Abstract
Thermal decomposition kinetics of Polypropylene (PP) waste is extremely important with respect to valorisation of waste plastics and production of utilizable components viz. chemicals, fuel oil & gas. The present research study focuses on pyrolysis kinetics of PP waste, which is present as a fraction of municipal plastic waste through distributed activation energy model (DAEM). The decomposition kinetics for PP follows a Gaussian distribution, where the normal distribution curves were centred corresponding to activation energy of 224 kJ/mol. The standard deviation of the distribution for the PP sample was found to be 22 kJ/mol indicating its wider distribution of decomposition range. The data validation has been carried out by comparing the rate parameter and extent of conversion values calculated through DAEM model with the Thermogravimetric analysis (TGA) experiments carried out for PP at various heating rates of 5, 10, 20 and 40 °C/min.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Plastics are the most innovative materials of 20th century where their consumption is increasing everyday due to properties of mechanical strength, inertness and durability. The demand for plastic materials will be doubled by 2025 which will have an additional burden on raw materials used for their production, more specifically on fossil fuel resources [1, 2]. This increasing demand along with human lifestyle changes results in large proportion of mismanaged plastic waste which is likely to end up in sinks. Waste management plays a vital role in addressing accumulation of plastic waste considering its biodegradability, where option of pyrolysis is viable for plastics in terms of producing of liquid, gas and solid char fractions considering energy investments into the process.
Pyrolysis kinetics is important with respect to upscaling of polymer recycling process at commercial scale [3]. Here the relative rates of decomposition, cracking and other polymerisation reactions affect the quality and quantity of oil produced during the process. Thermogravimetry Analysis (TGA) is used to study decomposition kinetics and subsequently used for evaluation of kinetic parameters. The present research study focuses on pyrolysis kinetics of polypropylene (PP) through distributed activation energy model where the accuracy and versatility of DAEM assists for reproducing kinetic parameters considering the complex pyrolysis phenomena. The activation energies at various conversion levels is assumed to follow Gaussian distribution, in which the current study helps in the estimation of kinetic parameters. Subsequently estimated kinetic parameters can be directly used for the prediction of rate of decomposition of polypropylene at various heating rates thereby avoiding the need of performing pyrolysis experiments.
2 Experimental
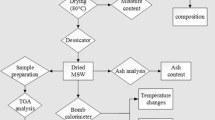
Commercial grade PP samples were purchased from Reliance Industries Limited in which decomposition studies were carried out at heating rate of 5, 10, 20 and 40 °C/min. PP beads were crushed to a particle size of 0.5 mm which was subsequently subjected for further experimentations and TGA analysis in temperature range of 30–600 °C. The TGA experiments were carried out in a Perkin Elmer differential thermal analyser—Diamond TG/DTA model of Perkin Elmer, USA, under non-isothermal conditions at Sophisticated Analytical Instrument Facility in IIT Bombay.
The amount of sample used for analysis was 15 mg having a particle size of 0.5 mm. Nitrogen flow of 50 ml/min was maintained through sample during experiments. The reproducibility of results obtained from experiments was ensured by repeatedly analysing the sample three times.
2.1 Theoretical Considerations
The rate of decomposition encountered in solid state kinetics is dependant up on Temperature T, extent of conversion (α) and heating rate (\( \beta = \frac{dT}{dt} \))
The extent of conversion α, is given by
where \( m_{0} \), \( m_{t} \) and \( m_{f} \) specify initial mass, mass at a time ‘t’ and final mass of the sample respectively.
The parameter \( k\left( T \right) \) signifies rate as a function of temperature which is represented by Arrhenius equation.
Combination of Eq. (23.1) and (23.3) results in explicit expression of reaction rate which can be written as
Rearranging Eq. (23.4) we will get
where \( x = \left( {\frac{{E_{a} }}{RT}} \right) \)
The temperature integral presented in Eq. 23.5 has no analytical solution, where fourth order Senum Yung approximation given in Eq. 23.6 is used to evaluate temperature integral. The rate of decomposition as represented by Eq. 23.4, where rate calculations are carried against temperature at similar extent of conversion at different heating rates. The representational form of Friedmann Iso-conversional method is given below.
2.1.1 Friedmann Method (FR)
Friedman’s method [4] is a differential iso-conversional method is obtained by taking logarithm on both sides of Eq. (23.4)
Plotting \( \ln \left( {\beta \frac{d\alpha }{dT}} \right) \) or \( \ln \left( {\frac{d\alpha }{dt}} \right) \) against \( \left( {\frac{1}{T}} \right) \) over the entire range of conversion will yield the value of activation energy.
2.1.2 Distributed Activation Energy Model
The distributed activation energy model assumes that decomposition of material is carried out through large number of independent reactions, each of them is having its own activation energy and frequency factor. It is further assumed that reactivity distribution follows a Gaussian distribution representing continuous distribution of activation energy. The rate of reaction represented in terms of this continuous distribution F(E) as
The true distribution of activation energy F(E) is represented through Gaussian distribution with mean activation energy E0 and having standard deviation of \( \sigma \) is mentioned below.
The comparison of experimental and simulated data is represented through minimization of objective function. (O.F)
3 Results and Discussion
The TGA curves for polypropylene are shown in Fig. 23.1. Thermograms are indicative of single stage devolatalization in temperature range of 300–600 °C, where it is characteristic of higher volatile content in the PP sample. The peaks in dTG curve shown in Fig. 23.2 indicate that reaction rate for solid state decomposition reaches a maximum at some intermediate stage of conversion. The temperatures for this maximum at different heating rates of 5, 10, 20 and 40 °C/min found from dTG peaks are 442, 453, 465, 481 °C for PP. This indicates the temperature level at which reactor has to be operated in order to maximise the conversion of feed plastics in a continuous reactor system.
3.1 Estimation of Activation Energy
Activation energy remains constant for most of single step reactions where rate constant is related to temperature. However, for solid state decomposition reactions, these kinetic parameters tend to vary with extent of conversion (α) [5]. Iso-conversional methods help to estimate the value of activation energy without the prior knowledge regarding reaction model or to hypothesize a form of kinetic equation, as any conversion function can fit TGA decomposition data by varying kinetic parameters [6]. This is presented as a significant disadvantage of model-based methods which rely on results of kinetic parameters obtained from Iso-conversional methods [7]. The mean value of activation energy obtained from Friedmann isoconversional plot for PP is 224.34 kJ/mol with the value of correlation coefficient representing exactness of fit are 0.9931.
The Eα vs α and corresponding k0 values obtained by Friedman’s method for PP is used for DAEM modelling. The plot of E vs V/V* and E vs f(E) is given in Figs. 23.3 and 23.4 respectively. It is observed that decomposition kinetics for PP follows a Gaussian distribution, where the normal distribution curves were centred corresponding to activation energy of 224 kJ/mol corresponding to the level for extent of conversion of 0.6. The standard deviation (\( \sigma ) \) for PP waste sample was found to be 22 kJ/mol which is indicative of its wider decomposition range.
Larger values of \( \sigma \) represent broader reaction profiles at constant heating rate and these parameters vary with respect polymers due to their structural heterogeneity. The isoconversional Friedmann method is used to estimate the value of activation energy and frequency factor at different levels of conversion. With the Gaussian distribution assumed for variation of activation energy represented by Eq. 23.9 the value of rate is calculated through DAEM model equation and compared with experimental value of rate \( \left( {\frac{d\alpha }{dT}} \right) \). The objective function (O.F) representing square of difference between the experimental and theoretical rate values are found to be minimum at various heating rates of 5, 10, 20 and 40 °C/min, which indicates that prediction of rate through DAEM model equation is closer to that of experimental values. The comparison between theoretical and experimental plots are given in Fig. 23.5 for various heating rates investigated in this manuscript.
The practical significance for estimation of kinetic parameters through DAEM involve predicting the rate of decomposition considering the complex nature of pyrolysis degradation process. Estimated kinetic parameters can be used for prediction of degradation model, which will be very helpful in dealing with the plastic wastes. In addition to this, the identified decomposition model expressed as a function of temperature and heating rate can be used to identify the operational temperature, where continuous reactor operation can be ensured with optimum heat input. This will prevent energy losses in tackling the wastes.
4 Conclusion
Thermal decomposition of PP has been carried out in present study at heating rates of 5, 10, 20 and 40 °C/min. The decomposition data so obtained is processed through Friedmann Isoconversional method to calculate the value of Activation Energy and Frequency factor at various conversion levels. Distributed Activation Energy Modelling (DAEM) was applied for decomposition of PP to calculate the rate of degradation, assuming Gaussian distribution for Activation Energy at various conversion levels. The normal distribution curves were centred corresponding to activation energy of 224 kJ/mol for the level of conversion of 0.6. The DAEM model is validated through the experimentally calculated values of rate parameter at various heating rates.
References
J.M.H. Army Lusher, P. Hollman, Microplastics in fisheries and aquaculture (2017)
L. Lebreton, A. Andrady, Future scenarios of global plastic waste generation and disposal, Palgrave Commun., 1–11 (2019)
Q.V. Bach, W.H. Chen, Pyrolysis characteristics and kinetics of microalgae viathermogravimetric analysis (TGA): A state of art review. Bioresource Technol. 246, 88–100 (2017)
H.L. Friedman, Kinetics of thermal degradation of char-forming plastics from thermogravimetry. application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp 6, 183–195 (1964)
P. Simon, Isoconversional methods—fundamentals meaning and application. J. Therm. Anal. Cal. 76, 123–132 (2004)
J.E. White, W.J. Catallo, B.L. Legendre, Biomass pyrolysis kinetics: a compartive critical review with relevant agricultural residue case studies. J. Appl. Anal. Pyrol. 91, 1–33 (2011)
J. Cai, D. Xu, Z. Dong, X. Yu, Y. Yang, S.W. Banks, A.V. Bridgwater, Proceessing of thermogravimetric data for Isoconversional kinetic analysis of lignocellulosic biomass pyrolysis 82, 2705–2715 (2018)
Acknowledgements
Authors are thankful to Bharuch Enviro Infrastructure Limited (BEIL) Ankleshwar, India for providing the financial support to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Kartik, S. et al. (2021). Distributed Activation Energy Model for Thermal Decomposition of Polypropylene Waste. In: Mporas, I., Kourtessis, P., Al-Habaibeh, A., Asthana, A., Vukovic, V., Senior, J. (eds) Energy and Sustainable Futures. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-030-63916-7_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-63916-7_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63915-0
Online ISBN: 978-3-030-63916-7
eBook Packages: EnergyEnergy (R0)