Abstract
Potassium (K) fertilizer recommendations for annual crops in the USA are generally founded in soil test results. The goal of this chapter is to highlight additional plant-related traits that may impact crop responses to K fertilization. This includes the role of tissue testing, the influence of luxury consumption, genetic improvement of K use efficiency, genotype × environment × management interactions on K uptake and yield, response to foliar K fertilization, intraplant K cycling, fungal associations and K uptake, the influence of K on crop quality, and the role of K in abiotic stress tolerance. Recognizing the potential role of these plant factors may help reconcile response inconsistencies based solely on soil test information, and improve future K recommendations. Finally, we hope to highlight knowledge gaps and opportunities for additional integrated soil–plant K research.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The impact of potassium (K) on growth and yield has been previously compiled and summarized for a wide array of agronomic and horticultural crop species (Munson 1985). In addition, the chemistry of K in soils, the K cycle, and K soil testing methods and corresponding recommendations are discussed at length elsewhere in this book, and as such, these details will not be discussed here. Collectively, these results and other research findings have been translated into an array of Extension publications used to guide soil fertility/plant nutrition practices of farmers (e.g., Vitosh et al. 1995; Buchholz et al. 2004). For K, this translation process continues in an effort to reconcile state-specific K recommendations and to resolve inconsistencies between these recommendations and incorporate findings from emerging research. The goal of this chapter is to broaden our understanding of K in crop performance by building on traditional concepts, expanding knowledge into nontraditional issues, and highlighting knowledge gaps and opportunities for additional research.
14.2 Soil and Tissue Testing
14.2.1 Soil Testing for Potassium
Traditionally, soil testing is used to predict yield responses to K fertilizer application, and this approach can work well (Slaton et al. 2010). However, the relationship between soil test K and seed yield can be inconsistent and not accurately predict crop response to fertilizer application. For example, Clover and Mallarino (2013) conducted K fertilizer response trials for maize (Zea mays L.) and soybean (Glycine max L. Merr.) yield on over 50 site-years in Iowa. The 16 site-years responsive to K fertilizer application had soil test K levels <173 mg K kg−1. However, another 18 site-years in this study also had soil test K levels below this “critical level” (<173 mg K kg−1 soil) and these were unresponsive to K fertilizer applications. Other work in Iowa with maize showed grain yield unresponsive to fertilizer K applications at 22 of 28 sites, even though soil test K concentrations ranged from 85 to 172 mg K kg−1 soil (Mallarino and Higashi 2009). Intensive sampling campaigns have revealed unforeseen temporal and spatial variation in soil test K estimates that can preclude an accurate assessment of soil test K, and negate an association with yield from being realized (Randall et al. 1997; Borges and Mallarino 1998; Lissbrant et al. 2010).
14.2.2 Tissue Testing for Potassium
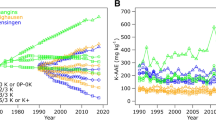
Tissue testing can supplement soil testing and augment efforts to predict crop responses to K fertilizer application, but results from tissue K testing can vary with species, stage of plant growth, and the tissue used for analysis (McNaught 1958; Page and Talibudeen 1982). Macy (1936) introduced the concept of “critical percentage” of each nutrient, above which there is “luxury consumption” and below which there is “poverty adjustment.” Results from a multistate K nutrition trial with maize (Hanway 1962; Fig. 14.1) identified a critical percentage of 10.3 g K kg−1 DM. Tissue K concentrations at most sites in this trial were above this critical concentration and would be considered in the luxury consumption category. Tyner (1947) estimated the critical leaf K concentration for maize to be 13.0 g K kg−1 DM. This generally agrees with more recent results where the critical concentration of K in maize vegetative tissues was reported to be in the range of 10.2–14.2 g K kg−1 DM (Page and Talibudeen 1982; Clover and Mallarino 2013; Stammer and Mallarino 2018) and the critical concentration identified in Fig. 14.1. While significant relationships were evident between soil test K levels and K concentrations in whole plants (at V5–V6), stems, and ear leaves, it was only the latter tissue whose K concentrations were associated with grain yield critical concentration (12.3 g K kg−1 DM, Mallarino and Higashi 2009). Like maize, leaf tissues, rather than whole-plant samples are more closely associated with soybean grain yield (Slaton et al. 2010; Clover and Mallarino 2013; Stammer and Mallarino 2018). The yield was reduced as leaf K concentrations of soybean sampled at ~R2 (late flowering) declined below a critical concentration range of 15.6–20.0 g K kg−1 DM. Extension recommendations (Vitosh et al. 1995; Kaiser et al. 2016; Brown 2017) generally identify higher critical K concentrations for maize ear (18.0–30.0 g K kg−1 DM) and soybean leaves (17.0–25.0 g K kg−1 DM) than the studies reported here. Opportunity clearly exists to broaden the implementation of tissue testing in K fertility recommendations by enhancing our understanding of critical K concentrations in select tissues of major crop species.
Relationship between aboveground dry weight and tissue K concentrations of maize at silking. Plant mass data were adjusted relative to the highest yield at each location-year. Linear-plateau regression was used to identify the critical K concentration for maize tissues in this study (10.3 g kg−1 DM) above which yield did not increase (e.g., luxury consumption). (Adapted from Hanway 1962)
14.2.3 Luxury Consumption of Potassium
Using tissue testing for K fertility management is complicated by a phenomenon known as luxury consumption (Hanway and Weber 1971). Luxury consumption occurs when fertilization increases tissue nutrient concentrations without a corresponding increase in biomass (Macy 1936). For example, modest rates of K fertilization (up to 180 kg K ha−1 year−1) increased yields, whereas higher K application rates (up to 270 kg K year−1) failed to further increase but resulted in high tissue K concentrations in alfalfa (Medicago sativa L.) (de Campos Bernardi et al. 2013). Luxury K consumption also has been reported in soybean and maize where K concentrations in vegetative tissues of nearly twice the critical value necessary for high grain yield have been reported (Clover and Mallarino 2013).
In a long-term maize K nutrition study, Qiu et al. (2014) reported that fertilizing with 225 kg K ha−1 failed to increase grain and stover yields and grain K concentrations over the intermediate K fertilizer rate (113 kg K ha−1), but did result in higher stover K concentrations. This luxury consumption reduced all measures of potassium use efficiency (KUE) including K harvest index, agronomic efficiency (kg kg−1), partial factor productivity (kg kg−1), and K recovery efficiency (%). In addition to reduced efficiencies, extremely high rates of K fertilization as KCl can reduce growth or even kill plants (Page and Talibudeen 1982). Smith and colleagues (Rominger et al. 1976; Smith et al. 1981) applied up to 3000 kg K ha−1 to alfalfa (Medicago sativa L.) and reported very high tissue K (up to 60 g K kg−1 DM) and Cl (up to 80 g Cl kg−1 DM) concentrations that were associated with reduced yield/plant death due to the high Cl concentrations. These extremely high rates of K fertilization are uneconomical, but also illustrate the negative agronomic consequences that can occur with extreme luxury consumption.
14.3 Factors Influencing Potassium Uptake
14.3.1 Genetics and Potassium Uptake
Interest in yield-K relationships has evolved from simple yield responses to issues focused on genetic (G), environmental (E), and management (M) strategies and their interactions (G × E × M) that alter K uptake and use in crops. This information underpins the current interest in 4R Nutrient Stewardship Programs (http://www.nutrientstewardship.com/). Unfortunately, less is known regarding the G × E × M effects on K nutrition than is known for nitrogen (N) and phosphorus (P), in part, because the potential environmental issues associated with N and P have stimulated more research on these nutrients. Early work by Kleese et al. (1968) reported significant G effects for seed K of wheat (Triticum aestivum L.), barley (Hordeum vulgare L.) and soybean, and little G × E effects despite large apparent (but unreported) differences in soil fertility between the two locations of the trial. They concluded that a single environment might suffice for screening genotypes for differences in mineral accumulation. Differences in soil K levels does not always alter seed K concentrations. Mallarino and Higashi (2009) observed no effect of a fourfold range in soil test K on maize grain K concentrations, although soil test K levels did alter K concentrations of vegetative tissue. Forage K concentrations of sorghum (Sorghum bicolor L.) hybrids exhibited a nearly twofold range (Gorz et al. 1987). General combining ability and additive gene action was more important than specific combining ability in determining herbage K concentrations of sorghum. Kaiser et al. (2014) analyzed leaf nutrient concentrations of flag leaves from spring wheat at heading in 18 site-years. They reported significant G, E, and G × E interaction effects for K (and most other nutrients). Flag leaf K concentrations ranged from 13.5 to 19.2 g K kg−1 DM in the 14 genotypes analyzed. Six genotypes responded consistently across environments, while the other eight, and especially the varieties Albany and Vantage, contributed extensively to the G × E effects. Because of this interaction, these authors suggested that critical concentrations/sufficiency ranges should be developed for individual genotypes or groups of varieties. This G × E for tissue K concentrations may contribute to the extensive variation often observed in tissue K-yield relationships, including those illustrated in Fig. 14.1.
14.3.2 Potassium Uptake and Yield
While direct genetic selection for increased KUE has not been reported, indirect improvement of KUE with selection for higher yield is viewed as a desirable outcome (Rengel and Damon 2008). Ciampitti and Vyn (2014) assembled a literature-based dataset to examine the relationship between maize grain yield and uptake of N, P, and K. These data were parsed into groups representing different eras of maize improvement (1880–1960, 1961–1975, 1976–1985, 1986–1995, 1996–2005, and 2006–2012) with each group containing from 59 to 455 individual observations. Data were further separated into that originating from the United States (US) and data from 31 other countries (“world”) exclusive of the US. Regression of plant K uptake and grain yield revealed a linear relationship inclusive of both old and new hybrids that were independent of geography (Fig. 14.2). The slope of this line indicates an uptake of 22.8 g K kg−1 of grain produced; a value that has remained effectively unchanged for over a century.
Relationship between maize grain yield and plant K uptake. Data points represent results from historical periods (1880–1960 [US only]; 1961–1975; 1976–1985; 1986–1996; 1996–2005; 2006–2012) and include from 59 to 455 observations per point. The linear regression includes all data. The dotted line illustrates a theoretical trend if K use efficiency had improved during this period of selection for high maize yields. (Adapted from Ciampitti and Vyn 2014)
A departure from linearity where modern hybrids produced high yields with less K uptake was not observed (Fig. 14.2, dotted line). This suggests that new hybrids had similar KUE as old hybrids; all hybrids required proportional amounts of K to produce grain. This conclusion is supported by estimates of the physiological efficiency of K (PE, grain yield per K content ratio at maturity) that remained largely unchanged irrespective of hybrid yield/era (Ciampitti and Vyn 2014). In a similar study, Morgounov et al. (2013) reported that spring wheat yield doubled between 1930s era varieties and those released after 1986. Seed N concentrations declined slightly, whereas grain K concentrations remained constant in response to selection for high yield. As with maize, K uptake scaled with grain yield, and KUE remained unchanged by selection for high grain yield. By comparison, total K uptake (kg ha−1) by cotton (Gossypium hirsutum L.) was unaffected as the lint yield of varieties released between the 1970s and 2006 increased (Rochester and Constable 2015). As a result, K uptake efficiency was improved with selection for yield from approximately 10–13 kg lint kg−1 K uptake.
The relatively conserved relationship described for K uptake-grain yield also is observed with K removal-forage yield (Berg et al. 2005, 2007). Analysis of alfalfa samples obtained from four annual harvests of 80 plots receiving 4 P (0–75 kg P ha−1) and 5 K (0–400 kg K ha−1) fertilizer rates over 7 years (28 total harvests, 2240 observations) revealed that, like maize (Fig. 14.2), uptake of K by alfalfa scales with herbage yield (Fig. 14.3). The R2 value of 0.76 indicates that irrespective of harvest date, stand age, and P-fertilization rate, forage yield remained the principle determinant of alfalfa K uptake. The slope of the regression line (0.022 kg K removed kg−1 DM agrees well with the “book value” of 0.021 kg K removed kg−1 DM previously reported (Vitosh et al. 1995).
Herbage K accumulation of alfalfa scales with alfalfa yield. Plots were fertilized with five K (0, 100, 200, 300, and 400 kg K ha−1 year−1) and four P (0, 25, 50, and 75 kg P ha−1 year−1) application rates and harvested four times in each of 7 years. Fertilizer applications were split with one-half the total annual amount applied after the first harvest in May and the other half applied after the final herbage harvest in September. (Adapted from Berg et al. 2005, 2007)
14.3.3 Potassium Uptake and Management
Genotype × Environment × Management (G × E × M) interactions can modify K uptake and KUE relationships. Parvej et al. (2016) compiled seed K and grain yields for soybean grown at 100 site-years in North America. This included K-fertilized and unfertilized plots and soil test K levels that ranged from 30 to 408 mg K kg−1 soil that together, resulted in a wide range in seed K concentrations (12.7–24.3 g K kg−1). Despite these differences, regression of seed K uptake and grain yield in soybean resulted in similar slopes for both fertilized and unfertilized treatments; approximately 18 g of K is removed per kg of seed (Fig. 14.4). The regression line for the “fertilized” plots was consistently above the “unfertilized” line (Note: intercepts were statistically similar) suggesting slightly higher seed K uptake by fertilized plants. This removal value agrees with results from Navarrete-Ganchozo (2014) who analyzed K removal of soybean receiving several K fertilizer rates/timings at five locations in Indiana USA over 7 years (n = 1049) and reported 19 g K removed kg−1 seed. In both studies, the nature of the yield-seed K removal relationship was virtually identical across vast differences in yield and environments suggesting few G × E × M interactions for this attribute. As with forage K accumulation in alfalfa, yield per se is the main determinant of seed K uptake in this species.
The relationship between seed yield and K uptake of soybean. Data represent 100 site-years located in North America collected primarily from 2002 to 2015 that included paired plots that were fertilized with K or left unfertilized. Thirty-three site-years were irrigated, while the rest were rainfed sites. Slopes of the linear regressions were statistically similar for the K-fertilized versus unfertilized plots. (Adapted from Parvej et al. 2016)
Interestingly, grain K concentrations are so conserved that G × E × M interactions often are inconsequential. For example, across species, K rates and application timings, and environments, the so-called “book values” for grain K concentrations were nearly as accurate at predicting K removal in grain as were lab-measured K concentrations and grain yields (Fig. 14.5). In all cases, the slopes of the relationship between measured and predicted K removal ranged from 1.0 to 1.1 and R2-values were 0.70–0.90. The option to use book values for grain K concentration estimates has enabled K use and K nutrient budgets to be determined at various spatial resolutions ranging from local to national scales (http://nugis.ipni.net/Publication/).
Comparison of observed K removal (grain yield × grain [K]) and K removal based on grain yield and “book values” for grain K (3.9 and 18.3 g K kg−1 grain DM for maize and soybean, respectively) across K fertilizer rates and species at 5 locations over 6 years. Locations include the Davis Purdue Ag Center (DPAC), the Northeast Purdue Ag Center (NePAC), Pinney Purdue Ag Center (PPAC), the Southeast Purdue Ag Center (SePAC), and Throckmorton Purdue Ag Center (TPAC). (Adapted from Navarrete-Ganchozo 2014)
Parvej et al. (2016) also explored the relationship between soil test K and seed K concentrations. Analysis including all site-years and fertilization groups revealed that seed K concentrations declined at soil test K levels below approximately 170 g K kg−1 soil; however, R2-values indicate that soil test K only explained 24 and 40% of the variability in seed K concentration for plots with and without K fertilizer, respectively. This illustrates the challenge of using soil testing in a site-specific manner to predict seed K concentrations.
14.4 Alternative Potassium Management Strategies
14.4.1 Foliar Fertilization with Potassium
An alternative management strategy for K fertilization is foliar fertilization. Unfortunately, most foliar fertilization research has used solutions containing N, P, K, and often micronutrients and not K alone (Garcia and Hanway 1976; Orlowski et al. 2016). Thus, positive responses of these fertilizer blends cannot be attributed solely to K (or any other macronutrient). Results of most studies with an array of species reveal inconsistent responses of yield to foliar fertilization, and when positive, modest yield increases (Thom et al. 1981; Giskin and Efron 1986; Umar et al. 1999; Ling and Silberbush 2002; Hu et al. 2008) with yield increases often attributed to N or P, and not K in the nutrient blend.
Among agronomic species, soybean has been the most studied with respect to foliar fertilization. Early multi-site/year studies showed inconsistent yield response of soybean to foliar fertilization (Haq and Mallarino 1998, 2000). When it occurred, the magnitude of yield enhancement generally did not offset the application costs of the foliar fertilizer. These authors also reported little consistent impact of foliar K on oil and protein concentrations in soybean seed (Haq and Mallarino 2005). Mallarino et al. (2001) reported a significant (P < 0.10) yield response of soybean to foliar fertilization in only 2 of 18 small plot trials and 1 of 8 larger strip trials. The responsive plots had relatively high soil test P and K (>30 mg P kg−1 and 133–213 mg K kg−1 soil), while plots with low P and K (9–13 kg P kg−1 soil; 97 mg K kg−1 soil) were surprisingly unresponsive to foliar applications. Moreira et al. (2017) also observed a yield response of soybean to foliar fertilization with KNO3 under certain environmental conditions and attributed this primarily to the N in the foliar spray. Although the application of KNO3 did not increase seed yield in this study, seed K concentrations were elevated slightly, suggesting that foliar K uptake was possible. However, other studies reveal no impact of foliar K application on seed K concentrations and grain yield, but high rates of foliar K resulted in increased damage to leaf tissues (Syverud et al. 1980).
Applying foliar K fertilizers with fungicides to soybean also has been evaluated. Foliar application of K as 0-0-30 or 0-0-62 (K2O) did not increase grain yield or ear leaf K concentrations over control plots (Shetley et al. 2015). Soil fertility levels were high at all locations and this may have precluded a positive response to foliar-applied K. Nelson et al. (2005, 2010) compared foliar K application to soybean to pre-plant K applications in soils with low-to-medium K availability. Consistent grain yield increases occurred in response to soil-applied K, and these were accompanied by higher tissue K concentrations. Foliar K application resulted in slightly higher yields in some site-years, especially if applied at vegetative growth stages, but soil-applied K resulted in the highest yields and profitability. These authors concluded that foliar K application to soybean is not a substitute for pre-plant K application. In a 57 site-year study in the Midwest USA, foliar application of a blend of nutrients including K did not significantly increase soybean yield and had a low probability of enhancing profitability (Orlowski et al. 2016). However, only five site-years in this study had soil test K levels below 100 mg K kg−1 soil, lessening the likelihood of a response to K application, including foliar applications. Nevertheless, the low probability of a positive yield response along with modest yield increases when they do occur indicates that foliar fertilization is an unreliable and likely unprofitable strategy for fertilizing crops with K.
14.4.2 Potassium Application Methods, Including Fertigation
A recent meta-analysis revealed that the K placement method has no effect on yield when K fertilizer was placed between 0 and 10 cm, and only a modest effect on yield when placed at depths greater than 10 cm (Nkebiwe et al. 2016). This conclusion is supported by a large number of multi-site studies where the yield of soybean and maize were unaffected by K placement and timing of application (e.g., Vyn and Janovicek 2001; Yin and Vyn 2002). The sub-soiling effect that occurs simultaneously with the deep placement of fertilizer can by itself increase yield. Mullins et al. (1994) showed that cotton lint yield was similar between the deep placement of K and subsoil tilled plots without additional K fertilization. This indicates that confounding between the effects of tillage associated with placement versus the effect of the fertilizer per se can obscure the underlying cause of yield increases when they occur.
An alternative to soil and foliar fertilization is fertigation; inclusion of K (and often N) in the irrigation water supplied to crops. These systems generally use drip or similar irrigation systems to provide water and nutrients near the root zone with the goal of increasing both water and nutrient use efficiency. Because of the infrastructure involved, this approach has been largely limited to high-value crops. Neilsen et al. (1999) reviewed fertigation practices in fruit trees. They concluded that fertigation of K resulted in similar depth of K movement into the soil (60–75 cm) but increased lateral K movement in soil when compared to broadcast K applications. Fertigation often increased tissue K concentrations and occasionally increased yield. The grain yield of rice was similar among application methods (fertigation, broadcasting, banding) (Ali et al. 2005). However, the timing of K application did impact yield. Applying K before transplanting or 50 days after transplanting (DAT) reduced rice grain yield for all application methods when compared to K applications made 25 DAT.
14.4.3 Recycling Potassium in Plants
The mobility of K in plants is a commonly observed phenomenon; under K-limited conditions, K is mobilized to younger, meristematic tissues where growth and development are occurring (Hoagland 1932). As a result, deficiency symptoms usually appear predominately in older vegetative tissues. In addition to this well-characterized K partitioning within the plant, there is a growing awareness that nutrient recycling from vegetative tissues to grain can partially meet the nutrient needs of developing seeds. This might occur because post-anthesis K uptake is low relative to other macronutrients. Van Duivenbooden et al. (1996) surveyed the literature (50–100 experiments) and found that wheat had the lowest post-anthesis K uptake (4% of total aboveground K), while maize, sorghum, millet, and rice had generally similar values (14–18%). Post-anthesis uptake of N and P tended to be higher than K, ranging from 18–35% for N and 10–47% for P. Remobilization of K from vegetative tissues to the seed may meet K needs during grain fill, negating the need for additional soil K uptake.
We estimated net changes in dry matter (DM), and mass of N, P, and K between silking and maturity for maize using data published by Ning et al. (2013) where mobilization patterns of senescing hybrids from the 1950s was compared to that of modern, stay-green hybrids (Fig. 14.6). As expected, grain was a net accumulator of DM, N, P, and K irrespective of hybrid group. Modern hybrids double the net accumulation of DM, P, and K, whereas net N accumulation increased about 67% over that reported for 1950s era hybrids. Net partitioning of DM and macronutrients to husks/cobs and were generally similar for both hybrid groups with net losses of N and P, and net gains in DM and K. However, the early-senescing hybrids of the 1950s exhibited net losses in DM and N, P, and K from all other tissues with especially large losses in K from stems. By comparison, these tissues of the stay-green hybrids had net gains in DM in most tissues between silking and maturity. In addition, stems and roots of these hybrids lost far less, and in some cases had net gains in DM and N, P, and K than the 1950s era plants. For K, these reductions in net K loss from roots and stems were offset, in part, by greater net K mobilization from leaf tissues. Summed over all tissues, net K losses of stay-green hybrids (539 mg plant−1) was 97% of the K accumulated in grain (553 mg plant−1). By comparison, only 40% of the K lost by vegetative tissues of the 1950’s era plants was accounted for by net accumulation in grain (259 mg/643 mg) indicating less efficient K recycling and loss of K from the plant system.
Net changes in dry matter (DM) and macronutrients on a per-plant basis of maize tissues between silking and maturity. Early-senescing hybrids released in the 1950s (left) are compared to modern stay-green hybrids released in the 2000s (right). Net gains in DM and nutrients are identified in green text, while net losses are highlighted in red text. (Adapted from Ning et al. 2013)
A similar analysis with low-yielding versus high-yielding plots of modern soybean lines revealed net gains in DM and masses of N, P, and K that scaled with grain yield (Fig. 14.7). Grain yield and N, P, and K in grain of high-yielding plots increased approximately 50% over that of the low-yielding plots between R3 and R8 growth stages. Irrespective of yield, leaf blades and petioles exhibited net losses of DM, N, P, and K during this period. Net gains of K in grain (63 and 91 kg ha−1) could be accounted for by net K losses in blades, petioles, and stems indicating that K recycling in the plant could meet grain K needs. By comparison, and irrespective of yield level, net losses of N and P could only account for approximately 40% of these nutrients in the grain, suggesting that internal recycling alone could not meet the N and P needs of soybean. In this study, leaf blades and petioles that fell to the ground between R3 and R8 returned up to 38 kg K ha−1 to the soil, with lesser amounts of N and a negligible amount of P returned to the soil.
Net changes in dry matter (DM) and macronutrients on a per-hectare basis of soybean tissues between growth stages R3 and R8. Patterns for relatively low-yielding plots are shown on the left, while trends for high-yielding soybean plots are on the right. Net gains in DM and nutrients are identified in green text, net losses are highlighted in red text, while loss to the soil are highlighted in blue. (Adapted from Gaspar et al. 2017a, b)
14.4.4 Crop Residues and Potassium Nutrition
Leaching of K from post-harvest residues can contribute significantly to soil test K and the cycling of K in agroecosystems. Oltmans and Mallarino (2015) reported 34 and 55 kg K ha−1 in soybean and maize residue, respectively, immediately after grain harvest. Two months post-harvest, approximately 50% of this K remained in maize residue, whereas only 19% of this K remained in soybean residue. In both species, reductions in stover K increased with post-harvest precipitation. Soil test K increased from fall to spring on average 27 and 24 mg K kg−1 for soybean and maize, respectively, and these values were positively associated with residue K losses (Fig. 14.8). This residue K input can impact crop yield. Singh et al. (2018) assembled apparent K budgets for rice-maize cropping systems in India for a 5-year period. In addition to fertilizing plots with K (620 kg K ha−1 for the 5-year period), they measured K inputs from alternative sources including crop residue (618–678 kg K ha−1) and roots/stubble (95–152 kg K ha−1). Only plots receiving K from all three of these sources maintained a positive K balance and high yields.
Impact of K leaching from stover of maize and soybean on soil test K (STK) levels. Stover was collected periodically after harvest in fall, K concentrations determined, and mass of K leached from stover calculated based on residue mass. Changes in STK between grain harvest in fall and the following April averaged 27 and 24 mg K kg−1 for soybean and maize, respectively. (Adapted from Oltmans and Mallarino 2015)
14.4.5 Fungal Associations and Potassium Nutrition
Symbiotic associations between plants and mycorrhizal fungi that enhance nutrient uptake have been well-documented for some nutrients, especially P. Less is known regarding the role of these fungi in augmenting plant K uptake. Rosendahl (1943) reported greater K uptake and enhanced plant growth of pine seedlings grown in orthoclase-amended sand when inoculated with Boletus felleus, a fungus known to form mycorrhizal associations with conifers. Recent reviews (Garcia and Zimmermann 2014; Dominguez-Nuñez et al. 2016) summarized the role of mycorrhizae in improving K nutrition of several plant species, especially under K-limited conditions as is often found in forest ecosystems. These authors also indicated that this symbiosis enhanced general abiotic stress tolerance. While the fundamental mechanisms controlling this symbiosis are poorly understood, overexpression of specific K channel genes in the genome of the ectomycorrhizal fungi Hebeloma cylindrosporum increased K accumulation in shoots of pine seedlings (Guerrero-Galán et al. 2018). These genes may serve as molecular targets for enhancing K uptake under low soil K conditions via this symbiotic association. Additional work is needed to more fully characterize the role and quantify the metabolic costs of mycorrhizae in providing K to plants.
Fungal endophytes form mutualistic associations with some plant species resulting in enhanced growth and abiotic stress tolerance. A classic example of this mutualism is endophyte-infected tall fescue (Schedonorus arundinaceus (Schreb.) Dumort.), where P uptake is enhanced in infected plants under low soil P conditions (Malinowski and Belesky 2000). Less is known regarding how endophyte infection impacts K uptake. Rahman and Saiga (2005) reported greater shoot growth and K uptake in tall fescue plants infected with the endophyte Neotyphodium coenophialum when compared to uninfected plants. Responses varied with soil type and tall fescue ecotype. Malinowski and Belesky (2000) also reported increased K absorption rates in one of two tall fescue ecotypes. Additional research, especially under low soil K conditions, is needed to fully understand the role of these fungal endophytes on K uptake. Further, it is unclear if the endophyte enhances shoot growth that then leads to greater K uptake (sink driven) or if greater K uptake occurs that leads to faster shoot growth (source driven).
14.5 Impact of Potassium on Crop Quality
14.5.1 Potassium Nutrition and Crop Quality
Given the large impact that K nutrition can have on physiological function and yield of crops, it is not surprising that crop composition also can be altered under K-deficient conditions. Usherwood (1985) and Mengel (1997) presented an overview of this topic previously. Rather than review the numerous recent papers published on this topic, the impact K deficiency can have on crop quality will be illustrated with key examples for crop categories.
14.5.2 Cereals
Unlike N and P, few reports exist describing the impact of K on grain quality of cereals. A recent review of N, P, and K on wheat performance (Duncan et al. 2018) noted the little consistent effect of K on grain protein concentration. These authors noted that balanced nutrition with N, P, and K maintained protein concentrations in yield-responsive environments. However, few of the 32 field studies reviewed assessed the impact of K on wheat grain quality per se. Holland et al. (2019) reported significant declines in crude protein concentration (%N) of wheat grain with even modest rates (12.5–25 kg K ha−1) of K fertilizer that was associated with increased grain yield. By comparison, the yield of triticale did not increase until a much higher K rate (100 kg K ha−1) was applied and only then did grain protein decline in one of two studies. This suggests that the decline in grain protein concentrations is a result of dilution of grain N as K-stimulated yield.
Rice (Oryza sativa L.) grain quality measured as gel consistency, amylose concentration, gelatinization temperature, and protein concentration can be altered by K fertilization (Bahmaniar and Ranjbar 2007). Some of these attributes interacted with K fertilization rate, plant genotype, and N fertilizer application making it challenging to develop a unified N and K fertilizer program targeting grain quality for rice cultivars used in this study.
In a multi-year study conducted at five locations, Brouder examined the impact of K on maize grain yield and quality (Fig. 14.9). Plots were fertilized with up to 180 kg K ha−1 annually or 360 kg K ha−1 biennially, and large differences in grain yield were observed at some locations (Navarrete-Ganchozo 2014). Despite a three-fold difference in grain K concentration, there was no significant impact of concentrations of starch, protein, or oil in grain. Maize grain amino acid concentrations were generally higher in plants provided adequate K (Usherwood 1985).
Relationship between grain K concentration and starch, protein, and oil concentration of maize grain. Data were obtained from 17 site-years in Indiana USA. Plants were fertilized with 0–180 kg K ha−1 annually or 0–360 kg K ha−1 biennially. Grain K concentrations were determined with an inductively coupled plasma spectrophotometer, while grain starch, protein, and oil were analyzed with a near-infrared reflectance spectrophotometer. (Adapted from unpublished data from S. Brouder, Purdue University)
14.5.3 Oilseeds
Bailey and Soper (1985) reported little consistent impact of K on the oil and protein composition of the seed of rape, flax, sunflower, and safflower. This agrees with more recent results with canola where seed oil and protein concentrations were unaffected by K fertilization rates that, in some environments, increased yield nearly twofold (Brennan and Bolland 2007). Oil and protein concentrations in soybean seeds also are often not responsive to K fertilizer application, especially if initial soil test K levels are adequate (Krueger et al. 2013). In some studies, seed oil concentrations increase with K, while seed protein concentrations declined (Yin and Vyn 2003). However, recent studies reveal that both protein and oil concentrations of soybean can increase in response to increasing K fertility (Abbasi et al. 2012; Bellaloui et al. 2013), including increases in specific fatty acids (Krueger et al. 2013). Potassium also can interact with P in determining oil and protein concentrations of soybean. Abbasi et al. (2012) applied P as single superphosphate and K as K2SO4 to a soil containing 3.4 mg kg−1 available P and 67 mg kg−1 exchangeable K. In addition to increasing soybean grain yield and nodulation, both grain oil and protein concentrations increased with K fertilizer application, with the highest concentrations achieved only when both P and K were adequate (Fig. 14.10). It is not clear if the addition of sulfur with the K to this soil containing 10 g kg−1 organic C also contributed to the changes in seed oil and protein.
Impact of potassium (K) and phosphorus (P) fertilizer application on the concentrations of protein and oil in soybean seed. Prior to fertilization, the clay loam soil (Humic Lithic Eutrudept) contained 3.7 and 67 mg kg−1 P and K, respectively. (Adapted from Abbasi et al. 2012)
14.5.4 Forage
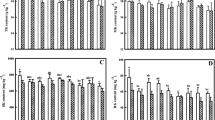
Numerous plant species can be used as forage, including silage produced from plants normally used for grain production like maize, sorghum, and small grains. Besides yield, K can alter plant morphology (e.g., leaf:stem ratio) and composition of cell walls that together, can impact forage intake, digestibility, and ultimately animal performance. Lissbrant et al. (2009) reported higher in vitro dry matter disappearance (IVDMD) and protein concentrations for low-yielding, K-deficient alfalfa when compared to forage from plants fertilized with K (Table 14.1). The greater digestibility was associated with lower concentrations of neutral detergent fiber (NDF), acid detergent fiber (ADF), and lignin of the K-deficient plants. However, the yield of both digestible nutrients (DNY) and protein ha−1 were greater in K-fertilized plants because of their greater forage yield. Similar trends for reduced forage protein concentrations with increased K fertilization have been reported (Macolino et al. 2013), but concentrations of NDF, ADF, and lignin were not increased in their study conducted in a high-K soil.
Most studies on forage grass quality have focused on N nutrition, with few studies exploring K impacts on forage grass quality. Balasko (1977) reported small, but significant increases in IVDMD in 4 of 8 winter harvests of tall fescue when N and P fertilizers were supplemented with K. Forage yield increased in 3 of 4 harvests where IVDMD increased. Similarly, when stargrass (Cynodon spp.) was adequately fertilized with P, yield responses to K fertilization were evident in 2 of 3 years and these higher yields were accompanied by increases in forage IVDMD, but reductions in forage protein (Pant et al. 2004). By comparison, Malhi et al. (2005) reported similar concentrations of protein, NDF, and ADF even in environments where timothy (Phleum pretense L.) forage yields were enhanced by K fertilization. Wheat and triticale (Triticosecale spp. Wittm. ex A. Camus) forage composition can be altered by K fertilization (Holland et al. 2019). Significant increases in dry matter disappearance, metabolizable energy, water-soluble carbohydrates, and reductions in NDF, ADF, and protein were observed in some environments and growth stages as a result of K fertilization.
In addition, because of luxury consumption discussed previously, forage can accumulate high-K concentrations leading to mineral imbalances that result in potentially fatal livestock diseases like hypomagnesemia and milk fever (Kayser and Isselstein 2005; Lunnan et al. 2018); thus, there is a potential anti-quality issue with forage K nutrition. The ratio of magnesium and calcium to K in forage, and limiting excess K also is a consideration for plants used for ruminant livestock feed.
14.5.5 Fiber
In cotton, K deficiency reduced most measures of fiber quality including fiber elongation, 50% span length, uniformity ratio, micronaire, fiber maturity, and perimeter in all genotypes studied (Pettigrew et al. 1996). This confirms earlier findings of Cassman et al. (1990) where most fiber attributes of cotton were positively associated with soil, leaf, and fiber K concentrations. Analysis of fiber composition of industrial hemp (Cannabis sativa L.) grown for six site-years in Canada revealed no effect of K fertilization (0–200 kg K ha−1) on cellulose, hemicellulose, or lignin concentrations (Aubin et al. 2015). However, fiber yield also was not influenced by K application, leading the authors to suggest that high initial soil test K levels (~200 kg K ha−1) may have prevented responses to K fertilization.
14.5.6 Tubers and Tuberous Roots
Starch synthase, a key enzyme in starch synthesis, requires K for proper activation (Murata and Akazawa 1968). Thus, it is not surprising that K influences the growth and composition of starch-rich tubers. Potato (Solanum tuberosum L.) quality is often influenced by both rate of K fertilization and K source. Tuber specific gravity, a trait positively associated with processing quality, was lower with K fertilization; however, specific gravity losses were less when K2SO4 rather than KCl is used as a K source (Panique et al. 1997). Stanley and Jewell (1989) reported a reduction in tuber dry matter with increasing K. Tubers of K-fertilized plants had lower reducing sugars that cause discoloration of fried food like chips and fries. Sensory evaluation of French fries made from K-fertilized tubers scored higher with consumers in several categories including color and texture. Other work on chipping potatoes revealed that chip yield was greatest, and residual chip oil content lowest for K2SO4-fertilized plants when compared to plants provided KCl as a fertilizer source (Kumar et al. 2007).
By comparison, increases in yield and quality of sweet potato (Ipomoea batatas L.) were not influenced by K source. In general, K fertilization increased the frequency of large tuberous roots in this species and slowed weight losses in storage; an index of quality (Nicholaides et al. 1985). In a 13 site-year study where the addition of K to N- and P-fertilized soil more than doubled tuber yields, John et al. (2013) reported increased tuber yield and a slight increase in tuber starch concentration for cassava (Manihot esculenta Crantz) with K fertilizer application. They also observed altered starch rheological characteristics including increased amylose content, granule size, pasting temperature, viscosity, and swelling volume with K fertilization. Concentrations of cyanogenic glycosides, a serious anti-quality attribute of this species, were reduced with K fertilizer application. By comparison, Obigbesan (1977) reported that cultivar and environment were more important that K fertilization in determining levels of cyanogenic glycosides in this species.
14.5.7 Fruits and Vegetables
Because this category contains numerous species whose K fertilizer practices are managed in diverse ways (source, timing, application methods, …), it is not possible to comprehensively represent general trends and effects here. An early review (Greenwood et al. 1980) indicated that K fertilization decreased the quality of some species (e.g., carrot, cauliflower, turnips), while improving the quality of others (spinach, parsnips). In a more recent review, Lester et al. (2010) summarized the impact of K nutrition on the quality of over 20 fruit/vegetable species. Fruit quality, measured as either compositional (e.g., sugar, acidity, vitamins, carotene, …) or physical attributes (e.g., color, firmness, texture, shelf-life, …) generally improved with K fertilization. In studies where results were not in agreement, this was attributed to variation in fertilizer application method and/or K source (Mikkelsen 2017). The underlying mechanisms involved in K-enriched fruit quality are emerging. For example, in pear (Pyrus communis L.) K regulates the expression of key genes involved in sugar and sorbitol metabolism in both sources and sink tissues that ultimately enhances sugar accumulation in and quality of pear fruits (Shen et al. 2017, 2018).
14.5.8 Human Nutrition and Health
A vast body of evidence regarding the role of K in human health and nutrition has been published. A few key examples are highlighted here. The positive impact of K on reducing blood pressure and related diseases has been a recurring research topic. Dietary K supplementation has been shown to reduce blood pressure in hypertensive individuals, particularly in high-sodium consumers not currently receiving hypertensive drug treatment, and those in the lowest category of K intake (Filippini et al. 2017). Adequate dietary K intake (~90 mmol day−1) is recommend to achieve blood pressure control. In a meta-analysis, D’Elia et al. (2011) reported that high dietary K intake is associated with low rates of stroke and might also reduce the risk of coronary heart disease and total cardiovascular disease. These results support recommendations for higher consumption of K-rich foods to prevent vascular diseases. Increased fruit and vegetable intake in the range commonly consumed is associated with a reduced risk of stroke (He et al. 2006). The protective effects of fruit and vegetables on stroke prevention have a strong biological basis, including the fact they are rich sources of dietary K. The positive effects of K on human health extend beyond blood pressure and heart disease to include reduction in osteoporosis (Lambert et al. 2015), insulin-resistant diabetes (Ekmekcioglu et al. 2016), kidney disease (Zhang et al. 2019), ulcerative colitis and Crohn’s disease (Khalili et al. 2016), and obesity and metabolic syndrome (Cai et al. 2016).
14.6 Plant Stress Tolerance and Potassium Nutrition
14.6.1 Potassium and Abiotic Stress Tolerance
Potassium has long been identified as a nutrient that is critical in alleviating the detrimental effects of abiotic stresses in plants. Several reviews have discussed the role of K in tolerance to drought, salinity, chilling, and freezing temperatures, flooding, and stresses associated with climate change (Ahmad et al. 2018; Amtmann et al. 2018; Anschütz et al. 2014; Cakmak 2005; Kant and Kafkafi 2002; Oosterhuis et al. 2014; Sardans and Peñuelas 2015; Wang et al. 2013). Rather than reiterate the details found in these reviews, a few key examples of how K functions in plants and imparts stress tolerance are outlined here.
The role of K in abiotic stress tolerance is exemplified by the regulation of stomatal aperture that influences both water loss from leaves and CO2 uptake in photosynthesis. Humble and Raschke (1971) used X-ray microprobe analyses to demonstrate the K accumulation in guard cells and subsequent water influx that ultimately opened stomata. This response was specific to K and highlights the key role it has in both transpiration and photosynthesis, processes central to plant water relations, and dry weight accumulation. For example, Pervez et al. (2004) reported increases in both photosynthesis and transpiration with increasing K fertilizer applications to cotton (Fig. 14.11). They also observed improved instantaneous water use efficiency (WUE, the ratio of photosynthesis to transpiration) and lower canopy temperatures with K fertilization. Lower leaf temperatures result from evaporative cooling associated with the latent heat of vaporization as liquid water evaporates from the leaf surface; a process critical to temperature regulation in plants.
Influence of K fertilizer application on rates of photosynthesis, transpiration, water use efficiency (WUE), and canopy temperature of cotton. Data are averaged over four cotton cultivars and two K sources. (Adapted from Pervez et al. 2004)
Potassium also influences the flooding tolerance of plants. Dwivedi et al. (2017) reported that K fertilization mitigated submergence-induced stress in rice. This included reducing membrane damage during flooding and improved post-flooding recovery of photosynthesis. This result confirms earlier findings with barley where the loss of membrane integrity and tissue K during waterlogging were associated with flooding intolerance (Zeng et al. 2014).
Long-term survival of perennial plants also is enhanced by K fertilization. Lissbrant et al. (2010) used cluster analysis to categorize plots from a 7-year-old P and K fertility study with alfalfa into groups that varied as a function of forage yield in May of the final yield of the study. The highest yielding plots in the High cluster had tissue K concentrations between 18.2 and 26.2 g K kg−1 dry matter (Table 14.2), whereas tissue K concentrations ranged from 7.5 to 15.7 g K kg−1 for the Very Low cluster. Plant populations declined with time in all clusters and averaged 9 and 51 plants m−2 for the Very Low and High clusters, respectively, in Years 6 and 7. Forage yields declined to 0 kg ha−1 for the Very Low cluster at the end of the study. Plants died during summer rather than winter (Berg et al. 2018); a finding that is contrary to the general understanding that K enhances winter survival of alfalfa. Additional work is necessary to identify the underlying cause(s) for the death of these alfalfa plants in summer.
14.6.2 Potassium and Biotic Stress Tolerance
Fewer reviews have summarized the role of K in biotic stress tolerance. In many cases, K application reduces disease incidence, but in some studies, disease prevalence is unaffected or even increases with K fertilization (Huber and Arny 1985). Others found insufficient data to conduct a thorough quantitative review of the role of K on disease incidence (Veresoglou et al. 2013). Amtmann et al. (2008) reported inconsistencies associated with plant- and experiment-specific interactions of K with plant physiological traits (growth, cell wall structure, metabolites, hormones, …) that are components of the disease-resistance response. In this study, response to K varied with pathogen group; reductions in fungal diseases more common with K application than were diseases caused by bacteria and viruses.
A meta-analysis of the role of K in insect resistance also revealed mixed results that were influenced by the insect group being studied (Butler et al. 2012). While insect populations generally showed a negative response to K fertilization, data were too sparse to identify significant effects. As with diseases, individual studies describe a positive impact of K fertilizer application on the reduction of insects, while other studies report no impact of K on insect stress (Kitchen et al. 1990; Myers and Gratton 2006; Myers et al. 2005). Closer collaboration among soil fertility/plant nutrition and entomologists/pathologists would advance our understanding of the role of K in biotic stress tolerance.
References
Abbasi MK, Tahir MM, Azam W, Abbas Z, Rahim N (2012) Soybean yield and chemical composition in response to phosphorus–potassium nutrition in Kashmir. Agron J 104:1476–1484. https://doi.org/10.2134/agronj2011.0379
Ahmad Z, Anjum S, Waraich EA, Ayub MA, Ahmad T, Tariq RMS, Ahmad R, Iqbal MA (2018) Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress–a review. J Plant Nutr 41:1734–1743. https://doi.org/10.1080/01904167.2018.1459688
Ali A, Zia MS, Hussain F, Salim M, Mahmood IA, Shahzad A (2005) Efficacy of different methods of potassium fertilizer application on paddy yield, K uptake and agronomic efficiency. Pak J Agric Sci 42:27–32
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691. https://doi.org/10.1111/j.1399-3054.2008.01075.x
Amtmann A, Armengaud P, Volkov V (2018) Potassium nutrition and salt stress. In: Annual Plant Reviews. Wiley, New Jersey, pp 328–379. https://doi.org/10.1002/9781119312994.apr0151
Anschütz U, Becker D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol 171:670–687. https://doi.org/10.1016/j.jplph.2014.01.009
Aubin MP, Seguin P, Vanasse A, Tremblay GF, Mustafa AF, Charron JB (2015) Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. Crop Forage Turfgrass Manage 1:1–10. https://doi.org/10.2134/cftm2015.0159
Bahmaniar MA, Ranjbar GA (2007) Response of rice (Oryza sativa L.) cooking quality properties to nitrogen and potassium application. Pak J Biol Sci 10:1880–1884
Bailey LD, Soper RJ (1985) Potassium nutrition of rape, flax, sunflower, and safflower. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Madison WI, pp 765–798
Balasko JA (1977) Effects of N, P, and K fertilization on yield and quality of tall fescue forage in winter. Agron J 69:425–428. https://doi.org/10.2134/agronj1977.00021962006900030023x
Bellaloui N, Yin X, Mengistu A, McClure AM, Tyler DD, Reddy KN (2013) Soybean seed protein, oil, fatty acids, and isoflavones altered by potassium fertilizer rates in the Midsouth. Amer J Plant Sci 4:976–988. https://doi.org/10.4236/ajps.2013.45121
Berg WK, Cunningham SM, Brouder SM, Joern BC, Johnson KD, Santini J, Volenec JJ (2005) Influence of phosphorus and potassium on alfalfa yield and yield components. Crop Sci 45:297–304. https://doi.org/10.2135/cropsci2005.0297
Berg WK, Cunningham SM, Brouder SM, Johnson KD, Joern BC, Volenec JJ (2007) The long-term impact of phosphorus and potassium fertilization on alfalfa yield and yield components. Crop Sci 47:2198–2209. https://doi.org/10.2135/cropsci2006.09.0576
Berg WK, Lissbrant S, Cunningham SM, Brouder SM, Volenec JJ (2018) Phosphorus and potassium effects on taproot C and N reserve pools and long-term persistence of alfalfa (Medicago sativa L.). Plant Sci 272:301–308. https://doi.org/10.1016/j.plantsci.2018.02.026
Borges R, Mallarino AP (1998) Variation of early growth and nutrient content of no-till corn and soybean in relation to soil phosphorus and potassium supplies. Comm Soil Sci Plant Anal 29:2589–2605. https://doi.org/10.1080/00103629809370136
Brennan RF, Bolland MDA (2007) Influence of potassium and nitrogen fertiliser on yield, oil and protein concentration of canola (Brassica napus L.) grain harvested in South-Western Australia. Austr J Exp Agric 47:976–983. https://doi.org/10.1071/EA06114
Brown C (2017) Agronomy guide for field crops publication 811. Ministry of agriculture, food and rural affairs, Queen’s printer for Ontario, Toronto, p 433. http://www.omafra.gov.on.ca/english/crops/pub811/pub811.pdf. Accessed 13 May 2020
Buchholz DD, Brown JR, Garret JD, Hanson RG, Wheaton HN (2004) Soil test interpretations and recommendations handbook. Univ of Missouri-College of Agric, Div Plant Sci, Columbia, MO
Butler J, Garratt MPD, Leather SR (2012) Fertilisers and insect herbivores: a meta-analysis. Ann Appl Biol 161:223–233. https://doi.org/10.1111/j.1744-7348.2012.00567.x
Cai X, Li X, Fan W, Yu W, Wang S, Li Z, Scott EM, Li X (2016) Potassium and obesity/metabolic syndrome: a systematic review and meta-analysis of the epidemiological evidence. Nutrients 8:183. https://doi.org/10.3390/nu8040183
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530. https://doi.org/10.1002/jpln.200420485
Cassman KG, Kerby TA, Roberts BA, Bryant DC, Higashi SL (1990) Potassium nutrition effects on lint yield and fiber quality of Acala cotton. Crop Sci 30:672–677. https://doi.org/10.2135/cropsci1990.0011183X003000030039x
Ciampitti IA, Vyn TJ (2014) Understanding global and historical nutrient use efficiencies for closing maize yield gaps. Agron J 106:2107–2117. https://doi.org/10.2134/agronj14.0025
Clover MW, Mallarino AP (2013) Corn and soybean tissue potassium content responses to potassium fertilization and relationships with grain yield. Soil Sci Soc Amer J 77:630–642. https://doi.org/10.2136/sssaj2012.0223
de Campos Bernardi, AC, Rassini JB, MendonÓa FC, de Paula Ferreira R (2013) Alfalfa dry matter yield, nutritional status and economic analysis of potassium fertilizer doses and frequency. Inter J Agron Plant Prod 4:389–98.
D’Elia L, Barba G, Cappuccio FP, Strazzullo P (2011) Potassium intake, stroke, and cardiovascular disease: a meta-analysis of prospective studies. J Amer College Cardio 57:1210–1219. https://doi.org/10.1016/j.jacc.2010.09.070
Dominguez-Nuñez JA, Benito B, Berrocal-Lobo M, Albanesi A (2016) Mycorrhizal fungi: role in the solubilization of potassium. In: Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 77–98
Duncan EG, O’Sullivan CA, Roper MM, Biggs JS, Peoples MB (2018) Influence of co-application of nitrogen with phosphorus, potassium and Sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat. Field Crops Res 226:56–65. https://doi.org/10.1016/j.fcr.2018.07.010
Dwivedi SK, Kumar S, Bhakta N, Singh SK, Rao KK, Mishra JS, Singh AK (2017) Improvement of submergence tolerance in rice through efficient application of potassium under submergence-prone rainfed ecology of Indo-Gangetic plain. Func Plant Biol 44:907–916. https://doi.org/10.1071/FP17054
Ekmekcioglu C, Elmadfa I, Meyer AL, Moeslinger T (2016) The role of dietary potassium in hypertension and diabetes. J Physiol Biochem 72:93–106. https://doi.org/10.1007/s13105-015-0449-1
Filippini T, Violi F, D’Amico R, Vinceti M (2017) The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Inter J Cardio 230:127–135. https://doi.org/10.1016/j.ijcard.2016.12.048
Garcia RL, Hanway JJ (1976) Foliar fertilization of soybeans during the seed-filling period. Agron J 68:653–657. https://doi.org/10.2134/agronj1976.00021962006800040030x
Garcia K, Zimmermann SD (2014) The role of mycorrhizal associations in plant potassium nutrition. Front Plant Sci 5:337. https://doi.org/10.3389/fpls.2014.00337
Gaspar AP, Laboski CA, Naeve SL, Conley SP (2017a) Dry matter and nitrogen uptake, partitioning, and removal across a wide range of soybean seed yield levels. Crop Sci 57:2170–2182. https://doi.org/10.2135/cropsci2016.05.0322
Gaspar AP, Laboski CA, Naeve SL, Conley SP (2017b) Phosphorus and potassium uptake, partitioning, and removal across a wide range of soybean seed yield levels. Crop Sci 57:2193–2204. https://doi.org/10.2135/cropsci2016.05.0378
Giskin M, Efron Y (1986) Planting date and foliar fertilization of corn grown for silage and grain under limited moisture. Agron J 78:426–429. https://doi.org/10.2134/agronj1986.00021962007800030005x
Gorz HJ, Haskins FA, Pedersen JF, Ross WM (1987) Combining ability effects for mineral elements in forage sorghum hybrids. Crop Sci 27:216–219. https://doi.org/10.2135/cropsci1987.0011183X002700020017x
Greenwood DJ, Cleaver TJ, Turner MK, Hunt J, Niendorf KB, Loquens SMH (1980) Comparison of the effects of phosphate fertilizer on the yield, phosphate content and quality of 22 different vegetable and agricultural crops. J Agric Sci 95:457–469. https://doi.org/10.1017/S0021859600039502
Guerrero-Galán C, Delteil A, Garcia K, Houdinet G, Conéjéro G, Gaillard I, Sentenac H, Zimmermann SD (2018) Plant potassium nutrition in ectomycorrhizal symbiosis: properties and roles of the three fungal TOK potassium channels in Hebeloma cylindrosporum. Envir Microbiol 20:1873–1887. https://doi.org/10.1111/1462-2920.14122
Hanway JJ (1962) Northcentral regional potassium studies. III. Field studies with corn. Res Bull (Iowa Agriculture and Home Economics Experiment Station) 34(503):Article 1
Hanway JJ, Weber CR (1971) Accumulation of N, P, and K by soybean (Glycine max (L.) Merrill) plants. Agron J 63:406–408. https://doi.org/10.2134/agronj1971.00021962006300030017x
Haq MU, Mallarino AP (1998) Foliar fertilization of soybean at early vegetative stages. Agron J 90:763–769. https://doi.org/10.2134/agronj1998.00021962009000060008x
Haq MU, Mallarino AP (2000) Soybean yield and nutrient composition as affected by early season foliar fertilization. Agron J 92:16–24. https://doi.org/10.2134/agronj2000.92116x
Haq MU, Mallarino A (2005) Response of soybean grain oil and protein concentrations to foliar and soil fertilization. Agron J 97:910–918. https://doi.org/10.2134/agronj2004.0215
He FJ, Nowson CA, MacGregor GA (2006) Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 367:320–326. https://doi.org/10.1016/S0140-6736(06)68069-0
Hoagland DR (1932) Mineral nutrition of plants. Annu Rev Biochem 1:618–636. https://doi.org/10.1146/annurev.bi.01.070132.003154
Holland JE, Hayes RC, Refshauge G, Poile GJ, Newell MT, Conyers MK (2019) Biomass, feed quality, mineral concentration and grain yield responses to potassium fertiliser of dual-purpose crops. New Zeal J Agric Res 62:476–494. https://doi.org/10.1080/00288233.2018.1512505
Hu Y, Burucs Z, Schmidhalter U (2008) Effect of foliar fertilization application on the growth and mineral nutrient content of maize seedlings under drought and salinity. Soil Sci Plant Nutr 54:133–141. https://doi.org/10.1111/j.1747-0765.2007.00224.x
Huber DM, Arny DC (1985) Interactions of potassium with plant disease. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Madison WI, pp 467–488
Humble GD, Raschke K (1971) Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol 48:447–453. https://doi.org/10.1104/pp.48.4.447
John KS, Ravindran CS, George J, Nair MM, Suja G (2013) Potassium: a key nutrient for high tuber yield and better tuber quality in cassava. Better Crops–South Asia 2013:26–27
Kaiser DE, Wiersma JJ, Anderson JJ (2014) Genotype and environment variation in elemental composition of spring wheat flag leaves. Agron J 106:324–336. https://doi.org/10.2134/agronj2013.0329
Kaiser DE, Rosen CJ, Lamb JA (2016) Potassium for crop production. Univ. of Minn. St. Paul MN https://extension.umn.edu/phosphorus-and-potassium/potassium-crop-production. Accessed 13 May 2020
Kant S, Kafkafi U (2002) Potassium and abiotic stresses in plants. In: Pasricha NS, Bansal SK (eds) Potassium for sustainable crop production. Potash Institute of India, Gurgaon, pp 233–251
Kayser M, Isselstein J (2005) Potassium cycling and losses in grassland systems: a review. Grass Forage Sci 60:213–224. https://doi.org/10.1111/j.1365-2494.2005.00478.x
Khalili H, Malik S, Ananthakrishnan AN, Garber JJ, Higuchi LM, Joshi A, Peloquin J, Richter JM, Stewart KO, Curhan GC, Awasthi A (2016) Identification and characterization of a novel association between dietary potassium and risk of Crohn’s disease and ulcerative colitis. Front Immun 7:554. https://doi.org/10.3389/fimmu.2016.00554
Kitchen NR, Buchholz DD, Nelson CJ (1990) Potassium fertilizer and potato leafhopper effects on alfalfa growth. Agron J 82:1069–1074. https://doi.org/10.2134/agronj1990.00021962008200060008x
Kleese RA, Rasmusson DC, Smith LH (1968) Genetic and environmental variation in mineral element accumulation in barley, wheat, and soybeans. Crop Sci 8:591–593. https://doi.org/10.2135/cropsci1968.0011183X000800050025x
Krueger K, Goggi AS, Mallarino AP, Mullen RE (2013) Phosphorus and potassium fertilization effects on soybean seed quality and composition. Crop Sci 53:602–610. https://doi.org/10.2135/cropsci2012.06.0372
Kumar P, Pandey SK, Singh BP, Singh SV, Kumar D (2007) Influence of source and time of potassium application on potato growth, yield, economics and crisp quality. Potato Res 50:1–13. https://doi.org/10.1007/s11540-007-9023-8
Lambert H, Frassetto L, Moore JB, Torgerson D, Gannon R, Burckhardt P, Lanham-New S (2015) The effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysis. Osteoporosis Inter 26:1311–1318. https://doi.org/10.1007/s00198-014-3006-9
Lester GE, Jifon JL, Makus DJ (2010) Impact of potassium nutrition on postharvest fruit quality: melon (Cucumis melo L) case study. Plant Soil 335:117–131. https://doi.org/10.1007/s11104-009-0227-3
Ling F, Silberbush M (2002) Response of maize to foliar vs. soil application of nitrogen–phosphorus–potassium fertilizers. J Plant Nutr 25:2333–2342. https://doi.org/10.1081/PLN-120014698
Lissbrant S, Stratton S, Cunningham SM, Brouder SM, Volenec JJ (2009) Impact of long-term phosphorus and potassium fertilization on alfalfa nutritive value–yield relationships. Crop Sci 49:1116–1124. https://doi.org/10.2135/cropsci2008.06.0333
Lissbrant S, Brouder SM, Cunningham SM, Volenec JJ (2010) Identification of fertility regimes that enhance long-term productivity of alfalfa using cluster analysis. Agron J 102:580–591. https://doi.org/10.2134/agronj2009.0300
Lunnan T, Øgaard AF, Krogstad T (2018) Potassium fertilization of timothy-based cut grassland—effects on herbage yield, mineral composition and critical K concentration on soils with different K status. Grass Forage Sci 73:500–509. https://doi.org/10.1111/gfs.12341
Macolino S, Lauriault LM, Rimi F, Ziliotto U (2013) Phosphorus and potassium fertilizer effects on alfalfa and soil in a non-limited soil. Agron J 105:1613–1618. https://doi.org/10.2134/agronj2013.0054
Macy P (1936) The quantitative mineral nutrient requirement of plants. Plant Physiol 11:749–764. https://doi.org/10.1104/pp.11.4.749
Malhi SS, Loeppky H, Coulman B, Gill KS, Curry P, Plews T (2005) Fertilizer nitrogen, phosphorus, potassium, and sulphur effects on forage yield and quality of timothy hay in the Parkland region of Saskatchewan. Can J Plant Nutr 27:1341–1360. https://doi.org/10.1081/PLN-200025834
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940. https://doi.org/10.2135/cropsci2000.404923x
Mallarino AP, Higashi SL (2009) Assessment of potassium supply for corn by analysis of plant parts. Soil Sci Soc Amer J 73:2177–2183. https://doi.org/10.2136/sssaj2008.0370
Mallarino AP, Haq MU, Wittry D, Bermudez M (2001) Variation in soybean response to early season foliar fertilization among and within fields. Agron J 93:1220–1226. https://doi.org/10.2134/agronj2001.1220
McNaught KJ (1958) Potassium deficiency in pastures: I. potassium content of legumes and grasses. New Zeal J Agric Res 1:148–181. https://doi.org/10.1080/00288233.1958.10431069
Mengel K (1997) Impact of potassium on crop yield and quality with regard to economical and ecological aspects. In: Johnston AE (ed) Food security in the WANA region, the essential need for balanced fertilization. International Potash Institute, Basle, pp 157–174
Mikkelsen R (2017) The importance of potassium management for horticultural crops. Indian J Fert 13(11):82–86
Moreira A, Moraes LAC, Schroth G, Becker FJ, Mandarino JMG (2017) Soybean yield and nutritional status response to nitrogen sources and rates of foliar fertilization. Agron J 109:629–635. https://doi.org/10.2134/agronj2016.04.0199
Morgounov AI, Belan I, Zelenskiy Y, Roseeva L, Tomoskozi S, Bekes F, Abugalieve A, Cakmak I, Vargas M, Crossa J (2013) Historical changes in grain yield and quality of spring wheat varieties cultivated in Siberia from 1900 to 2010. Can J Plant Sci 93:425–433. https://doi.org/10.4141/cjps2012-091
Mullins GL, Reeves DW, Burmester CH, Bryant HH (1994) In-row subsoiling and potassium placement effects on root growth and potassium content of cotton. Agron J 86:136–139. https://doi.org/10.2134/agronj1994.00021962008600010025x
Munson RD (1985) Potassium in agriculture. American Society of Agronomy, Madison, WI
Murata T, Akazawa T (1968) Enzymic mechanism of starch synthesis in sweet potato roots. I. Requirements of potassium ions for starch synthetase. Arch Biochem Biophys 126:873–879. https://doi.org/10.1016/0003-9861(68)90481-5
Myers SW, Gratton C (2006) Influence of potassium fertility on soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), population dynamics at a field and regional scale. Environ Entom 35:219–227. https://doi.org/10.1603/0046-225X-35.2.219
Myers SW, Gratton C, Wolkowski RP, Hogg DB, Wedberg JL (2005) Effect of soil potassium availability on soybean aphid (Hemiptera: Aphididae) population dynamics and soybean yield. J Econ Entom 98:113–120. https://doi.org/10.1093/jee/98.1.113
Navarrete-Ganchozo RJ (2014) Quantification of plant-available potassium (K) in a corn-soybean rotation: A long-term evaluation of K rates and crop K removal effects (Doctoral dissertation, Purdue University). (Order No. 3636478). Available from Agricultural & Environmental Science Database; Dissertations & Theses @ CIC Institutions; ProQuest Dissertations & Theses Global. (1615794935). Retrieved from https://search.proquest.com/docview/1615794935?accountid=13360. Accessed 13 May 2020
Neilsen GH, Neilsen D, Peryea F (1999) Response of soil and irrigated fruit trees to fertigation or broadcast application of nitrogen, phosphorus, and potassium. HortTechnol 9:393–401. https://doi.org/10.21273/HORTTECH.9.3.393
Nelson KA, Motavalli PP, Nathan M (2005) Response of no-till soybean [Glycine max (L.) Merr.] to timing of preplant and foliar potassium applications in a claypan soil. Agron J 97:832–838. https://doi.org/10.2134/agronj2004.0241
Nelson KA, Motavalli PP, Stevens WE, Dunn D, Meinhardt CG (2010) Soybean response to preplant and foliar-applied potassium chloride with strobilurin fungicides. Agron J 102:1657–1663. https://doi.org/10.2134/agronj2010.0065
Nicholaides JJ, Chancy HF, Mascagni HJ, Wilson LG, Eaddy DW (1985) Sweet potato response to K and P fertilization. Agron J 77:466–470. https://doi.org/10.2134/agronj1985.00021962007700030024x
Ning P, Li S, Yu P, Zhang Y, Li C (2013) Post-silking accumulation and partitioning of dry matter, nitrogen, phosphorus and potassium in maize varieties differing in leaf longevity. Field Crops Res 144:19–27. https://doi.org/10.1016/j.fcr.2013.01.020
Nkebiwe PM, Weinmann M, Bar-Tal A, Muller T (2016) Fertilizer placement to improved crop nutrient acquisition and yield. A review and meta-analysis. Field Crop Res 196:389–401. https://doi.org/10.1016/j.fcr.2016.07.018
Obigbesan GO (1977) Investigations on Nigerian root and tuber crops: effect of potassium on starch yields, HCN content and nutrient uptake of cassava cultivars (Manihot esculenta). J Agric Sci 89:29–34. https://doi.org/10.1017/S0021859600027167
Oltmans RR, Mallarino AP (2015) Potassium uptake by corn and soybean, recycling to soil, and impact on soil test potassium. Soil Sci Soc Am J 79:314–327. https://doi.org/10.2136/sssaj2014.07.0272
Oosterhuis DM, Loka DA, Kawakami EM, Pettigrew WT (2014) The physiology of potassium in crop production. Adv Agron 126:203–233. https://doi.org/10.1016/B978-0-12-800132-5.00003-1
Orlowski JM, Haverkamp BJ, Laurenz RG, Marburger D, Wilson EW, Casteel SN, Conley SP, Naeve SL, Nafziger ED, Roozeboom KL, Ross WJ (2016) High-input management systems effect on soybean seed yield, yield components, and economic break-even probabilities. Crop Sci 56:1988–2004. https://doi.org/10.2135/cropsci2015.10.0620
Page MB, Talibudeen O (1982) Critical potassium potentials for crops: 2. Potentials for wheat, maize, peas, beans and sugar beet in their early growth on a sandy loam. Eur J Soil Sci 33:771–778. https://doi.org/10.1111/j.1365-2389.1982.tb01806.x
Panique E, Kelling KA, Schulte EE, Hero DE, Stevenson WR, James RV (1997) Potassium rate and source effects on potato yield, quality, and disease interaction. Amer Potato J 74:379–398. https://doi.org/10.1007/BF02852777
Pant HK, Mislevy P, Rechcigl JE (2004) Effects of phosphorus and potassium on forage nutritive value and quantity: environmental implications. Agron J 96:1299–1305. https://doi.org/10.2134/agronj2004.1299
Parvej M, Slaton NA, Fryer MS, Roberts TL, Purcell LC (2016) Postseason diagnosis of potassium deficiency in soybean using seed potassium concentration. Soil Sci Soc Amer J 80:1231–1243. https://doi.org/10.2136/sssaj2016.02.0030
Pervez H, Ashraf M, Makhdum MI (2004) Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.). Photosynthetica 42:251–255. https://doi.org/10.1023/B:PHOT.0000040597.62743.5b
Pettigrew WT, Heitholt JJ, Meredith WR (1996) Genotypic interactions with potassium and nitrogen in cotton of varied maturity. Agron J 88:89–93. https://doi.org/10.2134/agronj1996.00021962008800010019x
Qiu S, Xie J, Zhao S, Xu X, Hou Y, Wang X, Zhou W, He P, Johnston AM, Christie P, Jin J (2014) Long-term effects of potassium fertilization on yield, efficiency, and soil fertility status in a rain-fed maize system in Northeast China. Field Crops Res 163:1–9. https://doi.org/10.1016/j.fcr.2014.04.016
Rahman MH, Saiga S (2005) Endophytic fungi (Neotyphodium coenophialum) affect the growth and mineral uptake, transport and efficiency ratios in tall fescue (Festuca arundinacea). Plant Soil 272:163–171. https://doi.org/10.1007/s11104-004-4682-6
Randall GW, Iragavarapu TK, Evans SD (1997) Long-term P and K applications: I. Effect on soil test incline and decline rates and critical soil test levels. J Prod Agric 10:565–571. https://doi.org/10.2134/jpa1997.0565
Rengel Z, Damon PM (2008) Crops and genotypes differ in efficiency of potassium uptake and use. Physiol Plant 133:624–636. https://doi.org/10.1111/j.1399-3054.2008.01079.x
Rochester IJ, Constable GA (2015) Improvements in nutrient uptake and nutrient use-efficiency in cotton cultivars released between 1973 and 2006. Field Crops Res 173:14–21. https://doi.org/10.1016/j.fcr.2015.01.001
Rominger RS, Smith D, Peterson LA (1976) Yield and chemical composition of alfalfa as influenced by high rates of K topdressed as KC1 and K2SO4 1. Agron J 68:573–577. https://doi.org/10.2134/agronj1976.00021962006800040010x
Rosendahl RO (1943) The effect of mycorrhizal and nonmycorrhizal fungi on the availability of difficultly soluble potassium and phosphorus. Soil Sci Soc Amer J 7:477–479. https://doi.org/10.2136/sssaj1943.036159950007000C0080x
Sardans J, Peñuelas J (2015) Potassium: a neglected nutrient in global change. Glob Ecol Biogeogr 24:261–275. https://doi.org/10.1111/geb.12259
Shen C, Wang J, Shi X, Kang Y, Xie C, Peng L, Dong C, Shen Q, Xu Y (2017) Transcriptome analysis of differentially expressed genes induced by low and high potassium levels provides insight into fruit sugar metabolism of pear. Front Plant Sci 8:938. https://doi.org/10.3389/fpls.2017.00938
Shen C, Li Y, Wang J, Al Shoffe Y, Dong C, Shen Q, Xu Y (2018) Potassium influences expression of key genes involved in sorbitol metabolism and its assimilation in pear leaf and fruit. J Plant Growth Reg 37:883–895. https://doi.org/10.1007/s00344-018-9783-1
Shetley J, Nelson KA, Stevens WG, Dunn D, Burdick B, Motavalli PP, English JT, Dudenhoeffer CJ (2015) Corn yield response to pyraclostrobin with foliar fertilizers. J Agric Sci 7:18. https://doi.org/10.5539/jas.v7n7p18
Singh VK, Dwivedi BS, Singh SK, Mishra RP, Shukla AK, Rathore SS, Shekhawat K, Majumdar K, Jat ML (2018) Effect of tillage and crop establishment, residue management and K fertilization on yield, K use efficiency and apparent K balance under rice-maize system in North-Western India. Field Crops Res 224:1–12. https://doi.org/10.1016/j.fcr.2018.04.012
Slaton NA, Golden BR, DeLong RE, Mozaffari M (2010) Correlation and calibration of soil potassium availability with soybean yield and trifoliolate potassium. Soil Sci Soc Amer J 74:1642–1651. https://doi.org/10.2136/sssaj2009.0197
Smith D, Dobrenz AK, Schonhorst MH (1981) Response of alfalfa seedling plants to high levels of chloride-salts. J Plant Nutr 4:143–174. https://doi.org/10.1080/01904168109362909
Stammer AJ, Mallarino AP (2018) Plant tissue analysis to assess phosphorus and potassium nutritional status of corn and soybean. Soil Sci Soc Amer J 82:260–270. https://doi.org/10.2136/sssaj2017.06.0179
Stanley R, Jewell S (1989) The influence of source and rate of potassium fertilizer on the quality of potatoes for French fry production. Potato Res 32:439–446. https://doi.org/10.1007/BF02358499
Syverud TD, Walsh LM, Oplinger ES, Kelling KA (1980) Foliar fertilization of soybeans (Glycine max L.). Comm Soil Sci Plant Anal 11:637–651. https://doi.org/10.1080/00103628009367069
Thom WO, Miller TC, Bowman DH (1981) Foliar fertilization of rice after midseason. Agron J 73:411–414. https://doi.org/10.2134/agronj1981.00021962007300030007x
Tyner EH (1947) The relation of corn yields to leaf nitrogen, phosphorus, and potassium content. Soil Sci Soc Amer J 11:317–323. https://doi.org/10.2136/sssaj1947.036159950011000C0059x
Umar S, Bansal SK, Imas P, Magen H (1999) Effect of foliar fertilization of potassium on yield, quality, and nutrient uptake of groundnut. J Plant Nutr 22:1785–1795. https://doi.org/10.1080/01904169909365754
Usherwood NR (1985) The role of potassium in crop quality. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Madison WI, pp 489–513
Van Duivenbooden N, deWit CT, Van Keulen H (1996) Nitrogen, phosphorus and potassium relations in five major cereals reviewed in respect to fertilizer recommendations using simulation modelling. Fert Res 44:37–49. https://doi.org/10.1007/BF00750691
Veresoglou SD, Barto EK, Menexes G, Rillig MC (2013) Fertilization affects severity of disease caused by fungal plant pathogens. Plant Path 62:961–969. https://doi.org/10.1111/ppa.12014
Vitosh ML, Johnson JW, Mengel DB (1995) Tri-state fertilizer recommendations for corn, soybeans, wheat and alfalfa. Ext Bull E-2567. Michigan State Univ East Lansing
Vyn TJ, Janovicek KJ (2001) Potassium placement and tillage system effects on corn response following long-term no till. Agron J 93:487–495. https://doi.org/10.2134/agronj2001.933487x
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Inter J Mol Sci 14:7370–7390. https://doi.org/10.3390/ijms14047370
Yin X, Vyn TJ (2002) Soybean responses to potassium placement and tillage alternatives following no-till. Agron J 94:1367–1374. https://doi.org/10.2134/agronj2002.1367
Yin X, Vyn TJ (2003) Potassium placement effects on yield and seed composition of no-till soybean seeded in alternate row widths. Agron J 95:126–132. https://doi.org/10.2134/agronj2003.1260
Zeng F, Konnerup D, Shabala L, Zhou M, Colmer TD, Zhang G, Shabala S (2014) Linking oxygen availability with membrane potential maintenance and K+ retention of barley roots: implications for waterlogging stress tolerance. Plant Cell Envir 37:2325–2338. https://doi.org/10.1111/pce.12422
Zhang Y, Chen P, Chen J, Wang L, Wei Y, Xu D (2019) Association of low serum potassium levels and risk for all-cause mortality in patients with chronic kidney disease: a systematic review and meta-analysis. Ther Apher Dial 23:22–31. https://doi.org/10.1111/1744-9987.12753
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Volenec, J.J., Brouder, S.M., Murrell, T.S. (2021). Broadening the Objectives of Future Potassium Recommendations. In: Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R., Thompson, M.L. (eds) Improving Potassium Recommendations for Agricultural Crops. Springer, Cham. https://doi.org/10.1007/978-3-030-59197-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-59197-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59196-0
Online ISBN: 978-3-030-59197-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)