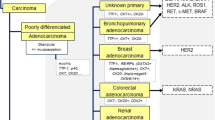
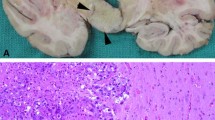
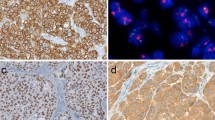
Abstract
In this chapter, we consider the diagnostic approach to brain metastases in a routine clinical setting. Here we review standard assessment of tissue from intraoperative frozen section analysis to conventional histological and immunohistochemical assays. Clinicopathological differential diagnoses particular to the central nervous system (CNS) axis are discussed as well as commonly used panels of antibodies. Finally, we consider the molecular testing that is currently employed at our own institution, and remark on how the trajectory of molecular pathology is likely to impact the evaluation of brain metastases in the near future.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Osborn AG, Salzman KL, Jhaveri MD, Barkovich J. Diagnostic imaging: brain. 3rd ed. Philadelphia: Elsevier; 2016.
Stark AM, Stohring C, Hedderich J, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: prognostic factors and survival in 309 patients with regard to patient age. J Clin Neurosci. 2011;18:34–8. https://doi.org/10.1016/j.jocn.2010.03.046.
Hwang TL, Close TP, Grego JM, Brannon WL, Gonzales F. Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer. 1996;77:1551–5. https://doi.org/10.1002/(SICI)1097-0142(19960415)77:8<1551::AID-CNCR19>3.0.CO;2-Z.
Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4:S209–19. https://doi.org/10.4103/2152-7806.111298.
Shapira Y, Hadelsberg UP, Kanner AA, Ram Z, Roth J. The ventricular system and choroid plexus as a primary site for renal cell carcinoma metastasis. Acta Neurochir. 2014;156:1469–74. https://doi.org/10.1007/s00701-014-2108-7.
Mampre D, et al. Propensity for different vascular distributions and cerebral edema of intraparenchymal brain metastases from different primary cancers. J Neurooncol. 2019;143:115–22. https://doi.org/10.1007/s11060-019-03142-x.
Cagney DN, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19:1511–21. https://doi.org/10.1093/neuonc/nox077.
Klotz S, et al. Clinical neuropathology image 6-2018: metastasis of breast carcinoma to meningioma. Clin Neuropathol. 2018;37:252–3. https://doi.org/10.5414/NP301150.
Takei H, Powell SZ. Tumor-to-tumor metastasis to the central nervous system. Neuropathology. 2009;29:303–8. https://doi.org/10.1111/j.1440-1789.2008.00952.x.
Hamlat A, et al. Malignant transformation of intra-cranial epithelial cysts: systematic article review. J Neurooncol. 2005;74:187–94. https://doi.org/10.1007/s11060-004-5175-4.
Freilich RJ, Thompson SJ, Walker RW, Rosenblum MK. Adenocarcinomatous transformation of intracranial germ cell tumors. Am J Surg Pathol. 1995;19:537–44.
Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary rules on population-based cancer survival. Cancer Causes Control. 2013;24:1231–42. https://doi.org/10.1007/s10552-013-0203-3.
Gurel B, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol. 2010;34:1097–105. https://doi.org/10.1097/PAS.0b013e3181e6cbf3.
Srodon M, Westra WH. Immunohistochemical staining for thyroid transcription factor-1: a helpful aid in discerning primary site of tumor origin in patients with brain metastases. Hum Pathol. 2002;33:642–5.
Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–10.
Ordonez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Adv Anat Pathol. 2012;19:140–51. https://doi.org/10.1097/PAP.0b013e318253465d.
Ho IC, et al. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991;10:1187–92.
Hoch RV, Thompson DA, Baker RJ, Weigel RJ. GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer. 1999;84:122–8.
Liu H, Shi J, Prichard JW, Gong Y, Lin F. Immunohistochemical evaluation of GATA-3 expression in ER-negative breast carcinomas. Am J Clin Pathol. 2014;141:648–55. https://doi.org/10.1309/AJCP0Q9UQTEESLHN.
Sangoi AR, Shrestha B, Yang G, Mego O, Beck AH. The novel marker GATA3 is significantly more sensitive than traditional markers mammaglobin and GCDFP15 for identifying breast cancer in surgical and cytology specimens of metastatic and matched primary tumors. Appl Immunohistochem Mol Morphol. 2016;24:229–37. https://doi.org/10.1097/PAI.0000000000000186.
Miettinen M, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13–22. https://doi.org/10.1097/PAS.0b013e3182a0218f.
Mete O, Kefeli M, Caliskan S, Asa SL. GATA3 immunoreactivity expands the transcription factor profile of pituitary neuroendocrine tumors. Mod Pathol. 2019;32:484–9. https://doi.org/10.1038/s41379-018-0167-7.
Ordonez NG. Value of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: a review and update. Hum Pathol. 2014;45:191–205. https://doi.org/10.1016/j.humpath.2013.02.007.
Tatsumori T, et al. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem Mol Morphol. 2014;22:377–82. https://doi.org/10.1097/PAI.0b013e3182980544.
Nguyen T, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma. Arch Pathol Lab Med. 2015;139:1028–34. https://doi.org/10.5858/arpa.2014-0479-OA.
Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expression in schwannoma; a study of 115 retroperitoneal and 22 peripheral schwannomas. Mod Pathol. 2006;19:115–21. https://doi.org/10.1038/modpathol.3800489.
Pratt D, et al. Re-evaluating TTF-1 immunohistochemistry in diffuse gliomas: expression is clone-dependent and associated with tumor location. Clin Neuropathol. 2017;36:263–71. https://doi.org/10.5414/NP301047.
Shibuya M. Welcoming the new WHO classification of pituitary tumors 2017: revolution in TTF-1-positive posterior pituitary tumors. Brain Tumor Pathol. 2018;35:62–70. https://doi.org/10.1007/s10014-018-0311-6.
Bielle F, et al. Chordoid gliomas of the third ventricle share TTF-1 expression with organum vasculosum of the lamina terminalis. Am J Surg Pathol. 2015;39:948–56. https://doi.org/10.1097/PAS.0000000000000421.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. https://doi.org/10.1038/nature11412.
Lambein K, Van Bockstal M, Denys H, Libbrecht L. 2013 update of the American Society of Clinical Oncology/College of American Pathologists guideline for human epidermal growth factor receptor 2 testing: impact on immunohistochemistry-negative breast cancers. J Clin Oncol. 2014;32:1856–7. https://doi.org/10.1200/JCO.2013.54.2530.
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–7. https://doi.org/10.1200/JOP.777003.
Aggarwal R, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36:2492–503. https://doi.org/10.1200/JCO.2017.77.6880.
Sepulveda AR, et al. Molecular biomarkers for the evaluation of colorectal cancer. Am J Clin Pathol. 2017. https://doi.org/10.1093/ajcp/aqw209.
Burgess EF, et al. Discordance of high PD-L1 expression in primary and metastatic urothelial carcinoma lesions. Urol Oncol. 2019;37:299.e219–25. https://doi.org/10.1016/j.urolonc.2019.01.002.
van de Nes J, et al. Targeted next generation sequencing reveals unique mutation profile of primary melanocytic tumors of the central nervous system. J Neurooncol. 2016;127:435–44. https://doi.org/10.1007/s11060-015-2052-2.
Wang L, et al. Consistent copy number changes and recurrent PRKAR1A mutations distinguish Melanotic Schwannomas from Melanomas: SNP-array and next generation sequencing analysis. Genes Chromosomes Cancer. 2015;54:463–71. https://doi.org/10.1002/gcc.22254.
Brastianos PK, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–77. https://doi.org/10.1158/2159-8290.CD-15-0369.
Capper D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–74. https://doi.org/10.1038/nature26000.
Moran S, et al. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:1386–95. https://doi.org/10.1016/S1470-2045(16)30297-2.
Orozco JIJ, et al. Epigenetic profiling for the molecular classification of metastatic brain tumors. Nat Commun. 2018;9:4627. https://doi.org/10.1038/s41467-018-06715-y.
Moss J, et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9:5068. https://doi.org/10.1038/s41467-018-07466-6.
Slieker RC, et al. DNA methylation landscapes of human fetal development. PLoS Genet. 2015;11:e1005583. https://doi.org/10.1371/journal.pgen.1005583.
Goodman AM, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–608. https://doi.org/10.1158/1535-7163.MCT-17-0386.
Leibold AT, Monaco GN, Dey M. The role of the immune system in brain metastasis. Curr Neurobiol. 2019;10:33–48.
Mansfield AS, et al. Contraction of T cell richness in lung cancer brain metastases. Sci Rep. 2018;8:2171. https://doi.org/10.1038/s41598-018-20622-8.
Sundstrom T, et al. Inhibition of mitochondrial respiration prevents BRAF-mutant melanoma brain metastasis. Acta Neuropathol Commun. 2019;7:55. https://doi.org/10.1186/s40478-019-0712-8.
Fischer GM, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9:628–45. https://doi.org/10.1158/2159-8290.CD-18-1489.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–60. https://doi.org/10.1016/j.devcel.2019.04.011.
Gonçalo Rodrigues, Ayuko Hoshino, Candia M. Kenific, Irina R. Matei, Loïc Steiner, Daniela Freitas, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nature Cell Biology. 2019;21(11):1403–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pisapia, D.J. (2020). Pathology of Brain Metastases. In: Ramakrishna, R., Magge, R., Baaj, A., Knisely, J. (eds) Central Nervous System Metastases. Springer, Cham. https://doi.org/10.1007/978-3-030-42958-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-42958-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42957-7
Online ISBN: 978-3-030-42958-4
eBook Packages: MedicineMedicine (R0)