Abstract
The paper attempts to investigate the changes in the brain network dynamics between alcoholic and non-alcoholic groups using electroencephalographic signals. A novel entropy-based technique is proposed in this study to understand the dynamics of the neural network for the two groups. To do this, we have examined for the two groups their time-varying instantaneous phase synchronization events when the subjects are engaged in an object recognition task. Next, we have characterized the complexity of the phase synchronization using Fuzzy Sample Entropy over different time scales, referred to as Multiscale Fuzzy Sample Entropy (MFSampEn). The temporal dynamics of phase synchronization arise due to the presence of deterministic characteristics in the time series and the results of surrogate analyses confirm that. Lastly, we check the applicability of Multiscale Fuzzy Sample Entropy (MFSampEn) as entropy-based features in the context of alcoholic and non-alcoholic object recognition classification.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Alcoholism
- EEG
- Fuzzy sample entropy
- Multiscale Fuzzy Sample Entropy
- SVM
- Complexity
- Dynamical Phase Synchronization
1 Introduction
The human brain comprises four basic modules: which often interact among themselves to execute a cognitive task, such as reasoning, learning, planning, and sensory-motor control/coordination. The present paper aims at studying the biological underpinnings of the brain dynamics with respect to functional connectivity between activated brain regions in alcoholics and non-alcoholics in an object recognition problem. The fact that the functional brain connectivity is not static and that it varies over time and for different pathological disorders has attracted the broad attention of scientists [15]. It is known that even a moderate amount of alcohol consumption may affect the brain causing slurred speech, decrease in anxiety and motor skills, etc. Therefore, it is important to compare the brain network dynamics of the two groups to investigate whether long term alcohol abuse causes any permanent damage of one/more brain regions resulting in significant deterioration of cognitive skills.
In recent years, considerable amount of researches [2, 3, 17] have focused on investigating how long term alcohol consumption affects the brain functionality. The existing literature also reveals that heavy drinking has negative implications on the short term memory and may sometimes even lead to blackouts [2]. It has also come to our notice that the drinking onset age may have some influence on alcohol-related neuro-toxicity [3]. However, all these findings have either physiological or philosophical basis of analysis. Understanding the biological insight of the brain functionality changes due to alcohol consumption is the new research era.
Although there exist recent works on functional Magnetic Resonance Imaging (fMRI) based study for discriminating the functional brain connectivity of the alcoholics as compared to the healthy controls [11], there is a dearth of literature which investigates the essence of integrating brain dynamics in functional connectivity analysis. The present work fills the void by an electroencephalographic (EEG) means of brain response acquisition. There are a considerable amount of work on EEG Event-Related Potentials (ERP) for alcoholic and non-alcoholic discrimination. For instance, beta power synchronization is observed among alcoholics as compared to non-alcoholics [17] in most of the existing studies. Numerous studies on gamma band analysis indicate that gamma oscillation is responsible for binding features of an object [17].
In this study, we set out to examine Electroencephalography (EEG) based time-varying instantaneous phase synchronization (referred to as dynamical phase synchronization [15]) of 10 alcoholic and 10 non-alcoholic subjects engaged in an object recognition task. We then investigate their dynamical pattern using multiscale fuzzy sample entropy analysis [6, 12, 23]. Furthermore, our experience of working with electroencephalography (EEG) signals [8, 9] reveals that for a given brain lobe the features extracted from the acquired EEG response vary widely across experimental instances of a given subject for similar stimulation. Due to the presence of fuzzy properties in the experimental instances, the logic of fuzzy sets can, therefore, be used to capture the dynamics of the brain. In the case of sample entropy (SampEn) calculation, a Heaviside function is used whose boundary is rigid. That is, the data points inside the boundary are considered equally, whereas the points marginally outside it are neglected. The rigid boundary condition causes discontinuity, and this may lead to abrupt fluctuations in the entropy values with a small deflection in the tolerance level, and may even fail to provide SampEn value if no template match can be found for a small tolerance value [23]. To circumvent this problem, Chen et al. developed a new complexity metric, Fuzzy Sample Entropy (FSampEn), and used a fuzzy membership function instead of the Heaviside function. In contrast to the Heaviside function, a fuzzy membership function has no rigid boundary. Furthermore, Fuzzy Sample Entropy is continuous and will not fluctuate dramatically with a slight change in the location parameter, as given by the rule of fuzzy membership function. Real biomedical results from [23] show that FSampEn handles noisy data better than its state-of-the-art competitors and is more capable of determining hidden complexity. The paper presents a novel strategy to enjoy the composite benefit of brain dynamics and FSampEn at different time scales (MFSampEn) [6, 7, 15] for changes in functional connectivity dynamics due to long-term alcoholism.
The paper is divided into 4 sections. In Sect. 2, we provide in detail the principles and methodology of the proposed technique. Section 3 deals with experiments on EEG data acquisition and results obtained thereof, and Sect. 4 discusses the conclusion.
2 Principles and Methodologies
2.1 Dynamical Phase Synchronization (DPS)
Phase Difference Eliminating Phase Slips. After preprocessing of the EEG signals acquired from the subjects’ scalp, the phase information of the EEG signals are extracted using the Hilbert transform. The instantaneous frequency (in Hz) is then derived from the obtained phase signal \(\phi (t)\) using first-order differences. The activity in the gamma band (30–50 Hz) shows prominent oscillations with a narrow bandwidth and is thus ideal for estimating instantaneous frequency taking into account the background noise [4]. Also, primarily, gamma-band activity is considered for effects of addiction in the behavior of people [17]. Thus, in this study we have focused on the gamma band oscillations.
However, there may lie a directional trend in the phase differences which affects the probability of two sets of simultaneous data points of length m having distance \({<}r\). To circumvent this, we derived an unwrapped phase signal \(\varPhi (t)(-\infty< \varPhi (t) <\infty )\). The phase differences across the electrodes indexed in time is then determined as:
where i, j denote the electrodes.
In order to eliminate the trend while calculating the multiscale fuzzy sample entropy (MFSampEn) as in [15], the obtained phase differences are wrapped into units of radians, as mod\((\varDelta \phi _{i,j}, 2\pi )\). If we expand the plot of mod\((\varDelta \phi _{ij}, 2\pi )\) versus time (in a sec) for an electrode pair, we obtain a pattern of dynamical synchronous and asynchronous phase differences as DPS [15].
2.2 Multiscale Fuzzy Sample Entropy
Let the input be a data matrix X of size \((N-m) \times m\), length N, pattern length m and location parameter R.
In sample entropy, self-matches are eliminated and X has \(N-m\) rows in order to match dimension in the m and \(m+1\) pattern lengths. These measures remove the bias in the estimations.
In order to determine the vectors similarity, we use a Gaussian fuzzy membership function [23], given by \(\mu (d(i,j),R,c)\), where d(i, j) denotes the distance between \((N-m)\) vectors as the maximum difference of their corresponding scalar components, R is the location parameter determining the location of the function, and c is the shape parameter determining the steepness of the function, with larger c implying a higher relaxation.
The B(i) term derived, represents the probability that there exists a pattern in the pattern space X that is similar in nature to the \(i^{th}\) pattern.
Hence, \(\phi _{m}\) = Probability that a given pair of patterns of length m are similar with each other. The output of the system, \({\ln }\phi _{m}-{\ln }{\phi _{m+1}}\), represents the rate at which new information is created by the underlying system.

Finally, in order to get the multiscale framework of fuzzy sample entropy [23], we take an approach as in [6, 7] to put the original time series through a “coarse-graining” process. The process is done in the sense that multiple coarse-grained time series are obtained by estimating the mean of the data points within non-overlapping windows of increasing length, \(\tau \) (where \(\tau \) represents the scale factor and \(1<j\le \frac{N}{\tau }\).) The length of each coarse-grained time series is \(\frac{N}{\tau }\). The rescaled coarse-grained time series can be calculated using the equation,
Next, the fuzzy sample entropy (FSampEn) [1, 23] is calculated from each coarse-grained time series and is plotted against each scale factor. The calculation of FSampEn for different coarse-grained time series have been done choosing the values of m and R as 2 and 0.15 respectively [6], where m denotes embedding dimension and R denotes location parameter.
2.3 Surrogate Analysis
For each subject, a surrogate data time series is estimated using the iterated amplitude-adjusted Fourier transform (IAAFT) [19] against the original phase difference \(\varDelta \phi _{i,j}(t)\) to search for the presence of nonlinear deterministic processes in DPS. IAAFT is an iterative variation of amplitude-adjusted Fourier transform (AAFT) [22] and making use of the iterative scheme to achieve the arbitrarily close approximation to the autocorrelation and the amplitude distribution [19]. The surrogate method follows a process of randomization of phase components of the signals.
2.4 Power Spectrum Analysis
A fast Fourier transform based average power calculation has been undertaken here. For the calculation, a Hanning window approach [10] has been employed for each epoch of 20 s. Power spectral density (PSD) (dB/Hz) has been calculated.
2.5 Classification of Alcoholic and Non-alcoholic Brain Responses Using Multiscale Fuzzy Sample Entropies as EEG Features
To classify the brain responses of alcoholic and non-alcoholic group, the multiscale fuzzy sample entropy (MFSampEn) [23] information are used as features to train and test a SVM classifier.
3 Experiments and Results
3.1 Experimental Dataset
The experimental data used in this paper has been obtained from the public EEG database [13, 14, 24] of the State University of New York Health Center and owned by Henri Begleiter. The recordings were collected from a pool of ten alcoholic and ten non-alcoholic (control) subjects while being engaged in a visual object recognition task. The data-set contains EEG recordings acquired with 64 electrodes, which includes 3 reference electrodes and the sampling rate of the EEG device used is 256 Hz. Total 260 objects were used to produce the memory set, where the object images were depicted from 1980 Snodgrass and Vandwart Picture set [20]. The subjects for the experiment were exposed to two consecutive pictures of objects from the picture set in such a way that the first picture differs from the second picture with regards to its semantic category, i.e., four footed animal, fruit, weapon, etc. The subjects’ mental task involved identifying whether the pictures shown are matching or non-matching. The detailed framework is explained in [24].
3.2 Data Pre-processing
In the pre-processing stage, the EEG signals are first band-pass filtered using elliptical band pass filter of order 6 with a pass band of 30–50 Hz. In the next step, eye-blinking artifacts are removed manually by checking for 100 \(\upmu \)V potential lasting for a period of 250 ms. Other environmental and physiological artefacts are removed following Independent Component Analysis (ICA) [5].
3.3 Feature Level Discrimination Between Alcoholic and Non-alcoholic Groups
The experiment is carried out to find a clear discrimination between alcoholic and non-alcoholic brain responses from the phase difference information obtained using Dynamical Phase Synchronization method. The experiment is carried out into two steps. In the first step, we extract the instantaneous phase information by Hilbert transform and instantaneous frequency by dividing the phase signal by \(2n\pi \). The instantaneous phase and frequency obtained for the electrode \(Fp_{1}\) and \(Fp_{2}\) for both the alcoholic and non-alcoholic groups are presented in Fig. 1.
In the second step, we compute the phase differences before and after phase-wrapping around mod\((\varDelta \theta , 2\pi )\), and frequency differences between each electrode pair. For instance, the phase difference before and after phase wrapping, and frequency difference between \(Fp_{1}\) and \(Fp_{2}\) for alcoholic and non-alcoholic control group are depicted in Fig. 2. It is evident from Fig. 2 that phase wrapping reduces the noise as a result of the removal of the trend, present in the EEG data, thus providing better discrimination between two classes: alcoholic and non-alcoholic.
3.4 Experimental Results
Multiscale Fuzzy Sample Entropy Analysis of DPS. The MFSampEn analysis of the surrogate data for the DPS with the phase components randomized and the original DPS profile for both the groups show that there is an overall increasing value of MFSampEn with respect to time scale. Furthermore, the FSampEn values of the surrogate is higher over all time scales, as shown in Fig. 3(a). Hence, we can conclude that nonlinear deterministic processes dictate DPS in brain network dynamics. Additionally, the group comparison indicated elevated FSampEn values of DPS for the alcoholic group across all scales in comparison to its control counterpart.
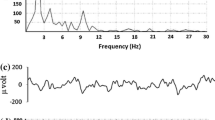
Power Spectral Density Analysis. After observing for each electrode, it is observed that the power spectral density of the control group is greater than the power spectral density of the alcoholic group. Figure 3(b) illustrates the variation of PSD for the alcoholic and non-alcoholic groups for Fp\(_{1}\).
Classifier Performance. In this section, we attempt to judge the feature extraction ability of Multiscale Fuzzy Sample Entropy (MFSampEn) [23] as features from alcoholic and non-alcoholic groups. Here, we have employed Support Vector Machine (SVM) [21] to compare the classification performances of MFSampEn with other entropy-based features, eg., FSampEn, MSampEn, SampEn [18], and ApEn [16]. The classification performances obtained are listed in Table 1.
4 Conclusion
This paper examines the complexity and power analysis of alcoholic and non-alcoholic subjects in the context of visual object recognition task. The complexity metric employed in this study is multiscale FSampEn (MFSampEn) whose merits lie in the fact that it gives more accurate entropy definition than MSampEn and ApEn. MFSampEn has more consistency and has less dependence on data. Experimental results indicate that the complexity of alcoholic subjects is more than those of non-alcoholic subjects. When both the groups are compared, it has been observed that the power spectral density of the alcoholic group is less than its non-alcoholic counterpart.
References
Azami, H., Fernández, A., Escudero, J.: Refined multiscale fuzzy entropy based on standard deviation for biomedical signal analysis. Med. Biol. Eng. Comput. 55(11), 2037–2052 (2017)
Balli, T., Palaniappan, R.: On the complexity and energy analyses in EEG between alcoholic and control subjects during delayed matching to sample paradigm. Int. J. Comput. Intell. Appl. 7(03), 301–315 (2008)
Brust, J.: Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public Health 7(4), 1540–1557 (2010)
Cohen, M.X.: Fluctuations in oscillation frequency control spike timing and coordinate neural networks. J. Neurosci. 34(27), 8988–8998 (2014)
Comon, P.: Independent component analysis, a new concept? Sig. Process. 36(3), 287–314 (1994)
Costa, M., Goldberger, A.L., Peng, C.K.: Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 89(6), 068102 (2002)
Costa, M., Goldberger, A.L., Peng, C.K.: Multiscale entropy analysis of biological signals. Phys. Rev. E 71(2), 021906 (2005)
Ghosh, L., Konar, A., Rakshit, P., Nagar, A.K.: Hemodynamic analysis for cognitive load assessment and classification in motor learning tasks using type-2 fuzzy sets. IEEE Trans. Emerg. Top. Comput. Intell. 3(3), 245–260 (2018)
Ghosh, L., Konar, A., Rakshit, P., Ralescu, A.L., Nagar, A.K.: EEG induced working memory performance analysis using inverse fuzzy relational approach. In: 2017 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE), pp. 1–6. IEEE (2017)
Harris, F.J.: On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE 66(1), 51–83 (1978)
Herting, M.M., Fair, D., Nagel, B.J.: Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage 54(4), 2582–2589 (2011)
Humeau-Heurtier, A.: The multiscale entropy algorithm and its variants: a review. Entropy 17(5), 3110–3123 (2015)
Ingber, L.: Statistical mechanics of neocortical interactions: canonical momenta indicators of electroencephalography. Phys. Rev. E 55(4), 4578 (1997)
Ingber, L.: Statistical mechanics of neocortical interactions: training and testing canonical momenta indicators of EEG. Math. Comput. Model. 27(3), 33–64 (1998)
Nobukawa, S., Kikuchi, M., Takahashi, T.: Changes in functional connectivity dynamics with aging: a dynamical phase synchronization approach. NeuroImage 188, 357–368 (2019)
Pincus, S.M., Gladstone, I.M., Ehrenkranz, R.A.: A regularity statistic for medical data analysis. J. Clin. Monit. 7(4), 335–345 (1991)
Porjesz, B., Begleiter, H.: Alcoholism and human electrophysiology. Alcohol Res. Health 27(2), 153–160 (2003)
Richman, J.S., Moorman, J.R.: Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 278(6), H2039–H2049 (2000)
Schreiber, T., Schmitz, A.: Improved surrogate data for nonlinearity tests. Phys. Rev. Lett. 77(4), 635 (1996)
Snodgrass, J.G., Vanderwart, M.: A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. Mem. 6(2), 174 (1980)
Subasi, A., Gursoy, M.I.: EEG signal classification using PCA, ICA, LDA and support vector machines. Expert Syst. Appl. 37(12), 8659–8666 (2010)
Theiler, J., Eubank, S., Longtin, A., Galdrikian, B., Farmer, J.D.: Testing for nonlinearity in time series: the method of surrogate data. Phys. D 58(1–4), 77–94 (1992)
Xie, H.B., Chen, W.T., He, W.X., Liu, H.: Complexity analysis of the biomedical signal using fuzzy entropy measurement. Appl. Soft Comput. 11(2), 2871–2879 (2011)
Zhang, X.L., Begleiter, H., Porjesz, B., Wang, W., Litke, A.: Event related potentials during object recognition tasks. Brain Res. Bull. 38(6), 531–538 (1995)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Chowdhury, A., Chakraborty, B., Ghosh, L., Dewan, D., Konar, A. (2019). A Dynamical Phase Synchronization Based Approach to Study the Effects of Long-Term Alcoholism on Functional Connectivity Dynamics. In: Deka, B., Maji, P., Mitra, S., Bhattacharyya, D., Bora, P., Pal, S. (eds) Pattern Recognition and Machine Intelligence. PReMI 2019. Lecture Notes in Computer Science(), vol 11942. Springer, Cham. https://doi.org/10.1007/978-3-030-34872-4_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-34872-4_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34871-7
Online ISBN: 978-3-030-34872-4
eBook Packages: Computer ScienceComputer Science (R0)