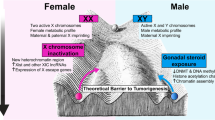
Abstract
Cancers are heterogeneous multifactorial diseases consisting of a major public health issue worldwide. Sex disparities are evidenced in cancer incidence, mortality, expression of prognosis factor, response to treatment, and survival. For both sexes, an interplay of intrinsic and environmental factors influences cancer cells and tumor microenvironment (TME) components. The TME cumulates both supportive and communicative functions, contributing to cancer development, progression, and metastasis dissemination. The frontline topics of this chapter are focused on the contribution of sex, via steroid hormones, such as estrogens and androgens, on the following components of the TME: cancer-associated fibroblasts (CAFs), extracellular matrix (ECM), blood and lymphatic endothelial cells, and immunity/inflammatory system.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Boese AC, Kim SC, Yin K-J, Lee J-P, Hamblin MH (2017) Sex differences in vascular physiology and pathophysiology: Estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 313(3):524–545
Miller VM (2014) Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol 306:781–788
Mauvais-Jarvis F, Bairey Merz N, Barnes PJ et al (2020) Sex and gender: Modifiers of health, disease, and medicine. Lancet 396(10250):565–582
Neigh G, Mitzelfelt M (2016) Sex differences in physiology, 1st edn
Paget S (1889) Distribution of secondary growths in cancer of the breast. Lancet 133(3421):571–573
Folkman J, Merler E, Abernathy C, Williams G (1971) Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133(2):275–288. https://doi.org/10.1084/jem.133.2.275
Nicolson GL (1988) Organ specificity of tumor metastasis: Role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev 7(2):143–188. https://doi.org/10.1007/BF00046483
Hart IR, Fidler IJ (1980) Role of organ selectivity in the determination of metastatic patterns of B16 melanoma - PubMed. Cancer Res 40(7):2281–2287. https://pubmed.ncbi.nlm.nih.gov/7388794/. Accessed 8 Oct 2020
Belli C, Trapani D, Viale G et al (2018) Targeting the microenvironment in solid tumors. Cancer Treat Rev 65:22–32. https://doi.org/10.1016/j.ctrv.2018.02.004
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hanahan D, Coussens LM (2012) Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Soysal SD, Tzankov A, Muenst SE (2015) Role of the tumor microenvironment in breast Cancer. Pathobiology 82(3–4):142–152. https://doi.org/10.1159/000430499
Ribeiro Franco PI, Rodrigues AP, de Menezes LB, Pacheco MM (2020) Tumor microenvironment components: Allies of cancer progression. Pathol Res Pract 216(1):152729. https://doi.org/10.1016/j.prp.2019.152729
Baghban R, Roshangar L, Jahanban-Esfahlan R et al (2020) Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signaling CCS 18(1). https://doi.org/10.1186/s12964-020-0530-4
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR (2019) Targeting tumor microenvironment for cancer therapy. Int J Mol Sci 20(4). https://doi.org/10.3390/ijms20040840
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125:5591–5596. https://doi.org/10.1242/jcs.116392
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. https://doi.org/10.1038/nature07205
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and Cancer. Cell 140(6):883–899. https://doi.org/10.1016/j.cell.2010.01.025
Becker A, Thakur B, Weiss J, Kim H, Peinado H, Lyden D (2016) Extracellular vesicles in Cancer: Cell-to-cell mediators of metastasis. Cancer Cell 30(6):836–848
Rubin JB, Lagas JS, Broestl L et al (2020) Sex differences in cancer mechanisms. Biol Sex Differ 11(1). https://doi.org/10.1186/s13293-020-00291-x
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Ben-Batalla I, Vargas-Delgado ME, Meier L, Loges S (2019) Sexual dimorphism in solid and hematological malignancies. Semin Immunopathol 41(2):251–263. https://doi.org/10.1007/s00281-018-0724-7
Mervic L (2012) Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS One 7(3). https://doi.org/10.1371/journal.pone.0032955
Meltzer S, Bakke KM, Rød KL et al (2020) Sex-related differences in primary metastatic site in rectal cancer; associated with hemodynamic factors? Clin Transl Radiat Oncol 21:5–10. https://doi.org/10.1016/j.ctro.2019.11.006
Ryu ES, Chang SJ, An J et al (2019) Sex-specific differences in risk factors of lymph node metastasis in patients with early gastric cancer. PLoS One 14(10). https://doi.org/10.1371/journal.pone.0224019
Pal SK, Hurria A (2010) Impact of age, sex, and comorbidity on Cancer therapy and disease progression. J Clin Oncol 28(26):4086–4093. https://doi.org/10.1200/JCO.2009.27.0579
Ishibashi H, Suzuki T, Suzuki S et al (2005) Progesterone receptor in non-small cell lung cancer - a potent prognostic factor and possible target for endocrine therapy. Cancer Res 65(14):6450–6458. https://doi.org/10.1158/0008-5472.CAN-04-3087
Holm B, Mellemgaard A, Skov T, Skov BG (2009) Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. J Clin Oncol 27(26):4254–4259. https://doi.org/10.1200/JCO.2008.18.8631
Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF (2011) Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomark Prev 20(8):1629–1637. https://doi.org/10.1158/1055-9965.EPI-11-0246
Kim HI, Lim H, Moon A (2018) Sex differences in cancer: Epidemiology, genetics and therapy. Biomol Ther 26(4):335–342. https://doi.org/10.4062/biomolther.2018.103
Sagerup CMT, Småstuen M, Johannesen TB, Helland Å, Brustugun OT (2011) Sex-specific trends in lung cancer incidence and survival: A population study of 40 118 cases. Thorax 66(4):301–307. https://doi.org/10.1136/thx.2010.151621
Shin JY, Jung HJ, Moon A (2019) Molecular markers in sex differences in cancer. Toxicol Res 35(4):331–341. https://doi.org/10.5487/TR.2019.35.4.331
Dong M, Cioffi G, Wang J et al (2020) Sex differences in Cancer incidence and survival: A Pan-Cancer analysis. Cancer Epidemiol Biomark Prev 29(7):1389–1397. https://doi.org/10.1158/1055-9965.EPI-20-0036
Cook MB, Dawsey SM, Freedman ND et al (2009) Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomark Prev 18(4):1174–1182. https://doi.org/10.1158/1055-9965.EPI-08-1118
McCartney G, Mahmood L, Leyland AH, Batty GD, Hunt K (2011) Contribution of smoking-related and alcohol-related deaths to the gender gap in mortality: Evidence from 30 European countries. Tob Control 20(2):166–168. https://doi.org/10.1136/tc.2010.037929
Liu-Smith F, Farhat AM, Arce A et al (2017) Sex differences in the association of cutaneous melanoma incidence rates and geographic ultraviolet light exposure. J Am Acad Dermatol 76(3):499–505. https://doi.org/10.1016/j.jaad.2016.08.027
Boibessot C, Toren P (2018) Sex steroids in the tumor microenvironment and prostate cancer progression. Endocr Relat Cancer 25(3):R179–R196. https://doi.org/10.1530/ERC-17-0493
Shang Y (2007) Hormones and cancer. Cell Res 17(4):277–279. https://doi.org/10.1038/cr.2007.26
Bolufer P, Collado M, Barragán E et al (2007) The potential effect of gender in combination with common genetic polymorphisms of drug-metabolizing enzymes on the risk of developing acute leukemia. Haematologica 92(3):308–314. https://doi.org/10.3324/haematol.10752
Tevfik Dorak M, Karpuzoglu E (2012) Gender differences in cancer susceptibility: An inadequately addressed issue. Front Genet 3(NOV):1–11. https://doi.org/10.3389/fgene.2012.00268
Schmetzer O, Flörcken A (2012) Sex differences in the drug therapy for oncologic diseases. Handb Exp Pharmacol 214:411–442. https://doi.org/10.1007/978-3-642-30726-3_19
Smida T, Bruno TC, Stabile LP (2020) Influence of estrogen on the NSCLC microenvironment: A comprehensive picture and clinical implications. Front Oncol 10(February):1–15. https://doi.org/10.3389/fonc.2020.00137
Östman A (2012) The tumor microenvironment controls drug sensitivity. Nat Med 18(9):1332–1334. https://doi.org/10.1038/nm.2938
Sun Y, Campisi J, Higano C et al (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 18(9):1359–1368. https://doi.org/10.1038/nm.2890
Wilson TR, Fridlyand J, Yan Y et al (2012) Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487(7408):505–509. https://doi.org/10.1038/nature11249
Acharyya S, Oskarsson T, Vanharanta S et al (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150(1):165–178. https://doi.org/10.1016/j.cell.2012.04.042
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR (2019) Targeting tumor microenvironment for cancer therapy. Int J Mol Sci 20(4). https://doi.org/10.3390/ijms20040840
Swartz MA, Iida N, Roberts EW et al (2012) Tumor microenvironment complexity: Emerging roles in cancer therapy. Am Assoc Cancer Res 72:2473–2480. https://doi.org/10.1158/0008-5472.CAN-12-0122
Bainbridge P (2013) Wound healing and the role of fibroblasts. J Wound Care 22(8):407–412. https://doi.org/10.12968/jowc.2013.22.8.407
Bourgot I, Primac I, Louis T, Noël A, Maquoi E (2020) Reciprocal interplay between Fibrillar collagens and collagen-binding Integrins: Implications in Cancer progression and metastasis. Front Oncol 10. https://doi.org/10.3389/fonc.2020.01488
Kendall RT, Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: Novel roles and mediators. Front Pharmacol 5(May):1–13. https://doi.org/10.3389/fphar.2014.00123
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H (2015) Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers (Basel) 7(4):2443–2458. https://doi.org/10.3390/cancers7040902
Orimo A, Weinberg RA (2007) Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Therapy 6(4):618–619. https://doi.org/10.4161/cbt.6.4.4255
Carnet O, Lecomte J, Masset A et al (2015) Mesenchymal stem cells shed Amphiregulin at the surface of lung carcinoma cells in a Juxtracrine manner. Neoplasia 17(7):552–563. https://doi.org/10.1016/j.neo.2015.07.002
Primac I, Maquoi E, Blacher S et al (2019) Stromal integrin α11 regulates PDGFRβ signaling and promotes breast cancer progression. J Clin Invest 129(11):4609–4628. https://doi.org/10.1172/JCI125890
Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D (2020) Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin Cancer Biol 62:166–181. https://doi.org/10.1016/j.semcancer.2019.08.004
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401
Tang D, Gao J, Wang S et al (2016) Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumor Biol 37(2):1889–1899
Monteran L, Erez N (2019) The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol 10(1835):1–15
Liu T, Han C, Wang S et al (2019) Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J Hematol Oncol 12(86):1–15
Cirillo F, Pellegrino M, Malivindi R et al (2017) GPER is involved in the regulation of the estrogen-metabolizing CYP1B1 enzyme in breast cancer. Oncotarget 8(63):106608–106624. https://doi.org/10.18632/oncotarget.22541
Zhang Y, Cong X, Li Z, Xue Y (2020) Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int Immunopharmacol 78. https://doi.org/10.1016/j.intimp.2019.105937
Daniels G, Lin Gellert L, Melamed J et al (2014) Decreased expression of stromal estrogen receptor α and β in prostate cancer. Am J Transl Res 6(2):140–146
Lai KP, Yamashita S, Huang CK, Yeh S, Chang C (2012) Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med 4(8):791–807. https://doi.org/10.1002/emmm.201101140
LeBleu VS, Kalluri R (2018) A peek into cancer-associated fibroblasts: Origins, functions and translational impact. Dis Model Mech 11:1–9
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E (2020) In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer 146(4):895–905
Liu T, Zhou L, Li D, Andl T, Zhang Y (2019) Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Dev Biol 7(60):1–14
Nishishita R, Morohashi S, Seino H et al (2018) Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol Lett 15(5):6195–6202. https://doi.org/10.3892/ol.2018.8097
Madeo A, Maggiolini M (2010) Nuclear alternate estrogen receptor GPR30 mediates 17β-estradiol - induced gene expression and migration in breast cancer - associated fibroblasts. Cancer Res 70(14):6036–6046. https://doi.org/10.1158/0008-5472.CAN-10-0408
Knower KC, Chand AL, Eriksson N et al (2013) Distinct nuclear receptor expression in stroma adjacent to breast tumors. Breast Cancer Res Treat 142(1):211–223. https://doi.org/10.1007/s10549-013-2716-6
Lappano R, Maggiolini M (2018) GPER is involved in the functional liaison between breast tumor cells and cancer-associated fibroblasts (CAFs). J Steroid Biochem Mol Biol 176:49–56. https://doi.org/10.1016/j.jsbmb.2017.02.019
Luo H, Yang G, Yu T et al (2014) GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr Relat Cancer 21(2):355–369. https://doi.org/10.1530/ERC-13-0237
Luo H, Liu M, Luo S et al (2016) Dynamic monitoring of GPER-mediated estrogenic effects in breast cancer associated fibroblasts: An alternative role of estrogen in mammary carcinoma development. Steroids 112:1–11. https://doi.org/10.1016/j.steroids.2016.03.013
Yu T, Yang G, Hou Y et al (2017) Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene 36(15):2131–2145. https://doi.org/10.1038/onc.2016.370
Avagliano A, Granato G, Ruocco MR et al (2018) Metabolic reprogramming of Cancer associated fibroblasts: The slavery of stromal fibroblasts. Biomed Res Int 2018:1–12. https://doi.org/10.1155/2018/6075403
De Francesco EM, Pellegrino M, Santolla MF et al (2014) GPER mediates activation of HIF1α/VEGF signaling by estrogens. Cancer Res 74(15):4053–4064. https://doi.org/10.1158/0008-5472.CAN-13-3590
Ren J, Guo H, Wu H et al (2015) GPER in CAFs regulates hypoxia-driven breast cancer invasion in a CTGF-dependent manner. Oncol Rep 33(4):1929–1937. https://doi.org/10.3892/or.2015.3779
Vivacqua A, Sebastiani A, Miglietta AM et al (2018) miR-338-3p is regulated by estrogens through GPER in breast cancer cells and cancer-associated fibroblasts (CAFs). Cells 7(11):203. https://doi.org/10.3390/cells7110203
De Marco P, Lappano R, De Francesco EM et al (2016) GPER signalling in both cancer-associated fibroblasts and breast cancer cells mediates a feedforward IL1β/IL1R1 response. Sci Rep 6(December 2015):1–14. https://doi.org/10.1038/srep24354
Hetzl AC, Montico F, Misitieri R et al (2013) Fibroblast growth factor, estrogen, and prolactin receptor features in different grades of prostatic adenocarcinoma in elderly men. Microsc Res Tech 76(3):321–330. https://doi.org/10.1002/jemt.22170
Salvin S, Yeh C-R, Da J et al (2014) Estrogen receptor α in cancer-associated fibroblasts suppresses prostate cancer invasion via modulation of thrombospondin 2 and matrix metalloproteinase 3. Carcinogenesis 35(6):1301–1309. https://doi.org/10.1093/carcin/bgt488
Yeh CR, Slavin S, Da J et al (2016) Estrogen receptor α in cancer associated fibroblasts suppresses prostate cancer invasion via reducing CCL5, IL6 and macrophage infiltration in the tumor microenvironment. Mol Cancer 15(1):1–14. https://doi.org/10.1186/s12943-015-0488-9
Den Boon JA, Pyeon D, Wang SS et al (2015) Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci U S A 112(25):E3255–E3264. https://doi.org/10.1073/pnas.1509322112
Kumar MM, Davuluri S, Poojar S et al (2016) Role of estrogen receptor alpha in human cervical cancer-associated fibroblasts: A transcriptomic study. Tumor Biol 37(4):4409–4420. https://doi.org/10.1007/s13277-015-4257-6
Neuwirt H, Bouchal J, Kharaishvili G et al (2020) Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun Signaling CCS 18(1):1–18. https://doi.org/10.1186/s12964-019-0505-5
Niu Y, Altuwaijri S, Yeh S et al (2008) Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A 105(34):12188–12193. https://doi.org/10.1073/pnas.0804701105
Yu S, Xia S, Yang D et al (2013) Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med Oncol 30(3). https://doi.org/10.1007/s12032-013-0674-9
Ammirante M, Shalapour S, Kang Y, Jamieson CAM, Karin M (2014) Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A 111(41):14776–14781. https://doi.org/10.1073/pnas.1416498111
Mishra R, Haldar S, Placencio V et al (2018) Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest 128(10):4472–4484. https://doi.org/10.1172/JCI99397
Clocchiatti A, Ghosh S, Procopio MG et al (2018) Androgen receptor functions as transcriptional repressor of cancer-associated fibroblast activation. J Clin Invest 128(12):5465–5478. https://doi.org/10.1172/JCI99159
Henshall SM, Quinn DI, Lee CS et al (2001) Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res 61(2):423–427
Ricciardelli C, Choong CS, Buchanan G et al (2005) Androgen receptor levels in prostate cancer epithelial and peritumoral stromal cells identify non-organ confined disease. Prostate 63(1):19–28. https://doi.org/10.1002/pros.20154
Wikström P, Marusic J, Stattin P, Bergh A (2009) Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate 69(8):799–809. https://doi.org/10.1002/pros.20927
Singh M, Jha R, Melamed J, Shapiro E, Hayward SW, Lee P (2014) Stromal androgen receptor in prostate development and cancer. Am J Pathol 184(10):2598–2607. https://doi.org/10.1016/j.ajpath.2014.06.022
Leach DA, Need EF, Toivanen R et al (2015) Erratum: Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome [Oncotarget. 2015; 6:16135-16150]. Oncotarget 6(34):36923. https://doi.org/10.18632/oncotarget.6263
Cioni B, Nevedomskaya E, Melis MHM et al (2018) Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol 12(8):1308–1323. https://doi.org/10.1002/1878-0261.12327
Liao C-P, Chen L-Y, Luethy A et al (2017) Androgen receptor in Cancer-associated fibroblasts influences Stemness in Cancer cells. Endocr Relat Cancer 24(4):157–170. https://doi.org/10.1530/ERC-16-0138
Nash C, Boufaied N, Mills IG, Franco OE, Hayward SW, Thomson AA (2018) Genome-wide analysis of AR binding and comparison with transcript expression in primary human fetal prostate fibroblasts and cancer associated fibroblasts. Mol Cell Endocrinol 471:1–14. https://doi.org/10.1016/j.mce.2017.05.006
Kular JK, Basu S, Sharma RI. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:1–17.
Theocharis, Achilleas D, Skandalis SS, Gialeli, Chrysostomi Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 1(97):4–27
Padhi A, Nain AS (2020) ECM in differentiation: A review of matrix structure, composition and mechanical properties. Ann Biomed Eng 48(3):1071–1089. https://doi.org/10.1007/s10439-019-02337-7
Barcus CE, Holt EC, Keely PJ, Eliceiri KW, Schuler LA (2015) Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PLoS One 10(1):1–22. https://doi.org/10.1371/journal.pone.0116891
Jallow F, O’Leary K, Rugowski D, Guerrero J, Ponik S, Schuler L (2019) Dynamic interactions between the extracellular matrix and estrogen activity in progression of ER+ breast cancer. Oncogene 38(43):6913–6925. https://doi.org/10.1038/s41388-019-0941-0
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3(12):1–24. https://doi.org/10.1101/cshperspect.a005058
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801. https://doi.org/10.1038/nrm3904
Venning FA, Wullkopf L, Erler JT (2015) Targeting ECM disrupts cancer progression. Front Oncol 5(OCT). https://doi.org/10.3389/fonc.2015.00224
Pankova D, Chen Y, Terajima M et al (2016) Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res 14(3):287–295. https://doi.org/10.1158/1541-7786.MCR-15-0307
Cox TR, Erler JT (2011) Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. DMM Dis Model Mech 4(2):165–178. https://doi.org/10.1242/dmm.004077
Gritsenko PG, Ilina O, Friedl P (2012) Interstitial guidance of cancer invasion. J Pathol 226(2):185–199. https://doi.org/10.1002/path.3031
Afratis NA, Klepfish M, Karamanos NK, Sagi I (2018) The apparent competitive action of ECM proteases and cross-linking enzymes during fibrosis: Applications to drug discovery. Adv Drug Deliv Rev 129:4–15. https://doi.org/10.1016/j.addr.2018.03.004
Winkler J, Abisoye-Ogunniyan A, Metcalf K, Werb Z (2020) Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11:1–19. https://doi.org/10.1038/s41467-020-18794-x
Arpino V, Brock M, Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44-46:247–254. https://doi.org/10.1016/j.matbio.2015.03.005
Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG (2020) Metalloproteinases and their inhibitors: Potential for the development of new therapeutics. Cell 9(5):1–34. https://doi.org/10.3390/cells9051313
Ren J, Niu G, Wang X, Song T, Hu Z, Ke C (2018) Overexpression of FNDC1 in gastric cancer and its prognostic significance. J Cancer 9(24):4586–4595. https://doi.org/10.7150/jca.27672
Izbicka E, Streeper RT, Michalek JE, Louden CL, Diaz A, Campos DR (2012) Plasma biomarkers distinguish non-small cell lung cancer from asthma and differ in men and women. CANCER GENOMICS PROTEOMICS 9(1):27–35
Andriani F, Landoni E, Mensah M et al (2018) Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer. BMC Cancer 18(1):1–14. https://doi.org/10.1186/s12885-018-4772-0
Kousidou OC, Berdiaki A, Kletsas D et al (2008) Estradiol-estrogen receptor: A key interplay of the expression of syndecan-2 and metalloproteinase-9 in breast cancer cells. Mol Oncol 2(3):223–232. https://doi.org/10.1016/j.molonc.2008.06.002
Nilsson UW, Garvin S, Dabrosin C (2007) MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res Treat 102(3):253–261. https://doi.org/10.1007/s10549-006-9335-4
Hirvonen R, Talvensaari-mattila A, Pääkkö P, Turpeenniemi-Hujanen T (2003) Matrix metalloproteinase-2 ( MMP-2 ) in T 1 – 2 N 0 breast carcinoma. Breast Cancer Res Treat 77:85–91. https://doi.org/10.1023/A:1021152910976
Li HC, Cao DC, Liu Y et al (2004) Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat 88(1):75–85. https://doi.org/10.1007/s10549-004-1200-8
Conklin MW, Eickhoff JC, Riching KM et al (2011) Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178(3):1221–1232. https://doi.org/10.1016/j.ajpath.2010.11.076
Kaushik S, Pickup MW, Weaver VM (2016) From transformation to metastasis: Deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev 35(4):655–667. https://doi.org/10.1007/s10555-016-9650-0
Barcus CE, O’Leary KA, Brockman JL et al (2017) Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res 19(1):1–13. https://doi.org/10.1186/s13058-017-0801-1
Zanconato F, Battilana G, Cordenonsi M, Piccolo S (2018) YAP/TAZ at the roots of cancer. Cancer Cell 29(6):26–33. https://doi.org/10.1016/j.ccell.2016.05.005.YAP/TAZ
Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QXA, Li B (2003) Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology 144(5):1656–1663. https://doi.org/10.1210/en.2002-0157
Li BY, Liao XB, Fujito A, Thrasher JB, Shen FY, Xu PY (2007) Dual androgen-response elements mediate androgen regulation of MMP-2 expression in prostate cancer cells. Asian J Androl 9(1):41–50. https://doi.org/10.1111/j.1745-7262.2007.00226.x
Hara T, Miyazaki H, Lee A, Tran CP, Reiter RE (2008) Androgen receptor and invasion in prostate cancer. Cancer Res 68(4):1128–1135. https://doi.org/10.1158/0008-5472.CAN-07-1929
Ghatak S, Hascall VC, Markwald RR, Misra S (2010) Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem 285(26):19821–19832. https://doi.org/10.1074/jbc.M110.104273
Ko CJ, Huang CC, Lin HY et al (2015) Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res 75(14):2949–2960. https://doi.org/10.1158/0008-5472.CAN-14-3297
Miyamoto H, Altuwaijri S, Cai Y, Messing EM, Chang C (2005) Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: Potential therapeutic approaches for prostate cancer. Mol Carcinog 44(1):1–10. https://doi.org/10.1002/mc.20121
Bonaccorsi L, Carloni V, Muratori M et al (2000) Androgen receptor expression in prostate carcinoma invasive phenotype *. Endocrinology 141(9):3172–3182
Cinar B, Koeneman KS, Edlund M, Prins GS, Zhau HE, Chung LWK (2001) Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res 61(19):7310–7317
Aalinkeel R, Nair MPN, Sufrin G et al (2004) Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res 64(15):5311–5321. https://doi.org/10.1158/0008-5472.CAN-2506-2
Di Zazzo E, Galasso G, Giovannelli P et al (2015) Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 7(1):193–208. https://doi.org/10.18632/oncotarget.6220
De Palma M, Biziato D, Petrova TV (2017) Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17(8):457–474. https://doi.org/10.1038/nrc.2017.51
Morfoisse F, Noel A (2019) Lymphatic and blood systems: Identical or fraternal twins? Int J Biochem Cell Biol 114(June):105562. https://doi.org/10.1016/j.biocel.2019.105562
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146(6):873–887. https://doi.org/10.1016/j.cell.2011.08.039
Bergers G, Brekken R, McMahon G et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744. https://doi.org/10.1038/35036374
Xi Wang RAK. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81(/):241–330. doi:https://doi.org/10.1016/bs.apha.2017.08.002.Matrix.
Garmy-Susini B, Varner JA (2008) Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res Biol 6(3–4):155–163. https://doi.org/10.1089/lrb.2008.1011
Hartman RJG, Kapteijn DMC, Haitjema S et al (2020) Intrinsic transcriptomic sex differences in human endothelial cells at birth and in adults are associated with coronary artery disease targets. Sci Rep 10(1). https://doi.org/10.1038/s41598-020-69451-8
Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW (2002) Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res 91(9):814–820. https://doi.org/10.1161/01.RES.0000038304.62046.4C
Gavin KM, Seals DR, Silver AE, Moreau KL (2009) Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94(9):3513–3520
Addis R, Campesi I, Fois M et al Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biol Sex Differ 2014, 5(1). https://doi.org/10.1186/s13293-014-0018-2
Kim KH, Bender JR (2009) Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol 308(1–2):3–8. https://doi.org/10.1016/j.mce.2009.03.025
Kleinert, H., Wallerath, T., Euchenhofer, C., Ihrig-Biedert, I., Li, H., Förstermann, U. (1998). Estrogens increase transcription of the human endothelial NO synthase gene analysis of the transcription factors involved. http://ahajournals.org.
Sumi, D., Ignarro, L. J. (2003). Estrogen-related receptor 1 up-Regulates endothelial nitric oxide synthase expression. www.pnas.orgcgidoi10.1073pnas.2235590100.
Martin, J. H. J., Alalami, O., Van Den Berg, H. W.. Reduced expression of endothelial and inducible nitric oxide synthase in a human breast cancer cell line which has acquired estrogen independence.
Martin, J. H. J., Begum, S., Alalami, O., Harrison, A., Scott, K. W. M. (2000). Endothelial nitric oxide synthase: Correlation with histologic grade, lymph node status and estrogen receptor expression in human breast cancer. Vol 21.www.karger.com/journals/tbi.
Fujimoto J, Toyoki H, Jahan I et al (2005) Sex steroid-dependent angiogenesis in uterine endometrial cancers. J Steroid Biochem Mol Biol 93:161–165. https://doi.org/10.1016/j.jsbmb.2004.12.021
Marquez-Garban DC, Mah V, Alavi M et al (2011) Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 76(9):910–920. https://doi.org/10.1016/j.steroids.2011.04.015
Dubois C, Rocks N, Blacher S et al (2019) Lymph/angiogenesis contributes to sex differences in lung cancer through oestrogen receptor alpha signalling. Endocr Relat Cancer 26(2). https://doi.org/10.1530/ERC-18-0328
Péqueux C, Raymond-Letron I, Blacher S et al (2012) Stromal estrogen receptor-α promotes tumor growth by normalizing an increased angiogenesis. Cancer Res 72(12):3010–3019. https://doi.org/10.1158/0008-5472.CAN-11-3768
Wang C, Li J, Ye S et al (2017) Oestrogen inhibits VEGF expression and angiogenesis in triple-negative breast cancer by activating GPER-1. J Ind Manag Optim 13(5):3802–3811. https://doi.org/10.7150/jca.29233
Death AK, McGrath KCY, Sader MA et al (2004) Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-κB-dependent pathway. Endocrinology 145(4):1889–1897. https://doi.org/10.1210/en.2003-0789
Schlesinger M, Bendas G (2015) Vascular cell adhesion molecule-1 (VCAM-1) – An increasing insight into its role in tumorigenicity and metastasis. Int J Cancer 136(11):2504–2514. https://doi.org/10.1002/ijc.28927
Piali, L., Fichtd, A., Terpe, H-J., Imhof, B. A., & Gisler, R. H.. Endothelial vascular cell adhesion molecule expression is suppressed by melanoma and carcinoma.
Dirkx, A. E. M., Oude Egbrink, G. A., Kuijpers, M. J. E., et al. (2003). Tumor angiogenesis modulates Leukocyte-Vessel wall interactions in vivo by reducing endothelial adhesion molecule expression 1. Vol 63. http://www.fdg.unimaas.nl/angiogenesislab.
Torres-Estay V, Carreño DV, San Francisco IF, Sotomayor P, Godoy AS, Smith GJ (2015) Androgen receptor in human endothelial cells. J Endocrinol 224(3):R131–R137. https://doi.org/10.1530/JOE-14-0611
Huo YN, Der YS, Sen LW (2019) Androgen receptor activation reduces the endothelial cell proliferation through activating the cSrc/AKT/p38/ERK/NFκB-mediated pathway. J Steroid Biochem Mol Biol 194. https://doi.org/10.1016/j.jsbmb.2019.105459
Nheu L, Nazareth L, Xu GY et al (2011) Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids 76(14):1590–1596. https://doi.org/10.1016/j.steroids.2011.09.015
Mabjeesh, N. J., Willard, M. T., Frederickson, C. E., Zhong, H., & Simons, J. W. (2003). Androgens stimulate Hypoxia-Inducible factor 1 activation via Autocrine loop of Tyrosine Kinase Receptor/Phosphatidylinositol 3-Kinase/Protein Kinase B in Prostate Cancer Cells 1.
Boddy JL, Fox SB, Han C et al (2005) The androgen receptor is significantly associated with vascular endothelial growth factor and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a, and the prolyl hydroxylases in human prostate cancer. Clin Cancer Res 11(21):7658–7663. https://doi.org/10.1158/1078-0432.CCR-05-0460
Kardideh B, Samimi Z, Norooznezhad F, Kiani S, Mansouri K (2019) Autophagy, cancer and angiogenesis: Where is the link? Cell Biosci 9(1). https://doi.org/10.1186/s13578-019-0327-6
Schaaf MB, Houbaert D, Meçe O, Agostinis P (2019) Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ 26(4):665–679. https://doi.org/10.1038/s41418-019-0287-8
Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438(7070):946–953. https://doi.org/10.1038/nature04480
Ma Q, Dieterich LC, Detmar M (2018) Multiple roles of lymphatic vessels in tumor progression. Curr Opin Immunol 53:7–12. https://doi.org/10.1016/j.coi.2018.03.018
Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14(3):159–172. https://doi.org/10.1038/nrc3677
Dieterich LC, Detmar M (2016) Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev 99:148–160. https://doi.org/10.1016/j.addr.2015.12.011
Fontaine C, Morfoisse F, Tatin F et al (2020) The impact of estrogen receptor in arterial and lymphatic vascular diseases. Int J Mol Sci 21(9). https://doi.org/10.3390/ijms21093244
Morfoisse F, Tatin F, Chaput B et al (2018) Lymphatic vasculature requires estrogen receptor-α signaling to protect from lymphedema. Arterioscler Thromb Vasc Biol 38(6):1346–1357. https://doi.org/10.1161/ATVBAHA.118.310997
Dubois C, Rocks N, Blacher S et al (2019) Lymph/angiogenesis contributes to sex differences in lung cancer through oestrogen receptor alpha signalling. Endocr Relat Cancer 26(2):201–216. https://doi.org/10.1530/ERC-18-0328
Niemiec J, Sas-Korczynska B, Harazin-Lechowska A, Martynow D, Adamczyk A (2015) Lymphatic and blood vessels in male breast cancer. Anticancer Res 35(2):1041–1048
Hunter S, Nault B, Ugwuagbo KC, Maiti S, Majumder M (2019) Mir526b and mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers (Basel) 11(7). https://doi.org/10.3390/cancers11070938
Vottero GV, Morfoisse F, Durré T et al (2019) Contralateral vascularized lymph node transfer: An optimized mouse model. J Reconstr Microsurg Open 04(02):e83–e91. https://doi.org/10.1055/s-0039-3400243
Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A (2019) Cancer-associated secondary lymphoedema. Nat Rev Dis Prim 5(1). https://doi.org/10.1038/s41572-019-0072-5
Parkin J, Cohen B (2001) Overview of the immune system. Lancet Oncol 357:1777–1789. https://doi.org/10.1016/B978-0-323-39981-4.00004-X
Oertelt-Prigione S (2012) The influence of sex and gender on the immune response. Autoimmun Rev 11(6–7). https://doi.org/10.1016/j.autrev.2011.11.022
Jaillon S, Berthenet K, Garlanda C (2019) Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol 56(3):308–321. https://doi.org/10.1007/s12016-017-8648-x
Furman D (2015) Sexual dimorphism in immunity: Improving our understanding of vaccine immune responses in men. Expert Rev Vaccines 14(3):461–471. https://doi.org/10.1586/14760584.2015.966694
Ortona E, Pierdominici M, Rider V (2019) Editorial: Sex hormones and gender differences in immune responses. Front Immunol 10(MAY). https://doi.org/10.3389/fimmu.2019.01076
Libert C, Dejager L, Pinheiro I (2010) The X chromosome in immune functions: When a chromosome makes the difference. Nat Rev Immunol. https://doi.org/10.1038/nri2815
Selmi C, Brunetta E, Raimondo MG, Meroni PL (2012) The X chromosome and the sex ratio of autoimmunity. Autoimmun Rev 11(6–7). https://doi.org/10.1016/j.autrev.2011.11.024
Ghorai A, Ghosh U (2014) miRNA gene counts in chromosomes vary widely in a species and biogenesis of miRNA largely depends on transcription or post-transcriptional processing of coding genes. Front Genet. https://doi.org/10.3389/fgene.2014.00100
Pinheiro I, Dejager L, Libert C (2011) X-chromosome-located microRNAs in immunity: Might they explain male/female differences?: The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays 33(11):791–802. https://doi.org/10.1002/bies.201100047
Fullwood MJ, Liu MH, Pan YF et al (2009) An oestrogen-receptor-α-bound human chromatin interactome. Nature 462(7269):58–64. https://doi.org/10.1038/nature08497
Atala A (2015) Re: Brain feminization requires active repression of masculinization via DNA methylation. J Urol 194(6):1823–1824. https://doi.org/10.1016/j.juro.2015.09.001
Castro A, Pyke RM, Zhang X et al (2020) Strength of immune selection in tumors varies with sex and age. Nat Commun 11(1). https://doi.org/10.1038/s41467-020-17981-0
Kovats S (2015) Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294(2):63–69. https://doi.org/10.1016/j.cellimm.2015.01.018
Moulton VR (2018) Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9(OCT):1–21. https://doi.org/10.3389/fimmu.2018.02279
Buskiewicz IA, Huber SA, Fairweather DL (2016) Sex hormone receptor expression in the immune system. In: Sex differences in physiology. Elsevier, pp 45–60. https://doi.org/10.1016/B978-0-12-802388-4.00004-5
Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B (2015) How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14(3):309–321. https://doi.org/10.1111/acel.12326
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16(10):626–638. https://doi.org/10.1038/nri.2016.90
Bupp MRG, Potluri T, Fink AL, Klein SL (2018) The confluence of sex hormones and aging on immunity. Front Immunol 9(JUN). https://doi.org/10.3389/fimmu.2018.01269
Ben-Batalla I, Vargas-Delgado ME, von Amsberg G, Janning M, Loges S (2020) Influence of androgens on immunity to self and foreign: Effects on immunity and Cancer. Front Immunol 11(July):1–20. https://doi.org/10.3389/fimmu.2020.01184
Hughes GC (2012) Progesterone and autoimmune disease. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2011.12.003
Scalerandi MV, Peinetti N, Leimgruber C et al (2018) Inefficient N2-like neutrophils are promoted by androgens during infection. Front Immunol 9(SEP). https://doi.org/10.3389/fimmu.2018.01980
Roved J, Westerdahl H, Hasselquist D (2017) Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav 88:95–105
Whitacre CC (2001) Sex differences in autoimmune disease. Nat Immunol. https://doi.org/10.1038/ni0901-777
Fish EN (2008) The X-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol 8:737–744
Tedeschi SK, Bermas B, Costenbader KH (2013) Sexual disparities in the incidence and course of SLE and RA. Clin Immunol 149(2):211–218. https://doi.org/10.1016/j.clim.2013.03.003
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555. https://doi.org/10.1016/S1471-4906(02)02302-5
Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers (Basel) 3(4):3856–3893. https://doi.org/10.3390/cancers3043856
Lin Y, Xu J, Lan H (2019) Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J Hematol Oncol 12(1):1–16. https://doi.org/10.1186/s13045-019-0760-3
Ciucci A, Zannoni GF, Buttarelli M et al (2016) Multiple direct and indirect mechanisms drive estrogen-induced tumor growth in high grade serous ovarian cancers. Oncotarget 7(7):8155–8171. https://doi.org/10.18632/oncotarget.6943
Gwak JM, Jang MH, Kim D, Seo AN, Park SY (2015) Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One 10(4):1–14. https://doi.org/10.1371/journal.pone.0125728
Campbell MJ, Tonlaar NY, Garwood ER et al (2011) Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat 128(3):703–711. https://doi.org/10.1007/s10549-010-1154-y
Gil M, Yue W, Santen RJ et al (1998) Macrophages, estrogen and the microenvironment of breast cancer. J Steroid Biochem Mol Biol 67(5–6):403–411. https://doi.org/10.1016/S0960-0760(98)00143-5
Siegfried J, Stabile LP (2014) Estrogenic steroid hormones in lung Cancer. Semin Oncol 41(1):5–16. https://doi.org/10.1053/j.seminoncol.2013.12.009
Svensson S, Abrahamsson A, Rodriguez GV et al (2015) CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer. Clin Cancer Res 21(16):3794–3805. https://doi.org/10.1158/1078-0432.CCR-15-0204
Ning C, Xie B, Zhang L et al (2016) Infiltrating macrophages induce ERα expression through an IL17A-mediated epigenetic mechanism to sensitize endometrial cancer cells to estrogen. Cancer Res 76(6):1354–1366. https://doi.org/10.1158/0008-5472.CAN-15-1260
Sun L, Chen B, Jiang R, Li J, Wang B (2017) Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol 311:86–93. https://doi.org/10.1016/j.cellimm.2016.11.002
Cioni B, Zaalberg A, van Beijnum JR, et al. Androgen receptor signalling in macrophages promotes TREM-1-mediated prostate cancer cell line migration and invasion. Nat Commun 2020;11(1):1–17. doi:https://doi.org/10.1038/s41467-020-18313-y.
Cioni B, Zwart W, Bergman AM (2018) Androgen receptor moonlighting in the prostate cancer microenvironment. Endocr Relat Cancer 25(6):R331–R349. https://doi.org/10.1530/ERC-18-0042
Izumi K, Fang LY, Mizokami A et al (2013) Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med 5(9):1383–1401. https://doi.org/10.1002/emmm.201202367
Fang LY, Izumi K, Lai KP et al (2013) Infiltrating macrophages promote prostate tumorigenesis via modulating androgen receptor-mediated CCL4-STAT3 signaling. Cancer Res 73(18):5633–5646. https://doi.org/10.1158/0008-5472.CAN-12-3228
Escamilla J, Schokrpur S, Liu C et al (2015) CSF1 receptor targeting in prostate Cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res 75(6):950–962. https://doi.org/10.1158/0008-5472.CAN-14-0992
Milette S, Hashimoto M, Perrino S et al (2019) Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat Commun 10(1):1–16. https://doi.org/10.1038/s41467-019-13571-x
Paharkova-Vatchkova V, Maldonado R, Kovats S (2004) Estrogen preferentially promotes the differentiation of CD11c + CD11b intermediate dendritic cells from bone marrow precursors. J Immunol 172(3):1426–1436. https://doi.org/10.4049/jimmunol.172.3.1426
Thompson MG, Peiffer DS, Larson M, Navarro F, Watkins SK (2017) FOXO3, estrogen receptor alpha, and androgen receptor impact tumor growth rate and infiltration of dendritic cell subsets differentially between male and female mice. Cancer Immunol Immunother 66(5):615–625. https://doi.org/10.1007/s00262-017-1972-4
Bupp MRG, Jorgensen TN (2018) Androgen-induced immunosuppression. Front Immunol 9(APR). https://doi.org/10.3389/fimmu.2018.00794
Jiang X, Ellison SJ, Alarid ET, Shapiro DJ (2007) Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene 26(28):4106–4114. https://doi.org/10.1038/sj.onc.1210197
Rothenberger NJ, Somasundaram A, Stabile LP (2018) The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci 19(2). https://doi.org/10.3390/ijms19020611
Veglia F, Gabrilovich DI (2017) Dendritic cells in cancer: The role revisited. Curr Opin Immunol 45:43–51. https://doi.org/10.1016/j.coi.2017.01.002
Fuertes MB, Kacha AK, Kline J et al (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med 208(10):2005–2016. https://doi.org/10.1084/jem.20101159
Capone I, Marchetti P, Ascierto PA, Malorni W, Gabriele L (2018) Sexual dimorphism of immune responses: A new perspective in cancer immunotherapy. Front Immunol 9(MAR):1–8. https://doi.org/10.3389/fimmu.2018.00552
Fridlender ZG, Sun J, Kim S et al (2010) Polarization of TAN phenotype by TGFb: “N1” versus “N2” TAN. Cancer Cell 16(3):183–194. https://doi.org/10.1016/j.ccr.2009.06.017.Polarization
Habib P, Dreymueller D, Rösing B et al (2018) Estrogen serum concentration affects blood immune cell composition and polarization in human females under controlled ovarian stimulation. J Steroid Biochem Mol Biol 178(November 2017):340–347. https://doi.org/10.1016/j.jsbmb.2018.02.005
Chung HH, Or YZ, Shrestha S et al (2017) Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution. Sci Rep 7(November 2016):1–13. https://doi.org/10.1038/srep46485
Markman JL, Porritt RA, Wakita D et al (2020) Loss of testosterone impairs anti-tumor neutrophil function. Nat Commun 11(1). https://doi.org/10.1038/s41467-020-15397-4
Chuang KH, Altuwaijri S, Li G et al (2009) Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med 206(5):1181–1199. https://doi.org/10.1084/jem.20082521
Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C (2012) Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am J Pathol 181(5):1504–1512. https://doi.org/10.1016/j.ajpath.2012.07.008
Fridman, Wolf Herman Pagès F, Sautès-Fridman, Catherine Galon J (2012) The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 12:298–306
Buskiewicz IA, Huber SA, Fairweather D (2016) Sex hormone receptor expression in the immune system. In: Sex differences in physiology, pp 45–60
Dorak T, Mehmet Karpuzoglu E (2012) Gender differences in cancer susceptibility: An inadequately addressed issue. Front Genet
Fish EN (2008) The X-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol 8(9):737–744. https://doi.org/10.1038/nri2394
Dannenfelser R, Nome M, Tahiri A et al (2017) Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, ER activity, and genomic complexity. Oncotarget 8(34):57121–57133. https://doi.org/10.18632/oncotarget.19078
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD (2011) Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 58(7):1107–1116. https://doi.org/10.1111/j.1365-2559.2011.03846.x
Ali HR, Provenzano E, Dawson SJ et al (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol 25(8):1536–1543. https://doi.org/10.1093/annonc/mdu191
Audun Werner Haabeth OA, Lorvik B, Hammarström KC, Donaldson C, Haraldsen IM, Guttorm Bogen B, Corthaya A (2011) Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 15(2)
DeNardo DG, Barreto JB, Andreu P et al (2009) CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing Protumor properties of macrophages. Cancer Cell 16(2):91–102. https://doi.org/10.1016/j.ccr.2009.06.018
Gabriele L, Buoncervello M, Ascione B (2016) Bellenghi Carè, Maria Matarrese P, Carè a. the gender perspective in cancer research and therapy: Novel insights and on-going hypotheses. Ann Ist Super Sanita 52(2):213–222
Dakup PP, Porter KI, Little AA, Zhang H, Gaddameedhi S (2020) Sex differences in the association between tumor growth and T cell response in a melanoma mouse model. Cancer Immunol Immunother 69(10):2157–2162. https://doi.org/10.1007/s00262-020-02643-3
van Rooijen JM, Qiu SQ, Timmer-Bosscha H et al (2018) Androgen receptor expression inversely correlates with immune cell infiltration in human epidermal growth factor receptor 2–positive breast cancer. Eur J Cancer 103:52–60. https://doi.org/10.1016/j.ejca.2018.08.001
Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F (2009) Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 348(1–2):9–17. https://doi.org/10.1016/j.jim.2009.06.004
Mercader M, Bodner BK, Moser MT et al (2001) T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 98(25):14565–14570. https://doi.org/10.1073/pnas.251140998
Vasanthakumar A, Chisanga D, Blume J et al (2020) Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579(7800):581–585. https://doi.org/10.1038/s41586-020-2251-7
Ishikawa A, Wada T, Nishimura S et al (2020) Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS One 15(4). https://doi.org/10.1371/journal.pone.0230885
Kissick HT, Sanda MG, Dunn LK et al (2014) Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A 111(27):9887–9892. https://doi.org/10.1073/pnas.1402468111
Li C, Jiang P, Wei S, Xu X, Wang J (2020) Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol Cancer 19(1). https://doi.org/10.1186/s12943-020-01234-1
Josefowicz SZ, Lu LF, Rudensky AY (2012) Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol 30:531–564. https://doi.org/10.1146/annurev.immunol.25.022106.141623
Ohkura N, Kitagawa Y, Sakaguchi S (2013) Development and maintenance of regulatory T cells. Immunity 38(3):414–423. https://doi.org/10.1016/j.immuni.2013.03.002
Panduro M, Benoist C, Mathis D (2016) Tissue Tregs. Annu Rev Immunol 34:609–633. https://doi.org/10.1146/annurev-immunol-032712-095948
Bellenghi M, Puglisi R, Pontecorvi G, De Feo A, Carè A, Mattia G (2020) Sex and gender disparities in melanoma. Cancers (Basel) 12(7):1–23. https://doi.org/10.3390/cancers12071819
Del Mar GM, Gulfo J, Camps N et al (2017) Modulation of SHBG binding to testosterone and estradiol 4 by sex and morbid. Obesity
Generali D, Bates G, Berruti A et al (2009) Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res 15(3):1046–1051. https://doi.org/10.1158/1078-0432.CCR-08-1507
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep 5(June):1–9. https://doi.org/10.1038/srep15179
Fijak M, Schneider E, Klug J et al (2011) Testosterone replacement effectively inhibits the development of experimental autoimmune Orchitis in rats: Evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol 186(9):5162–5172. https://doi.org/10.4049/jimmunol.1001958
Tipton AJ, Sullivan JC (2014) Sex and gender differences in T cells in hypertension. Clin Ther 36(12):1882–1900. https://doi.org/10.1016/j.clinthera.2014.07.011.Sex
Jia M, Dahlman-Wright K, Gustafsson JÅ (2015) Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 29(4):557–568. https://doi.org/10.1016/j.beem.2015.04.008
Zhou JH, Kim KB, Myers JN et al (2014) Immunohistochemical expression of hormone receptors in melanoma of pregnant women, nonpregnant women, and men. Am J Dermatopathol 36(1):74–79. https://doi.org/10.1097/DAD.0b013e3182914c64
De Giorgi V, Gori A, Gandini S et al (2013) Oestrogen receptor beta and melanoma: A comparative study. Br J Dermatol 168(3):513–519. https://doi.org/10.1111/bjd.12056
Donley GM, Liu WT, Pfeiffer RM et al (2019) Reproductive factors, exogenous hormone use and incidence of melanoma among women in the United States. Br J Cancer 120(7):754–760. https://doi.org/10.1038/s41416-019-0411-z
Botteri E, Støer NC, Weiderpass E, Pukkala E, Ylikorkala O, Lyytinen H (2019) Menopausal hormone therapy and risk of melanoma: A nationwide register-based study in Finland. Cancer Epidemiol Biomark Prev 28(11):1857–1860. https://doi.org/10.1158/1055-9965.EPI-19-0554
Hicks BM, Kristensen KB, Pedersen SA, Hölmich LR, Pottegård A (2019) Hormone replacement therapy and the risk of melanoma in post-menopausal women. Hum Reprod 34(12):2418–2429. https://doi.org/10.1093/humrep/dez222
Ulrich BC, Guibert N (2018) Immunotherapy efficacy and gender: Discovery in precision medicine. Transl Lung Cancer Res 7:S211–S213. https://doi.org/10.21037/tlcr.2018.08.05
Momtaz P, Postow MA (2014) Immunologic checkpoints in cancer therapy: Focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers Med 7:357–365. https://doi.org/10.2147/PGPM.S53163
Dinesh RK, Hahn BH, Singh RP (2010) PD-1, gender, and autoimmunity. Autoimmun Rev 9(8):583–587. https://doi.org/10.1016/j.autrev.2010.04.003
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med 50(12). https://doi.org/10.1038/s12276-018-0191-1
Botticelli, A., Elisa Onesti, C., Zizzari, I., et al. (2017). The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Vol 8. www.impactjournals.com/oncotarget.
Riella LV, Liu T, Yang J et al (2012) Deleterious effect of CTLA4-Ig on a TREG-dependent transplant model. Am J Transplant 12(4):846–855. https://doi.org/10.1111/j.1600-6143.2011.03929.x
Özdemir BC, Dotto GP (2019) Sex hormones and anticancer immunity. Clin Cancer Res 25(15):4603–4610. https://doi.org/10.1158/1078-0432.CCR-19-0137
Conforti F, Pala L, Bagnardi V et al (2019) Sex-based differences of the tumor mutational burden and T-cell inflammation of the tumor microenvironment. Ann Oncol 30(4):653–655. https://doi.org/10.1093/annonc/mdz034
Grassadonia A, Sperduti I, Vici P et al (2018) Effect of gender on the outcome of patients receiving immune checkpoint inhibitors for advanced Cancer: A systematic review and meta-analysis of phase III randomized clinical trials. J Clin Med 7(12):542. https://doi.org/10.3390/jcm7120542
Wallis CJD, Butaney M, Satkunasivam R et al (2019) Association of Patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: A systematic review and meta-analysis. JAMA Oncol 5(4):529–536. https://doi.org/10.1001/jamaoncol.2018.5904
Irelli A, Sirufo MM, D’Ugo C, Ginaldi L, De Martinis M (2020) Sex and gender influences on cancer immunotherapy response. Biomedicine 8(7):1–23. https://doi.org/10.3390/BIOMEDICINES8070232
Duma N, Abdel-Ghani A, Yadav S et al (2019) Sex differences in tolerability to anti-programmed cell Death protein 1 therapy in patients with metastatic melanoma and non-small cell lung Cancer: Are we all equal? Oncologist 24(11). https://doi.org/10.1634/theoncologist.2019-0094
Wizemann TM, Pardue M-L Exploring the biological contributions to human health. National Academies Press, Washington, DC, 2001. https://doi.org/10.17226/10028
Taylor KE, Vallejo-Giraldo C, Schaible NS, Zakeri R, Miller VM. Reporting of sex as a variable in cardiovascular studies using cultured cells. Biol Sex Differ 2011;1(11):1–7.
Beery AK, Zucker I (2011) Sex Bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35(3):565–572
Lee S, Kwak H, Kang M, Park Y, Jeong G (2008) Fibroblast-associated tumour microenvironment induces vascular structure-networked tumouroid. Sci Rep 8(2365):1–12
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wuidar, V., Gillot, L., Dias Da Silva, I., Lebeau, A., Gallez, A., Pequeux, C. (2021). Sex-Based Differences in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1329. Springer, Cham. https://doi.org/10.1007/978-3-030-73119-9_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-73119-9_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73118-2
Online ISBN: 978-3-030-73119-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)