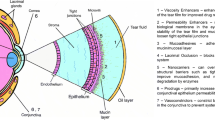
Abstract
Ophthalmic emulsions are formulation described in the literature since more than 30 years and officially listed in the US and European pharmacopeias as appropriated ocular dosage forms. However, while once was expecting that emulsion would be used as drug vehicle, surprisingly a new category of products emerged from the market, the artificial tears based on emulsions. Those products are the new generation of artificial tears being now as widely used in place of hydrogels. These eye drops provide several advantages over hydrogels or saline solutions as they supplement the tears with lipids acting as lubricant and more importantly as a barrier against evaporation and tear film stabilizer. On the other hand, emulsions as drug carriers were very rare to reach the market. About 35 active ingredients were tested in emulsions and described in more than 55 scientific articles, leading to only four prescription products in the USA and Europe. Restasis reached the US market in 2003, followed by Durezol in 2006, Ikervis in 2015 in Europe, and Xelpros in the USA in 2018. Some other products under clinical stage may reach the market within the next 5 years. This chapter is giving an outlook on the field of ophthalmic emulsions, providing the last scientific updates for the two categories of products. The different products marketed and under development are described and discussed, and the technical gaps to fill are pointed out to facilitate other developments to emphasize the importance of that complex but very useful dosage form.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AT:
-
Artificial tears
- AUC:
-
Area under curve
- BAK:
-
Benzalkonium chloride
- BUT:
-
Tear breakup time
- CK:
-
Cetalkonium chloride
- CsA:
-
Cyclosporin A
- DED:
-
Dry eye disease
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- HMW:
-
High molecular weight
- HPMC:
-
Hydroxypropyl methylcellulose
- MCT:
-
Medium-chain triglycerides
- MGD:
-
Meibomian gland dysfunction
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- o/w:
-
Oil in water
- PC:
-
Phosphatidylcholine
- SEDDSs:
-
Self-emulsifying drug delivery systems
- SH:
-
Sodium hyaluronate
- TFLL:
-
Tear film lipid layer
- v/v:
-
Volume/volume
- VKC:
-
Vernal keratoconjunctivitis
- w/v:
-
Weight/volume
- w/w:
-
Weight/weight
References
Acheampong AA, Shackleton M, Tang-Liu DD, Ding S, Stern ME, Decker R. Distribution of cyclosporin a in ocular tissues after topical administration to albino rabbits and beagle dogs. Curr Eye Res. 1999;18(2):91–103.
Acheampong A, Tang-Liu DD, Chang JN, Power DF. Methods of providing therapeutic effects using cyclosporin components. United States. Patent US8629111B2; 2014.
Aguilar AJ, Marquez MI, Albera PA, Tredicce JL, Berra A. Effects of Systane((R)) balance on noninvasive tear film break-up time in patients with lipid-deficient dry eye. Clin Ophthalmol. 2014;8:2365–72.
Amrane M, Creuzot-Garcher C, Robert PY, Ismail D, Garrigue JS, Pisella PJ, Baudouin C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease - a randomised comparative study. J Fr Ophtalmol. 2014;37(8):589–98.
Angelov O, Wiese A, Yuan Y, Andersen J, Acheampong A, Brar B. Preclinical safety studies of cyclosporine ophthalmic emulsion. Adv Exp Med Biol. 1998;438:991–5.
Anselem S, Beilin M, Garty N. Submicron emulsion as ocular delivery system for adaprolol maleate, a soft b-blocker. Pharm Res. 1993;10(Suppl):S205.
Ashara KC, Shah KV. Emulsion of chloramphenicol: an overwhelming approach for ocular delivery. Folia Med (Plovdiv). 2017;59(1):23–30.
Aydemir O, Demir T, Türkçüoğlu P, Turgut B, Çeliker Ü (2008) Efficiency of additional topical ophthalmic phospholipid-based microemulsion (Lipimix) in the treatment of chronic blepharitis with dry eye. Paper presented at the 9th congress of the International Society of Dacryology and dry eye, Istanbul, Turkey,
Baspinar Y, Bertelmann E, Pleyer U, Buech G, Siebenbrodt I, Borchert HH. Corneal permeation studies of everolimus microemulsion. J Ocul Pharmacol Ther. 2008;24(4):399–402.
Bausch & Lomb. Lacrinmune (product information; in Spanish); 2009.
Beilin M, Bar-Ilan A, Amselem S. Ocular retention time of submicron emulsion (SME) and the miotic response to pilocarpine delivered in SME. Invest Ophthalmol Vis Sci. 1995;36:S166.
Bouyer E, Mekhloufi G, Rosilio V, Grossiord J-L, Agnely F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: alternatives to synthetic surfactants in the pharmaceutical field? Int J Pharm. 2012;436(1–2):359–78.
Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–60.
Bucolo C, Marrazzo G, Platania CB, Romano GL, Drago F, Salomone S. Effects of topical indomethacin, bromfenac and nepafenac on lipopolysaccharide-induced ocular inflammation. J Pharm Pharmacol. 2014;66(7):954–60.
Buech G, Bertelmann E, Pleyer U, Siebenbrodt I, Borchert HH. Formulation of sirolimus eye drops and corneal permeation studies. J Ocul Pharmacol Ther. 2007;23(3):292–303.
Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids--a review. Curr Eye Res. 2008;33(5):405–20.
Calvo P, Alonso MJ, Vila-Jato JL, Robinson JR. Improved ocular bioavailability of indomethacin by novel ocular drug carriers. J Pharm Pharmacol. 1996a;48(11):1147–52.
Calvo P, Vila-Jato JL, Alonso MJ. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J Pharm Sci. 1996b;85(5):530–6.
Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9(1):87–93.
Cohen T, Sauvageon-Martre H, Brossard D, D’Hermies F, Bardin C, Chast F, Chaumeil JC. Amphotericin B eye drops as a lipidic emulsion. Int J Pharm. 1996;137:249–54.
Cwiklik L. Tear film lipid layer: a molecular level view. Biochim Biophys Acta. 2016;1858:2421–30.
Cwiklik L, Melcrova A, Daull P, Garrigue J. Tear film break-up: a molecular level view by employing in silico approach. Paper presented at the ARVO, Baltimore; 2017.
Czajkowska-Kosnik A, Sznitowska M, Mirkowska K. Self-emulsifying oils for ocular drug delivery. II. In vitro release of indomethacin and hydrocortisone. Acta Pol Pharm. 2012;69(2):309–17.
Daull P, Garrigue JS. reservative-free cationic nanoemulsion of latanoprost. Ophthalmology times Europe. 2013 [Epub ahead of print].
Daull P, Buggage R, Lambert G, Faure MO, Serle J, Wang RF, Garrigue JS. A comparative study of a preservative-free Latanoprost cationic emulsion (Catioprost) and a BAK-preserved Latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012;28(5):515–23.
Daull P, Lallemand F, Philips B, Lambert G, Buggage R, Garrigue JS. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits. Cornea. 2013a;32(3):345–54.
Daull P, Paterson CA, Kuppermann BD, Garrigue JS. A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. J Ocul Pharmacol Ther. 2013b;29:258–69.
Daull P, Lallemand F, Garrigue JS. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. JPP. 2014;66(4):531–41.
Daull P, Feraille L, Elena PP, Garrigue JS. Comparison of the anti-inflammatory effects of artificial tears in a rat model of corneal scraping. J Ocul Pharmacol Ther. 2016;32:109–18.
Daull P, Amrane M, Garrigue JS. Novasorb® cationic nanoemulsion and latanoprost: the ideal combination for glaucoma management? Glauc Open Access. 2017;2(1):107.
Daull P, Guenin S, Hamon de Almeida V, Garrigue JS. Anti-inflammatory activity of CKC-containing cationic emulsion eye drop vehicles. Mol Vis. 2018;24:459–70.
DEWS. The epidemiology of dry eye disease: report of the epidemiology Subcommittee of the International dry eye WorkShop (2007). Ocul Surf. 2007;5:93–107.
Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin Pharmacother. 2012;
Dong Y, Hengst L, Patel D, Hunt R, Qu H, Choi S, Ashraf M, Cruz CN, Xu X. A kinetic approach to determining drug distribution in complex biphasic systems. J Pharm Sci. 2019;108:2002–11.
Donnenfeld ED. Difluprednate for the prevention of ocular inflammation postsurgery: an update. Clin Ophthalmol. 2011;5:811–6.
European Medicines Agency. Ikervis authorisation. 2015. https://www.ema.europa.eu/documents/assessment-report/ikervis-epar-public-assessment-report_en.pdf. Accessed 11 Feb 2019.
European Medicines Agency. Verkazia authorisation. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/verkazia. Accessed 11 Feb 2019.
Faulds D, Goa KL, Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45(6):953–1040.
Fialho SL, da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Exp Ophthalmol. 2004;32(6):626–32.
Fogt JS, Kowalski MJ, King-Smith PE, Epitropolous AT, Hendershot AJ, Lembach C, Maszczak JP, Jones-Jordan LA, Barr JT. Tear lipid layer thickness with eye drops in meibomian gland dysfunction. Clin Ophthalmol. 2016;10:2237–43.
Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52(4):369–74.
Gallarate M, Gasco MR, Trotta M. Influence of octanoic acid on membrane permeability of timolol from solutions and from microemulsions. Acta pharmaceutica technologica. 1988;34(2):102–5.
Gallarate M, Gasco MR, Trotta M, Chetoni P, Saettone M. Preparation and evaluation in vitro of solutions and o/w microemulsions containing levobunolol as ion-pair. Int J Pharm. 1993;100(1–3):219–25.
Gallarate M, Chirio D, Bussano R, Peira E, Battaglia L, Baratta F, Trotta M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm. 2013;440(2):126–34.
Garg P, Tuteja N, Qayum S. To study the efficacy of difluprednate ophthalmic emulsion and prednisolone acetate ophthalmic suspension on post-operative inflammation in cataract surgery. J Clin Diagn Res. 2016;10(12):NC05–8.
Garrigue JS, Amrane M, Faure MO, Holopainen JM, Tong L. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharmacol Ther. 2017;33(9):647–61.
Garty N, Lusky M, Zalish M, Rachmiel R, Greenbaum A, Desatnik H, Neumann R, Howes J, Melamed S. Pilocarpine in submicron emulsion formulation for treatment of ocular hypertension-a phase-ii clinical-trial. In: Investigative ophthalmology & visual science, vol. 4. Philadelphia: Lippincott-Raven; 1994. p. 2175.
Gasco MR, Gallarate M, Trotta M, Bauchiero L, Gremmo E, Chiappero O. Microemulsions as topical delivery vehicles: ocular administration of timolol. J Pharm Biomed Anal. 1989;7(4):433–9.
Gehrmann S, Bunjes H. Preparation of nanoemulsions by premix membrane emulsification: which parameters have a significant influence on the resulting particle size? J Pharm Sci. 2017;106(8):2068–76.
Georgiev GA, Yokoi N, Ivanova S, Tonchev V, Daull P. Surface chemistry of the interactions of cationic nanoemulsions with human meibum films. Paper presented at the ARVO, Seattle, 1–5 May 2016.
Georgiev GA, Yokoi N, Nencheva Y, Peev N, Daull P. Surface chemistry interactions of Cationorm with films by human Meibum and tear film compounds. Int J Mol Sci. 2017;18(7):1558.
Glasser CA, Vila MM, Pereira JC, Chaud MV, Oliveira Junior JM, Tubino M, Balcao VM. Development of a water-in-oil-in-water multiple emulsion system integrating biomimetic aqueous-core lipid nanodroplets for protein entity stabilization. Part II: process and product characterization. Drug Dev Ind Pharm. 2016;42(12):1990–2000.
Gore A, Pujara C, Attar M, Neervannan S. Ocular emulsions and dry eye: a case study of a non-biological complex drug product delivered to a complex organ to treat a complex disease. GABI J. 2017;6(1):13–23.
Goto E, Shimazaki J, Monden Y, Takano Y, Yagi Y, Shimmura S, Tsubota K. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109(11):2030–5.
Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004;45(7):2342–7.
Gulsen D, Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int J Pharm. 2005;292(1–2):95–117.
Hagigit T, Nassar T, Behar-Cohen F, Lambert G, Benita S. The influence of cationic lipid type on in-vitro release kinetic profiles of antisense oligonucleotide from cationic nanoemulsions. Eur J Pharm Biopharm. 2008;70(1):248–59.
Hagigit T, Abdulrazik M, Orucov F, Valamanesh F, Lambert M, Lambert G, Behar-Cohen F, Benita S. Topical and intravitreous administration of cationic nanoemulsions to deliver antisense oligonucleotides directed towards VEGF KDR receptors to the eye. J Control Release. 2010;145(3):297–305.
Hamdi Y, Lallemand F, Benita S. Drug-loaded nanocarriers for back-of-the-eye diseases-formulation limitations. J Drug Deliv Sci Technol. 2015;30:331–41.
Hasegawa T, Amako H, Yamamoto T, Tazawa M, Sakamoto Y. Corneal-protective effects of an artificial tear containing sodium hyaluronate and castor oil on a porcine short-term dry eye model. J Vet Med Sci. 2014;76(9):1219–24.
Ibrahim SS, Awad GA, Geneidi A, Mortada ND. Comparative effects of different cosurfactants on sterile prednisolone acetate ocular submicron emulsions stability and release. Colloids Surf B Biointerfaces. 2009;69(2):225–31.
Irimia T, Dinu-Pirvu CE, Ghica MV, Lupuleasa D, Muntean DL, Udeanu DI, Popa L. Chitosan-based in situ gels for ocular delivery of therapeutics: a state-of-the-art review. Mar Drugs. 2018;16(10):373.
Joussen AM, Rohrschneider K, Reichling J, Kirchhof B, Kruse FE. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp Eye Res. 2000;71(5):483–7.
Kaercher T, Thelen U, Brief G, Morgan-Warren RJ, Leaback R. A prospective, multicenter, noninterventional study of Optive Plus((R)) in the treatment of patients with dry eye: the prolipid study. Clin Ophthalmol. 2014;8:1147–55.
Kakimoto H, Takamura Y, Arimura S, Miyake S, Matsumura T, Gozawa M, Iwasaki K, Morioka M, Yamada Y, Inatani M. Effect of 0.05% difluprednate ophthalmic emulsion on proinflammatory cytokine levels after retinal laser photocoagulation in rabbits. J Ocul Pharmacol Ther. 2018;34(5):410–5.
Kesavan K, Kant S, Singh PN, Pandit JK. Mucoadhesive chitosan-coated cationic microemulsion of dexamethasone for ocular delivery: in vitro and in vivo evaluation. Curr Eye Res. 2013;38(3):342–52.
Khanal S, Tomlinson A, Pearce EI, Simmons PA. Effect of an oil-in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26(2):175–81.
Kimura M, Yasueda S-I, Yamaguchi M, Inada K. Compositions containing difluprednate. Patent EP0878197 of 1998; 2000.
Kinnunen K, Kauppinen A, Piippo N, Koistinen A, Toropainen E, Kaarniranta K. Cationorm shows good tolerability on human HCE-2 corneal epithelial cell cultures. Exp Eye Res. 2014;120:82–9.
Klang S, Baszkin A, Benita S. The stability of piroxicam incorporated in a positively-charged submicron emulsion for ocular administration. Int J Pharm. 1996;132(1–2):33–44.
Klang S, Abdulrazik M, Benita S. Influence of emulsion droplet surface charge on indomethacin ocular tissue distribution. Pharm Dev Technol. 2000;5(4):521–32.
Kurup TRR, Wan LSC, Chan LW. Preservative requirements in emulsions. Pharm Acta Helv. 1992;67(7):204–8.
Kuwano M, Ibuki H, Morikawa N, Ota A, Kawashima Y. Cyclosporine a formulation affects its ocular distribution in rabbits. Pharm Res. 2002;19(1):108–11.
Labbe A, Baudouin C, Ismail D, Amrane M, Garrigue JS, Leonardi A, Figueiredo FC, Van Setten G, Labetoulle M. Pan-European survey of the topical ocular use of ciclosporin a. J Fr Ophtalmol. 2017;40:187–95.
Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Cyclosporine a delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56(3):307–18.
Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. Successfully improving ocular drug delivery using the cationic nanoemulsion Novasorb. J Drug Deliv. 2012;2012:604204. https://doi.org/10.1155/2012/604204.
Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine a delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28.
Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55(2):289–98.
Lefebvre G, Riou J, Bastiat G, Roger E, Frombach K, Gimel JC, Saulnier P, Calvignac B. Spontaneous nano-emulsification: process optimization and modeling for the prediction of the nanoemulsion’s size and polydispersity. Int J Pharm. 2017;534(1–2):220–8.
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–8.
Leonardi A, Van Setten G, Amrane M, Ismail D, Garrigue J-S, Figueiredo FC, Baudouin C. Efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–96.
Li CC, Abrahamson M, Kapoor Y, Chauhan A. Timolol transport from microemulsions trapped in HEMA gels. J Colloid Interface Sci. 2007;315(1):297–306.
Li X, Muller RH, Keck CM, Bou-Chacra NA. Mucoadhesive dexamethasone acetate-polymyxin B sulfate cationic ocular nanoemulsion--novel combinatorial formulation concept. Pharmazie. 2016;71(6):327–33.
Liang H, Brignole-Baudouin F, Rabinovich-Guilatt L, Mao Z, Riancho L, Faure MO, Warnet JM, Lambert G, Baudouin C. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: an in vivo study in rabbits. Mol Vis. 2008;14:204–16.
Liang H, Baudouin C, Daull P, Garrigue JS, Brignole-Baudouin F. In vitro and in vivo evaluation of a preservative-free cationic emulsion of latanoprost in corneal wound healing models. Cornea. 2012;31(11):1319–29.
Liu Y, Lin X, Tang X. Lipid emulsions as a potential delivery system for ocular use of azithromycin. Drug Dev Ind Pharm. 2009;35(7):887–96.
Mafi R, Gray C, Pelton R, Ketelson H, Davis J. On formulating ophthalmic emulsions. Colloids Surf B: Biointerfaces. 2014;122:7–11.
Maichuk Iu F, Karimov MK, Lapshina NA. [Ketoconazole in the treatment of ocular mycoses]. Vestn oftalmol. 1990;106(1):44–6.
McCann LC, Tomlinson A, Pearce EI, Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea. 2012;31(1):1–5.
Meehan K, Vollmer L, Sowka J. Intraocular pressure elevation from topical difluprednate use. Optometry. 2010;81(12):658–62.
Mercado-Sesma A, Contreras-Rubio A, Baiza-Duran L, Olvera-Montano O, Miranda-Robles M, Bonilla-Garcia J. Bioavailability of generic 0.05% difluprednate emulsion in the aqueous humor, cornea, and conjunctiva of New Zealand rabbits after a single dose compared with commercial difluprednate. J ophthalmic Inflamm Infect. 2017;7(1):10.
Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45.
Mochizuki H, Yamada M, Hatou S, Tsubota K. Turnover rate of tear-film lipid layer determined by fluorophotometry. Br J Ophthalmol. 2009;93(11):1535–8.
Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419–33.
Muchtar S, Almog S, Torracca MT, Saettone MF, Benita S. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic Res. 1992;24(3):142–9.
Muchtar S, Abdulrazik M, Frucht-Pery J, Benita S. Ex-vivo permeation study of indomethacin from a submicron emulsion through albino rabbit cornea. J Control Release. 1997;44(1):55–64.
Mulki L, Foster CS. Difluprednate for inflammatory eye disorders. Drugs Today (Barc). 2011;47(5):327–33.
Myint M, Bucki R, Janmey PA, Diamond SL. Synthesis and structure-activity relationships of novel cationic lipids with anti-inflammatory and antimicrobial activities. Bioorg Med Chem Lett. 2015;25(14):2837–43.
Naveh N, Muchtar S, Benita S. Pilocarpine incorporated into a submicron emulsion vehicle causes an unexpectedly prolonged ocular hypotensive effect in rabbits. J Ocul Pharmacol. 1994;10(3):509–20.
Naveh N, Weissman C, Muchtar S, Benita S, Mechoulam R. A submicron emulsion of HU-211, a synthetic cannabinoid, reduces intraocular pressure in rabbits. Graefes Arch Clin Exp Ophthalmol. 2000;238(4):334–8.
NDA 206185 (Revised: 09/2018) XelPros(r): full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206185s000lbl.pdf. Accessed 22 Feb 2019.
Nencheva Y, Olzynska A, Melcrova A, As Georgiev G, Daull P, Garrigue J-S, Cwiklik L. Improving stability of tear film lipid layer via concerted action of two drug molecules: a biophysical view. Biophys J. 2018;114(3):104a.
Pandey D, Kesharwani P, Jain D. Entrapment of drug-sorbate complex in submicron emulsion: a potential approach to improve antimicrobial activity in bacterial corneal infection. J Drug Deliv Sci Technol. 2019;49:455–62.
Pathak MK, Chhabra G, Pathak K. Design and development of a novel pH triggered nanoemulsified in-situ ophthalmic gel of fluconazole: ex-vivo transcorneal permeation, corneal toxicity and irritation testing. Drug Dev Ind Pharm. 2013;39:780–90.
Patil SM, Li V, Peng J, Kozak D, Xu J, Cai B, Keire DA, Chen K. A simple and noninvasive DOSY NMR method for droplet size measurement of intact oil-in-water emulsion drug products. J Pharm Sci. 2019;108(2):815–20.
Peng CC, Bengani LC, Jung HJ, Leclerc J, Gupta C, Chauhan A. Emulsions and microemulsions for ocular drug delivery. J Drug Deliv Sci Technol. 2011;21(1):111–21.
Popovic M, Chan C, Lattanzio N, El-Defrawy S, Schlenker MB. Comparative cost evaluation of brand name and generic ophthalmology medications in Ontario. Can J Ophthalmol. 2018;53(2):173–87.
Praestegaard M, Steele F, Gomez F. Pharmacokinetic characterization of a novel ocular formulation of ciclosporin. Invest Ophthalmol Vis Sci. 2016;57(12):5388.
Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. Ocul Surf. 2015;13(1):26–42.
Rabinovich-Guilatt L, Couvreur P, Lambert G, Dubernet C. Cationic vectors in ocular drug delivery. J Drug Target. 2004;12(9–10):623–33.
Rahman Z, Xu X, Katragadda U, Krishnaiah YS, Yu L, Khan MA. Quality by design approach for understanding the critical quality attributes of cyclosporine ophthalmic microemulsion. Mol Pharm. 2014;11(3):787–99.
Rantamaki AH, Telenius J, Koivuniemi A, Vattulainen I, Holopainen JM. Lessons from the biophysics of interfaces: lung surfactant and tear fluid. Prog Retin Eye Res. 2011;30(3):204–15.
Rieger G. Lipid-containing eye drops: a step closer to natural tears. Ophthalmologica. 1990;201(4):206–12.
Robert P-Y, Cochener B, Amrane M, Ismail D, Garrigue J-S, Pisella P-J, Baudouin C. Efficacy and safety of a cationic emulsion in the treatment of signs and symptoms of moderate-to-severe dry eye disease: a prospective randomised controlled study. Eur J Opthalmol. 2016;26:546–55.
Robin JS, Ellis PP. Ophthalmic ointments. Surv Ophthalmol. 1978;22(5):335–40.
Rodriguez-Aller M, Guinchard S, Guillarme D, Pupier M, Jeannerat D, Rivara-Minten E, Veuthey JL, Gurny R. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur J Pharm Biopharm. 2015;95(Pt B):203–14.
Royle L, Matthews E, Corfield A, Berry M, Rudd PM, Dwek RA, Carrington SD. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25(8):763–73.
Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology. 2000;107(4):631–9.
Santen SAS. Phase III, Multinational, Multicenter, Investigator-Masked, Randomised, Active-Controlled Trial, comparing the efficacy and safety of DE-130A with Xalatan® in Patients with Open-Angle Glaucoma or Ocular Hypertension over a 3-Month period, followed by a 12-Month Follow-Up with Open-Label DE-130A Treatment. 2017. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-004262-95. Accessed 15 Feb 2019.
Scaffidi RC, Korb DR. Comparison of the efficacy of two lipid emulsion eyedrops in increasing tear film lipid layer thickness. Eye Contact Lens. 2007;33(1):38–44.
Schulz MB, Daniels R. Hydroxypropylmethylcellulose (HPMC) as emulsifier for submicron emulsions: influence of molecular weight and substitution type on the droplet size after high-pressure homogenization. Eur J Pharm Biopharm. 2000;49(3):231–6.
Scifo C, Barabino S, De Pasquale G, Blanco AR, Mazzone MG, Rolando M. Effects of a new lipid tear substitute in a mouse model of dry eye. Cornea. 2010;29(7):802–6.
Sebba F. The behaviour of minute oil droplets encapsulated in a water film. Colloid Polym Sci. 1979;257(4):392–6.
Selek H, Unlu N, Orhan M, Irkec M. Evaluation of retinoic acid ophthalmic emulsion in dry eye. Eur J Ophthalmol. 2000;10(2):121–7.
Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984;29(2):117–28.
Shen JQ, Gan Y, Gan L, Zhu CL, Zhu JB. [Ion-sensitive nanoemulsion-in situ gel system for ophthalmic delivery of flurbiprofen axetil]. Yao Xue Xu Bao. 2010;45(1):120–5.
Shen J, Gan L, Zhu C, Zhang X, Dong Y, Jiang M, Zhu J, Gan Y. Novel NSAIDs ophthalmic formulation: flurbiprofen axetil emulsion with low irritancy and improved anti-inflammation effect. Int J Pharm. 2011;412(1–2):115–22.
Siebenbrodt I, Keipert S. Poloxamer-systems as potential Ophthalmics II. Microemulsïons. Eur J Pharm Biopharm. 1993;39(1):25–30.
Sila-on W, Vardhanabhuti N, Ongpipattanakul B, Kulvanich P. Influence of incorporation methods on partitioning behavior of lipophilic drugs into various phases of a parenteral lipid emulsion. AAPS PharmSciTech. 2008;9(2):684–92.
Silva-Cunha A, da Silva GR, de Castro WV, Fialho SL. Evaluation of the pharmacokinetics and ocular tolerance of a microemulsion containing tacrolimus. J Ocul Pharmacol Ther. 2014;30(1):59–65.
Sindt CW, Foulks GN. Efficacy of an artificial tear emulsion in patients with dry eye associated with meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1713–22.
Small DS, Acheampong A, Reis B, Stern K, Stewart W, Berdy G, Epstein R, Foerster R, Forstot L, Tang-Liu DD. Blood concentrations of cyclosporin a during long-term treatment with cyclosporin a ophthalmic emulsions in patients with moderate to severe dry eye disease. J Ocul Pharmacol Ther. 2002;18(5):411–8.
Steele F. An ophthalmic composition GB. Patent; 2012.
Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin a ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin a phase 2 study group. Ophthalmology. 2000;107(5):967–74.
Suresh PK, Dewangan D. Ophthalmic delivery system for dexamethasone: an overview. Int J Innov Pharm Res. 2011;2(4):161–5.
Sznitowska M, Janicki S, Dabrowska EA, Gajewska M. Physicochemical screening of antimicrobial agents as potential preservatives for submicron emulsions. Eur J Pharm Sci. 2002;15(5):489–95.
Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004;58(2):357–68.
Tiwari R, Pandey V, Asati S, Soni V, Jain D. Therapeutic challenges in ocular delivery of lipid based emulsion. Egypt J Basic Appl Sci. 2018;5(2):121–9.
US Food and Drug Administration. Restasis drug approval package. 2002. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-023_Restasis.cfm. Accessed 11 Feb 2019.
US Food and Drug Administration. Durezol drug approval package. 2008. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022212s000TOC.cfm. Accessed 11 Feb 2019.
Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21(1):15–34.
Walenga RL, Babiskin AH, Zhang X, Absar M, Zhao L, Lionberger RA. Impact of vehicle physicochemical properties on modeling-based predictions of cyclosporine ophthalmic emulsion bioavailability and tear film breakup time. J Pharm Sci. 2019;108(1):620–9.
Wang Y-D, Tang X-L, Ye C-T, Wu W. Determination of tacrolimus ophthalmic emulsion by HPLC. Chin Pharm J. 2010;45(3):221–3.
Wang X, Patil SM, Keire DA, Xu X, Chen K. Application of ultra-centrifugation and bench-top (19)F NMR for measuring drug phase partitioning for the ophthalmic oil-in-water emulsion products. AAPS PharmSciTech. 2018;19(4):1647–51.
Wehrle P, Korner D, Benita S. Sequential statistical optimization of a positively-charged submicron emulsion of miconazole. Pharm Dev Technol. 1996;1(1):97–111.
Yamaguchi M, Yasueda S, Isowaki A, Yamamoto M, Kimura M, Inada K, Ohtori A. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int J Pharm. 2005;301(1–2):121–8.
Yamaguchi M, Ueda K, Isowaki A, Ohtori A, Takeuchi H, Ohguro N, Tojo K. Mucoadhesive properties of chitosan-coated ophthalmic lipid emulsion containing indomethacin in tear fluid. Biol Pharm Bull. 2009;32(7):1266–71.
Yin J, Xiang C, Lu G. Cationic lipid emulsions as potential bioadhesive carriers for ophthalmic delivery of palmatine. J Microencapsul. 2016;33(8):718–24.
Zhai J, Gu J, Yuan J, Chen J. Tacrolimus in the treatment of ocular diseases. BioDrugs. 2011;25(2):89–103.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Lallemand, F., Garrigue, JS. (2021). Emulsions for Topical Eye Delivery: State of the Art and Future Perspectives. In: Neervannan, S., Kompella, U.B. (eds) Ophthalmic Product Development. AAPS Advances in the Pharmaceutical Sciences Series, vol 37. Springer, Cham. https://doi.org/10.1007/978-3-030-76367-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-76367-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76366-4
Online ISBN: 978-3-030-76367-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)