G (2018) LncRNA GAS5 overexpression reverses LPS-induced inflammatory injury and apoptosis through up-regulating KLF2 expression in ATDC5 chondrocytes. Cell Physiol Biochem 45(3):1241–1251. https://doi.org/10.1159/000487455
Li G, Liu Y, Meng F et al (2018) Tanshinone IIA promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by up-regulating lncRNA GAS5. Biosci Rep 38(5). https://doi.org/10.1042/BSR20180626
Ma C, Wang W, Li P (2019) LncRNA GAS5 overexpression downregulates IL-18 and induces the apoptosis of fibroblast-like synoviocytes. Clin Rheumatol 38(11):3275–3280. https://doi.org/10.1007/s10067-019-04691-2
Li M, Wang N, Shen Z, Yan J (2020) Long non-coding RNA growth arrest-specific transcript 5 regulates rheumatoid arthritis by targeting homeodomain-interacting protein kinase 2. Clin Exp Rheumatol 38:1145
Ghafouri-Fard S, Esmaeili M, Taheri M (2020) H19 lncRNA: roles in tumorigenesis. Biomed Pharmacother 123:109774. https://doi.org/10.1016/j.biopha.2019.109774
Steck E, Boeuf S, Gabler J et al (2012) Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med (Berl) 90(10):1185–1195. https://doi.org/10.1007/s00109-012-0895-y
Hu Y, Li S, Zou Y (2019) Knockdown of LncRNA H19 relieves LPS-induced damage by modulating miR-130a in osteoarthritis. Yonsei Med J 60(4):381–388. https://doi.org/10.3349/ymj.2019.60.4.381
Zhang X, Liu X, Ni X, Feng P, Wang YU (2019) Long non-coding RNA H19 modulates proliferation and apoptosis in osteoarthritis via regulating miR-106a-5p. J Biosci 44(6):128
Yang B, Xu L, Wang S (2020) Regulation of lncRNA-H19/miR-140-5p in cartilage matrix degradation and calcification in osteoarthritis. Ann Palliat Med 9(4):1896–1904. https://doi.org/10.21037/apm-20-929
Tan F, Wang D, Yuan Z (2020) The fibroblast-like Synoviocyte derived Exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 Axis. Inflammation 43(4):1498–1509. https://doi.org/10.1007/s10753-020-01227-8
Stuhlmüller B, Kunisch E, Franz J et al (2003) Detection of oncofetal h19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol 163(3):901–911. https://doi.org/10.1016/S0002-9440(10)63450-5
Yang J, Li Y, Wang L, Zhang Z, Li Z, Jia Q (2020) LncRNA H19 aggravates TNF-α-induced inflammatory injury via TAK1 pathway in MH7A cells. Biofactors. https://doi.org/10.1002/biof.1659
Liu F, Liu X, Yang Y et al (2020) NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol Int 44(4):947–957. https://doi.org/10.1002/cbin.11291
Tu Y, Ma T, Wen T et al (2020) MicroRNA-377-3p alleviates IL-1β-caused chondrocyte apoptosis and cartilage degradation in osteoarthritis in part by downregulating ITGA6. Biochem Biophys Res Commun 523(1):46–53. https://doi.org/10.1016/j.bbrc.2019.11.186
Li D, Sun Y, Wan Y, Wu X, Yang W (2020) LncRNA NEAT1 promotes proliferation of chondrocytes via down-regulation of miR-16-5p in osteoarthritis. J Gene Med:e3203. https://doi.org/10.1002/jgm.3203
Wang Z, Hao J, Chen D (2019) Long noncoding RNA Nuclear enriched abundant transcript 1 (NEAT1) regulates proliferation, apoptosis, and inflammation of chondrocytes via the miR-181a/Glycerol-3-phosphate dehydrogenase 1-like (GPD1L) Axis. Med Sci Monit 25:8084–8094. https://doi.org/10.12659/MSM.918416
Shui X, Chen S, Lin J, Kong J, Zhou C, Wu J (2019) Knockdown of lncRNA NEAT1 inhibits Th17/CD4. J Cell Physiol 234(12):22477–22484. https://doi.org/10.1002/jcp.28811
Borsani G, Tonlorenzi R, Simmler MC et al (1991) Characterization of a murine gene expressed from the inactive X chromosome. Nature 351(6324):325–329. https://doi.org/10.1038/351325a0
Plath K, Mlynarczyk-Evans S, Nusinow DA, Panning B (2002) Xist RNA and the mechanism of X chromosome inactivation. Annu Rev Genet 36:233–278. https://doi.org/10.1146/annurev.genet.36.042902.092433
Marshall EA, Stewart GL, Sage AP, Lam WL, Brown CJ (2019) Beyond sequence homology: cellular biology limits the potential of XIST to act as a miRNA sponge. PLoS One 14(8):e0221371. https://doi.org/10.1371/journal.pone.0221371
Lambert NC (2019) Nonendocrine mechanisms of sex bias in rheumatic diseases. Nat Rev Rheumatol 15(11):673–686. https://doi.org/10.1038/s41584-019-0307-6
Kanaan SB, Onat OE, Balandraud N et al (2016) Evaluation of X chromosome inactivation with respect to HLA genetic susceptibility in rheumatoid arthritis and systemic sclerosis. PLoS One 11(6):e0158550. https://doi.org/10.1371/journal.pone.0158550
Lin J, He Y, Chen J, Zeng Z, Yang B, Ou Q (2017) A critical role of transcription factor YY1 in rheumatoid arthritis by regulation of interleukin-6. J Autoimmun 77:67–75. https://doi.org/10.1016/j.jaut.2016.10.008
Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G (2005) A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil 13(9):769–781. https://doi.org/10.1016/j.joca.2005.04.014
Hame SL, Alexander RA (2013) Knee osteoarthritis in women. Curr Rev Musculoskelet Med 6(2):182–187. https://doi.org/10.1007/s12178-013-9164-0
Xiang S, Li Z, Bian Y, Weng X (2019) Identification of changed expression of mRNAs and lncRNAs in osteoarthritic synovium by RNA-sequencing. Gene 685:55–61. https://doi.org/10.1016/j.gene.2018.10.076
Li L, Lv G, Wang B, Kuang L (2018) The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem Biophys Res Commun 503(4):2555–2562. https://doi.org/10.1016/j.bbrc.2018.07.015
Sun P, Wu Y, Li X, Jia Y (2020) miR-142-5p protects against osteoarthritis through competing with lncRNA XIST. J Gene Med 22(4):e3158. https://doi.org/10.1002/jgm.3158
Lian LP, Xi XY (2020) Long non-coding RNA XIST protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced injury via regulating miR-653-5p/SIRT1 axis. J Biol Regul Homeost Agents 34(2):379–391. https://doi.org/10.23812/19-549-A-65
Wang T, Liu Y, Wang Y, Huang X, Zhao W, Zhao Z (2019) Long non-coding RNA XIST promotes extracellular matrix degradation by functioning as a competing endogenous RNA of miR-1277-5p in osteoarthritis. Int J Mol Med 44(2):630–642. https://doi.org/10.3892/ijmm.2019.4240
Liu Y, Liu K, Tang C, Shi Z, Jing K, Zheng J (2020) Long non-coding RNA XIST contributes to osteoarthritis progression via miR-149-5p/DNMT3A axis. Biomed Pharmacother 128:110349. https://doi.org/10.1016/j.biopha.2020.110349
Chen H, Yang S, Shao R (2019) Long non-coding XIST raises methylation of TIMP-3 promoter to regulate collagen degradation in osteoarthritic chondrocytes after tibial plateau fracture. Arthritis Res Ther 21(1):271. https://doi.org/10.1186/s13075-019-2033-5
Li L, Lv G, Wang B, Kuang L (2020) XIST/miR-376c-5p/OPN axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J Cell Physiol 235(1):281–293. https://doi.org/10.1002/jcp.28968
Ghafouri-Fard S, Taheri M (2019) Maternally expressed gene 3 (MEG3): a tumor suppressor long non coding RNA. Biomed Pharmacother 118:109129. https://doi.org/10.1016/j.biopha.2019.109129
Mondal T, Subhash S, Vaid R et al (2015) MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nat Commun 6:7743. https://doi.org/10.1038/ncomms8743
Su W, Xie W, Shang Q, Su B (2015) The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int 2015:356893. https://doi.org/10.1155/2015/356893
Xu J, Xu Y (2017) The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci 7:69. https://doi.org/10.1186/s13578-017-0195-x
Chen K, Zhu H, Zheng MQ, Dong QR (2019) LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 Axis. Cartilage 1947603519855759. https://doi.org/10.1177/1947603519855759
Wang Z, Chi X, Liu L et al (2018) Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells. Biomed Pharmacother 100:240–249. https://doi.org/10.1016/j.biopha.2018.02.018
Li X, Tang C, Wang J et al (2018) Methylene blue relieves the development of osteoarthritis by upregulating lncRNA MEG3. Exp Ther Med 15(4):3856–3864. https://doi.org/10.3892/etm.2018.5918
Wang A, Hu N, Zhang Y et al (2019) MEG3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med Genet 12(1):201. https://doi.org/10.1186/s12920-019-0649-6
Li G, Liu Y, Meng F et al (2019) LncRNA MEG3 inhibits rheumatoid arthritis through miR-141 and inactivation of AKT/mTOR signalling pathway. J Cell Mol Med 23(10):7116–7120. https://doi.org/10.1111/jcmm.14591
Lu X, Qian J (2019) Downregulated MEG3 participates in rheumatoid arthritis via promoting proliferation of fibroblast-like synoviocytes. Exp Ther Med 17(3):1637–1642. https://doi.org/10.3892/etm.2018.7100
Wang KC, Yang YW, Liu B et al (2011) A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472(7341):120–124. https://doi.org/10.1038/nature09819
Shang A, Wang W, Gu C et al (2019) Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J Exp Clin Cancer Res 38(1):411. https://doi.org/10.1186/s13046-019-1394-6
Ghafouri-Fard S, Dashti S, Taheri M (2020) The HOTTIP (HOXA transcript at the distal tip) lncRNA: review of oncogenic roles in human. Biomed Pharmacother 127:110158. https://doi.org/10.1016/j.biopha.2020.110158
Kim D, Song J, Han J, Kim Y, Chun CH, Jin EJ (2013) Two non-coding RNAs, MicroRNA-101 and HOTTIP contribute cartilage integrity by epigenetic and homeotic regulation of integrin-α1. Cell Signal 25(12):2878–2887. https://doi.org/10.1016/j.cellsig.2013.08.034
Mao G, Kang Y, Lin R et al (2019) Long non-coding RNA HOTTIP promotes CCL3 expression and induces cartilage degradation by sponging miR-455-3p. Front Cell Dev Biol 7:161. https://doi.org/10.3389/fcell.2019.00161
Hu X, Tang J, Bao P et al (2020) Silencing of long non-coding RNA HOTTIP reduces inflammation in rheumatoid arthritis by demethylation of SFRP1. Mol Ther Nucleic Acids 19:468–481. https://doi.org/10.1016/j.omtn.2019.11.015
Onagoruwa OT, Pal G, Ochu C, Ogunwobi OO (2020) Oncogenic role of PVT1 and therapeutic implications. Front Oncol 10:17. https://doi.org/10.3389/fonc.2020.00017
Li Y, Li S, Luo Y, Liu Y, Yu N (2017) LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol 36(7):571–580. https://doi.org/10.1089/dna.2017.3678
Zhao Y, Zhao J, Guo X, She J, Liu Y (2018) Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep 38(5). https://doi.org/10.1042/BSR20180576
Lu X, Yu Y, Yin F et al (2020) Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharmacol 79:106052. https://doi.org/10.1016/j.intimp.2019.106052
Ding LB, Li Y, Liu GY et al (2020) Long non-coding RNA PVT1, a molecular sponge of miR-26b, is involved in the progression of hyperglycemia-induced collagen degradation in human chondrocytes by targeting CTGF/TGF. Innate Immun 26(3):204–214. https://doi.org/10.1177/1753425919881778
Xu K, Meng Z, Xian XM et al (2020) LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-α in synoviocytes by sponging miR-211-3p. Mol Cell Probes 52:101560. https://doi.org/10.1016/j.mcp.2020.101560
Wang J, Kong X, Hu H, Shi S (2020) Knockdown of long non-coding RNA PVT1 induces apoptosis of fibroblast-like synoviocytes through modulating miR-543-dependent SCUBE2 in rheumatoid arthritis. J Orthop Surg Res 15(1):142. https://doi.org/10.1186/s13018-020-01641-6
Zhang CW, Wu X, Liu D et al (2019) Long non-coding RNA PVT1 knockdown suppresses fibroblast-like synoviocyte inflammation and induces apoptosis in rheumatoid arthritis through demethylation of. J Biol Eng 13:60. https://doi.org/10.1186/s13036-019-0184-1
Zhou H, Sun L, Wan F (2019) Molecular mechanisms of TUG1 in the proliferation, apoptosis, migration and invasion of cancer cells. Oncol Lett 18(5):4393–4402. https://doi.org/10.3892/ol.2019.10848
Tang LP, Ding JB, Liu ZH, Zhou GJ (2018) LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev Med Pharmacol Sci 22(24):8574–8581. https://doi.org/10.26355/eurrev_201812_16620
Liang Z, Ren C (2018) Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed Pharmacother 103:897–902. https://doi.org/10.1016/j.biopha.2018.04.085
Ghafouri-Fard S, Taheri M (2019) UCA1 long non-coding RNA: an update on its roles in malignant behavior of cancers. Biomed Pharmacother 120:109459. https://doi.org/10.1016/j.biopha.2019.109459
Wang G, Bu X, Zhang Y et al (2017) LncRNA-UCA1 enhances MMP-13 expression by inhibiting miR-204-5p in human chondrocytes. Oncotarget 8(53):91281–91290. https://doi.org/10.18632/oncotarget.20108
Yan ZF, Zhao XY, Liu W, Liu XP (2018) UCA1 impacts progress of rheumatoid arthritis by inducing the apoptosis of fibroblast-like synoviocyte. Eur Rev Med Pharmacol Sci 22(4):914–920. https://doi.org/10.26355/eurrev_201802_14370
Baldinu P, Cossu A, Manca A et al (2004) Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat 23(4):318–326. https://doi.org/10.1002/humu.20015
Palmieri G, Paliogiannis P, Sini MC et al (2017) Long non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol 111:31–38. https://doi.org/10.1016/j.critrevonc.2017.01.003
Sun Y, Kang S, Pei S, Sang C, Huang Y (2020) MiR93-5p inhibits chondrocyte apoptosis in osteoarthritis by targeting lncRNA CASC2. BMC Musculoskelet Disord 21(1):26. https://doi.org/10.1186/s12891-019-3025-y
Huang T, Wang J, Zhou Y, Zhao Y, Hang D, Cao Y (2019) LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci Rep 39(5). https://doi.org/10.1042/BSR20182454
Liu C, Guo X, Bai S, Zeng G, Wang H (2020) lncRNA CASC2 downregulation participates in rheumatoid arthritis, and CASC2 overexpression promotes the apoptosis of fibroblast-like synoviocytes by downregulating IL-17. Mol Med Rep 21(5):2131–2137. https://doi.org/10.3892/mmr.2020.11018
Congrains A, Kamide K, Ohishi M, Rakugi H (2013) ANRIL: molecular mechanisms and implications in human health. Int J Mol Sci 14(1):1278–1292. https://doi.org/10.3390/ijms14011278
Kotake Y, Nakagawa T, Kitagawa K et al (2011) Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 30(16):1956–1962. https://doi.org/10.1038/onc.2010.568
Kong Y, Hsieh CH, Alonso LC (2018) A lncRNA at the CDKN2A/B locus with roles in cancer and metabolic disease. Front Endocrinol (Lausanne) 9:405. https://doi.org/10.3389/fendo.2018.00405
Li X, Huang TL, Zhang GD, Jiang JT, Guo PY (2019) LncRNA ANRIL impacts the progress of osteoarthritis via regulating proliferation and apoptosis of osteoarthritis synoviocytes. Eur Rev Med Pharmacol Sci 23(22):9729–9737. https://doi.org/10.26355/eurrev_201911_19535
Zhang TP, Zhu BQ, Tao SS et al (2019) Long non-coding RNAs genes polymorphisms and their expression levels in patients with rheumatoid arthritis. Front Immunol 10:2529. https://doi.org/10.3389/fimmu.2019.02529
Ye S, Zhu S, Feng L (2020) LncRNA ANRIL/miR-125a axis exhibits potential as a biomarker for disease exacerbation, severity, and inflammation in bronchial asthma. J Clin Lab Anal 34(3):e23092. https://doi.org/10.1002/jcla.23092
Wang X, Sun W, Shen W et al (2016) Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol 64(6):1283–1294. https://doi.org/10.1016/j.jhep.2016.01.019
Huang J, Liu L, Yang J, Ding J, Xu X (2019) lncRNA DILC is downregulated in osteoarthritis and regulates IL-6 expression in chondrocytes. J Cell Biochem 120(9):16019–16024. https://doi.org/10.1002/jcb.28880
Wang G, Tang L, Zhang X, Li Y (2019) LncRNA DILC participates in rheumatoid arthritis by inducing apoptosis of fibroblast-like synoviocytes and down-regulating IL-6. Biosci Rep 39(5). https://doi.org/10.1042/BSR20182374
Xu D, Jiang Y, Yang L et al (2017) Long noncoding RNAs expression profile and functional networks in rheumatoid arthritis. Oncotarget 8(56):95280–95292. https://doi.org/10.18632/oncotarget.20036
Zhang P, Sun J, Liang C et al (2020) lncRNA IGHC. Mediat Inflamm 2020:9743037. https://doi.org/10.1155/2020/9743037
Chen S, Liang H, Yang H et al (2017) LincRNa-p21: function and mechanism in cancer. Med Oncol 34(5):98. https://doi.org/10.1007/s12032-017-0959-5
Tang L, Ding J, Zhou G, Liu Z (2018) LncRNA-p21 promotes chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-451. Mol Med Rep 18(6):5295–5301. https://doi.org/10.3892/mmr.2018.9506
Spurlock CF, Tossberg JT, Matlock BK, Olsen NJ, Aune TM (2014) Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol 66(11):2947–2957. https://doi.org/10.1002/art.38805
Zimta AA, Tigu AB, Braicu C, Stefan C, Ionescu C, Berindan-Neagoe I (2020) An emerging class of long non-coding RNA with oncogenic role arises from the snoRNA host genes. Front Oncol 10:389. https://doi.org/10.3389/fonc.2020.00389
Thin KZ, Tu JC, Raveendran S (2019) Long non-coding SNHG1 in cancer. Clin Chim Acta 494:38–47. https://doi.org/10.1016/j.cca.2019.03.002
Lei J, Fu Y, Zhuang Y, Zhang K, Lu D (2019) LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci Rep 39(9). https://doi.org/10.1042/BSR20191523
Li Z, Chao TC, Chang KY et al (2014) The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A 111(3):1002–1007. https://doi.org/10.1073/pnas.1313768111
Liu G, Wang Y, Zhang M, Zhang Q (2019) Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol 66:354–361. https://doi.org/10.1016/j.intimp.2018.11.038
Moharamoghli M, Hassan-Zadeh V, Dolatshahi E, Alizadeh Z, Farazmand A (2019) The expression of GAS5, THRIL, and RMRP lncRNAs is increased in T cells of patients with rheumatoid arthritis. Clin Rheumatol 38(11):3073–3080. https://doi.org/10.1007/s10067-019-04694-z
Liang Y, Li H, Gong X, Ding C (2020) Long non-coding RNA THRIL mediates cell growth and inflammatory response of fibroblast-like Synoviocytes by activating PI3K/AKT signals in rheumatoid arthritis. Inflammation 43(3):1044–1053. https://doi.org/10.1007/s10753-020-01189-x
Dong D, Mu Z, Zhao C (2018) Sun M. : a novel tumor-related long non-coding RNA. Cancer Cell Int 18:125. https://doi.org/10.1186/s12935-018-0623-y
Ye Y, Gao X, Yang N (2018) LncRNA ZFAS1 promotes cell migration and invasion of fibroblast-like synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum Cell 31(1):14–21. https://doi.org/10.1007/s13577-017-0179-5
Gergianaki I, Bortoluzzi A, Bertsias G (2018) Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 32(2):188–205. https://doi.org/10.1016/j.berh.2018.09.004
Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD (2017) Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev 16(3):258–268. https://doi.org/10.1016/j.autrev.2017.01.007
Lewis MJ, Jawad AS (2017) The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford) 56(suppl_1):i67–i77. https://doi.org/10.1093/rheumatology/kew399
Ferretti C, La Cava A (2016) Chapter 8 - overview of the pathogenesis of systemic lupus erythematosus. In: Tsokos GC (ed) Systemic lupus erythematosus. Academic, pp 55–62
Sawalha AH (2016) Chapter 15 - neutrophils in systemic lupus erythematosus. In: Basic, applied and clinical aspects
Jacquemin C, Blanco P (2016) Chapter 16 - the role of dendritic cells in systemic lupus erythematosus. In: Tsokos GC (ed) Systemic lupus erythematosus. Academic, pp 131–136
Drijvers JM, Pillai S (2016) Chapter 14 - integrating current thinking on peripheral B-cell tolerance in lupus. In: Tsokos GC (ed) Systemic lupus erythematosus. Academic, pp 121–126
Tsokos GC (2020) Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 21(6):605–614. https://doi.org/10.1038/s41590-020-0677-6
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE (2016) New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12(12):716–730. https://doi.org/10.1038/nrrheum.2016.186
Hagberg N, Rönnblom L (2016) Chapter 19 - the interferon system in lupus erythematosus. In: Tsokos GC (ed) Systemic lupus erythematosus. Academic, pp 153–158
Luan M, Shang Z, Teng Y et al (2017) The shared and specific mechanism of four autoimmune diseases. Oncotarget 8(65):108355–108374. https://doi.org/10.18632/oncotarget.19383
Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC (2017) Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol Med 23(7):615–635. https://doi.org/10.1016/j.molmed.2017.05.006
Moulton VR (2016) Chapter 17 - Cytokines. In: Tsokos GC (ed) Systemic lupus erythematosus. Academic, pp 137–141
Sunahori K, Nagpal K, Hedrich CM, Mizui M, Fitzgerald LM, Tsokos GC (2013) The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in T-cells from controls and systemic lupus erythematosus patients. J Biol Chem 288(30):21936–21944. https://doi.org/10.1074/jbc.M113.467266
Ye H, Wang X, Wang L et al (2019) Full high-throughput sequencing analysis of differences in expression profiles of long noncoding RNAs and their mechanisms of action in systemic lupus erythematosus. Arthritis Res Ther 21(1):70. https://doi.org/10.1186/s13075-019-1853-7
Luo Q, Li X, Xu C et al (2018) Integrative analysis of long non-coding RNAs and messenger RNA expression profiles in systemic lupus erythematosus. Mol Med Rep 17(3):3489–3496. https://doi.org/10.3892/mmr.2017.8344
Li J, Wu GC, Zhang TP et al (2017) Association of long noncoding RNAs expression levels and their gene polymorphisms with systemic lupus erythematosus. Sci Rep 7(1):15119. https://doi.org/10.1038/s41598-017-15156-4
Wu GC, Hu Y, Guan SY, Ye DQ, Pan HF (2019) Differential plasma expression profiles of long non-coding RNAs reveal potential biomarkers for systemic lupus erythematosus. Biomol Ther 9(6). https://doi.org/10.3390/biom9060206
Xu H, Chen W, Zheng F et al (2020) Reconstruction and analysis of the aberrant lncRNA-miRNA-mRNA network in systemic lupus erythematosus. Lupus 29(4):398–406. https://doi.org/10.1177/0961203320908927
Li C, Pan S, Song Y, Li Y, Qu J (2019) Silence of lncRNA MIAT protects ATDC5 cells against lipopolysaccharides challenge via up-regulating miR-132. Artif Cells Nanomed Biotechnol 47(1):2521–2527. https://doi.org/10.1080/21691401.2019.1626410
Ali MA, Shaker OG, Khalefa AA et al (2020) Serum long noncoding RNAs FAS-AS1 & PVT1 are novel biomarkers for systemic lupus erythematous. Br J Biomed Sci:1–5. https://doi.org/10.1080/09674845.2020.1765459
Zhu JK, He TD, Wei ZX, Wang YM (2018) LncRNA FAS-AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur Rev Med Pharmacol Sci 22(10):2966–2972. https://doi.org/10.26355/eurrev_201805_15051
Wang JB, Li J, Zhang TP et al (2019) Expression of several long noncoding RNAs in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Adv Med Sci 64(2):430–436. https://doi.org/10.1016/j.advms.2019.08.002
Wang Y, Chen S, Du J et al (2018) Long noncoding RNA expression profile and association with SLEDAI score in monocyte-derived dendritic cells from patients with systematic lupus erythematosus. Arthritis Res Ther 20(1):138. https://doi.org/10.1186/s13075-018-1640-x
Xuan J, Xiong Y, Shi L, Aramini B, Wang H (2019) Do lncRNAs and circRNAs expression profiles influence discoid lupus erythematosus progression?-a comprehensive analysis. Ann Transl Med 7(23):728. https://doi.org/10.21037/atm.2019.12.10
Guo G, Chen A, Ye L et al (2020) TCONS_00483150 as a novel diagnostic biomarker of systemic lupus erythematosus. Epigenomics 12(11):973–988. https://doi.org/10.2217/epi-2019-0265
Li L-J, Zhao W, Tao S-S et al (2017) Comprehensive long non-coding RNA expression profiling reveals their potential roles in systemic lupus erythematosus. Cell Immunol 319:17–27. https://doi.org/10.1016/j.cellimm.2017.06.004
Yang H, Liang N, Wang M et al (2017) Long noncoding RNA MALAT-1 is a novel inflammatory regulator in human systemic lupus erythematosus. Oncotarget 8(44):77400–77406. https://doi.org/10.18632/oncotarget.20490
Gao F, Tan Y, Luo H (2020) MALAT1 is involved in type I IFNs-mediated systemic lupus erythematosus by up-regulating OAS2, OAS3, and OASL. Braz J Med Biol Res 53(5):e9292. https://doi.org/10.1590/1414-431X20209292
Wu GC, Li J, Leng RX et al (2017) Identification of long non-coding RNAs GAS5, linc0597 and lnc-DC in plasma as novel biomarkers for systemic lupus erythematosus. Oncotarget 8(14):23650–23663. https://doi.org/10.18632/oncotarget.15569
Suo QF, Sheng J, Qiang FY, Tang ZS, Yang YY (2018) Association of long non-coding RNA GAS5 and miR-21 levels in CD4. Exp Ther Med 15(1):345–350. https://doi.org/10.3892/etm.2017.5429
Zhang F, Wu L, Qian J et al (2016) Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun 75:96–104. https://doi.org/10.1016/j.jaut.2016.07.012
Dong G, Yang Y, Li X et al (2020) Granulocytic myeloid-derived suppressor cells contribute to IFN-I signaling activation of B cells and disease progression through the lncRNA NEAT1-BAFF axis in systemic lupus erythematosus. Biochim Biophys Acta Mol basis Dis 1866(1):165554. https://doi.org/10.1016/j.bbadis.2019.165554
Zhang LH, Xiao B, Zhong M et al (2020) LncRNA NEAT1 accelerates renal mesangial cell injury via modulating the miR-146b/TRAF6/NF-κB axis in lupus nephritis. Cell Tissue Res. https://doi.org/10.1007/s00441-020-03248-z
Rider V, Abdou NI, Kimler BF, Lu N, Brown S, Fridley BL (2018) Gender bias in human systemic lupus erythematosus: a problem of steroid receptor action? Front Immunol 9:611. https://doi.org/10.3389/fimmu.2018.00611
Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC (2016) Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A 113(14):E2029–E2038. https://doi.org/10.1073/pnas.1520113113
Martin AR, Syrett CM, Myles A, Atchison ML, Anguera MC (2018) Atypical Xist RNA localization to the inactive X in a female-biased murine model of systemic lupus erythematosus. J Immunol 200(1 Supplement):40.18–40.18
Syrett CM, Paneru B, Sandoval-Heglund D et al (2019) Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight 4(7). https://doi.org/10.1172/jci.insight.126751
Zhang Y, Li X, Gibson A, Edberg J, Kimberly RP, Absher DM (2020) Skewed allelic expression on X-chromosome associated with aberrant expression of XIST on systemic lupus erythematosus lymphocytes. Hum Mol Genet. https://doi.org/10.1093/hmg/ddaa131
Lee JT, Davidow LS, Warshawsky D (1999) Tsix, a gene antisense to Xist at the X-inactivation Centre. Nat Genet 21(4):400–404. https://doi.org/10.1038/7734
Loos F, Maduro C, Loda A et al (2016) Xist and Tsix transcription dynamics is regulated by the X-to-autosome ratio and Semistable transcriptional states. Mol Cell Biol 36(21):2656–2667. https://doi.org/10.1128/MCB.00183-16
Cao HY, Li D, Wang YP, Lu HX, Sun J, Li HB (2020) Clinical significance of reduced expression of lncRNA TUG1 in the peripheral blood of systemic lupus erythematosus patients. Int J Rheum Dis 23(3):428–434. https://doi.org/10.1111/1756-185X.13786
Xu Y, Deng W, Zhang W (2018) Long non-coding RNA TUG1 protects renal tubular epithelial cells against injury induced by lipopolysaccharide via regulating microRNA-223. Biomed Pharmacother 104:509–519. https://doi.org/10.1016/j.biopha.2018.05.069
Cao HY, Li D, Wang YP, Lu HX, Sun J, Li HB (2020) The protection of NF-κB inhibition on kidney injury of systemic lupus erythematosus mice may be correlated with lncRNA TUG1. Kaohsiung J Med Sci 36(5):354–362. https://doi.org/10.1002/kjm2.12183
Jiang CR, Li TH (2018) Circulating UCA1 is highly expressed in patients with systemic lupus erythematosus and promotes the progression through the AKT pathway. Eur Rev Med Pharmacol Sci 22(8):2364–2371. https://doi.org/10.26355/eurrev_201804_14828
Deng Y, Luan S, Zhang Q, Xiao Y (2018) Long noncoding RNA THRIL contributes in lipopolysaccharide-induced HK-2 cells injury by sponging miR-34a. J Cell Biochem. https://doi.org/10.1002/jcb.27354
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wijesinghe, S.N., Lindsay, M.A., Jones, S.W. (2022). Long Non-coding RNAs in Rheumatology. In: Carpenter, S. (eds) Long Noncoding RNA. Advances in Experimental Medicine and Biology, vol 1363. Springer, Cham. https://doi.org/10.1007/978-3-030-92034-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-92034-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92033-3
Online ISBN: 978-3-030-92034-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)