Abstract
A delay in the diagnosis of acute compartment syndrome (ACS) is associated with a potentially devastating outcome for the patient. A key risk factor for ACS is youth, with more than two-thirds of cases associated with an underlying fracture. Pain is noted as the index or key sign associated with ACS. However, clinical assessment alone has been documented to have poor diagnostic performance characteristics, with the sensitivity quoted in the literature 13–54%. Although combining symptoms and signs can improve the sensitivity, this will often mean irreversible disability has developed e.g. paralysis. Intracompartmental pressure monitoring of at risk patients has recently been found to have a high sensitivity (94%) and specificity (98%) when utilising a slit catheter technique and a differential pressure threshold of 30 mmHg for more than two hours.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Background to the Problem
-
It is well established that the expedient diagnosis of acute compartment syndrome (ACS), followed by urgent fasciotomy and decompression, provides the best outcome for the patient by avoiding irreversible tissue ischaemia and necrosis [1,2,3,4].
-
Delay in the diagnosis of ACS can lead to potentially catastrophic outcomes for the patient [5,6,7,8,9], as well as being associated with high medical costs [10] and medicolegal indemnity cases [11, 12]. Complications include infection, muscle necrosis/contractures, nerve injury, chronic pain, fracture non-union and even amputation.
-
Factors associated with a delay/failure of diagnosis are inadequate experience of medical personnel, regional or general anaesthesia (GA), polytrauma patients, soft tissue injuries as well as the use of clinical signs alone when making the diagnosis [4, 13,14,15,16,17,18,19,20].
-
There is currently no universally agreed reference standard for the diagnosis of ACS, and the prevalence documented in literature is below 30%, meaning the diagnostic performance characteristics of any test is by definition limited [21,22,23].
-
The use of intra-compartmental pressure (ICP) monitoring continues to be debated, with one study using it as the primary diagnostic tool in only 11.7% of 386 tibial shaft fractures [23], whilst a recent survey of US trauma surgeons reported that clinical assessment should be utilised in the awake patient, with monitoring recommended in the obtunded or unconscious patient [24]
What Is Recommended?
Which Patients Should Be Monitored?
The incidence of acute compartment syndrome (ACS) is documented to be 3.1 per 100,000 population/year [21]. Males are more frequently affected than females (10:1) [21, 25], and the mean age is quoted at just over 30 years, with males younger than females [21, 26, 27]. Table 5.1 details those patients in whom compartment pressure monitoring is recommended. These can be considered risk factors and/or high-risk patients for the development of ACS, as well as factors known to be associated with a delayed diagnosis of ACS [4, 13,14,15,16,17,18,19,20].
Youth is the principal risk factor for developing ACS, with the highest prevalence documented to be in the second and third decades [29]. One proposed explanation for this is that young patients have a higher muscle bulk and thus a limited capacity for swelling in a fixed compartment. Sarcopenia and an associated increased perfusion pressure due to hypertension can also possibly explain the protective effects of ageing. The important caveat for youth as a risk factor are cases of ACS secondary to soft tissue injuries, which make up almost a quarter of all cases [1, 30, 31]. For these cases, it is noted that the mean age is significantly older than those who develop ACS following a fracture [32]. Soft tissue causes of ACS include crush injuries, crush syndrome, drug overdose and anticoagulant medications [16, 21, 27, 33,34,35,36,37,38,39,40].
Tibial diaphyseal fractures account for a third of all ACS cases [21]. Despite some previous literature suggesting that intramedullary nailing was associated with the development of ACS [7, 41,42,43,44,45], other studies have found this not to be the case [45, 46], and more recently, youth, males and diaphyseal fractures are noted to be the key risk factors [4, 22]. Recent literature has reported an increased risk of ACS following tibial plateau fractures [47], particularly the more complex higher-energy Schatzker VI types [47, 48]. Forearm diaphyseal fractures and fractures of the distal radius, particularly high-energy, are also associated with ACS.
The current literature suggests a high rate of ACS following closed low-energy rather than open high-energy fractures of the tibial shaft [21, 49,50,51]. The reason for this could be due to the theory of ‘auto-decompression’ of the fascial boundaries at the time of injury. However, there is data to certainly support an increased rate of ACS following high-energy forearm and femoral fractures [21, 25, 38, 52]. One study has reported a lower limb ACS rate of 20% in critically injured patients, with increased lactate levels and base deficit, as well as a transfusion need associated with the diagnosis [53].
What Are the Techniques Available?
The advantages and disadvantages of the various invasive monitoring techniques available are found in Table 5.2. The needle manometer [54,55,56] was an early method of pressure monitoring and is a simple and cheap technique, but there are noted problems with the tip blocking and major concerns associated with the large volume of fluid infused, which could induce or exacerbate compartment syndrome. The wick catheter was a modification of this [57, 58] and provides a large surface area for pressure measurement, whilst also reducing the blocking risk. However, false low measurements have been noted if a blockage (e.g. blood clot or air bubble) does occur.
The slit catheter is like the wick catheter [59,60,61] and is the technique we use in our centre [62]. Again, a large surface area is available for measurement via an axial cut at the catheter end [59]. Patency can be assessed when the catheter is in place by applying light pressure to the compartment, which should give an immediate transient elevation in the pressure reading. The data suggests that the slit catheter is superior to the needle manometer method [60] and comparable to the wick catheter [61].
A solid-state transducer intra-compartmental catheter (STIC) can also be used to measure compartment pressures [63,64,65]. This method employs a pressure transducer within the catheter lumen. Good correlations with conventional techniques have been reported [64]; however, this method is expensive/labour intensive, and less modern designs can require an infusion to maintain patency [65]. There is also the Stryker ICP™ monitor (Stryker, Kalamazoo, MI), which is commonly used in North America for compartment pressure monitoring. The accuracy of this monitor has been shown to be limited as regards inter-observer variability [66].
Where Should the Catheter Be Placed?
The recommended catheter placement location for the upper and lower limb sites at risk of ACS is found in Table 5.3. Accurate catheter placement within the affected compartment is carried out using a strict aseptic technique [67]. In the presence of a fracture, the literature would suggest that the catheter tip should be placed within 5 cm of the level of the fracture, as this will give the peak measure reading within the compartment [4, 68,69,70]. Others advocate this results in a false high reading due to the fracture haematoma [71]. It is essential that the transducer is secured at the level of the compartment as the readings will to change with the height relative to the compartment.
Current data would suggest the lower leg anterior compartment should be used as it is the most commonly involved compartment and is easily accessible [51, 72]. However, some authors advocate concomitant monitoring of the deep posterior compartment due to the possibility of missing an isolated deep ACS. It should be noted that this is often uncomfortable and cumbersome for the patient [5, 68].
What Is the Pressure Threshold for Decompression?
There has been much debate when using compartment pressure monitoring regarding the pressure threshold for diagnosing ACS and proceeding to fasciotomy. Should we use the absolute compartment pressure in isolation? Is the differential pressure or perfusion pressure (∆P) the best thing to use?
Early data suggested using an absolute ICP threshold of 30–40 mmHg [30, 50, 54, 58, 73,74,75]. However, it was subsequently noted that a patient’s tolerance for an absolute pressure reading does vary widely and was intrinsically linked with the systemic blood pressure or perfusion pressure [51, 69, 76,77,78]. Whitesides et al. documented the use of the differential pressure (∆P), calculated as diastolic pressure – intra-compartmental pressure [76]. Following on from this, data then proposed a differential pressure of 10–35 mmHg as diagnostic [69, 78, 79]. However, it has been noted that the differential pressure will possibly be increased in traumatised or ischaemic muscle.
There is now clinical and experimental data supporting a differential pressure of ≤30 mmHg as diagnostic for ACS requiring fasciotomy [6, 51, 67, 80]. In a study from our centre, there were 116 patients with an acute fracture of the tibial shaft [51] that underwent immediate continuous pressure monitoring of the anterior compartment for a minimum of 24 hours. The authors used a differential pressure of ≤30 mmHg for more than 2 hours as diagnostic, with 3 patients requiring fasciotomy. No unnecessary fasciotomies were noted, and there were no missed cases of ACS and no related sequelae at a final mean follow-up of just over a year [51].
This protocol was subsequently validated in our centre by White et al. in a study of 101 tibial diaphyseal fractures. In this series, 41 patients had an absolute pressure reading of greater than 30 mmHg for more than 6 hours continuously, but with a normal differential pressure of >30 mmHg. These patients were compared with 60 patients who all had an absolute reading of less than 30 mmHg throughout. In the year following intervention, no significant difference in isometric muscle analysis or in return to function was found between these two groups [67].
Janzing et al. assessed a monitoring protocol in a prospective study of 95 patients with a tibial shaft fracture that underwent continuous pressure monitoring [81]. There was a 14.4% fasciotomy rate reported in the series. The authors found that the optimal combined sensitivity and specificity was clinical symptoms and differential pressure of <30 mmHg (61%, 97%), with a differential pressure of ≤30 mmHg performing best when using monitoring alone (89%, 65%). The authors suggested that an increased fasciotomy rate could occur with continuous pressure monitoring, but this study does not completely consider the trend of the differential pressure over time.
Is Continuous Monitoring Important?
Time to fasciotomy is established to be a key factor in predicting patient outcome [5,6,7,8,9]. All the available data clearly determines that timing is of critical importance in the development of muscle damage [73, 75, 82, 83]. However, it is also necessary to contemplate the trend over time for compartment pressure monitoring in order to confirm the diagnosis of ACS and determine the need to proceed to fasciotomy, with the exception of severe or extreme cases that obviously need to proceed to theatre immediately. The current data suggests that if a single pressure reading is used, then this will most probably result in an increased rate of unnecessary fasciotomies (overtreatment). One study reported a false-positive rate of 35% if a one-off differential pressure reading of ≤30 mmHg was used as diagnostic and if the trend over time was not considered [84].
Kakar et al. reported a prospective study of 242 tibial shaft fractures treated with intramedullary nailing under general anaesthesia (GA) [85]. They found that although the preoperative diastolic blood pressure was related to the post-operative pressure, a significant difference was found with the intraoperative pressure. This work emphasises the need to use serial continuous measurements and that intraoperative and immediate post-operative readings should be used with caution. This is certainly the experience in our centre too.
The protocol we use in our centre is well documented in the literature, and when employing a differential pressure of ≤30 mmHg over a 2 hour period as diagnostic [62], we have reported a reduction in the time to fasciotomy and complication rate, whilst not significantly increasing the rate of fasciotomies [51]. We would suggest that if the differential pressure is below 30 mmHg, but the absolute pressure is decreasing (and thus the differential pressure is increasing), then it is most likely safe to closely observe the patient in the expectation of the differential pressure returning to safe levels within a short period of time.
How Do Clinical Signs Compare with Pressure Monitoring?
To determine whether pressure measurement is a good surrogate for ischaemia, it is important to consider what the alternatives are, namely, clinical assessment. The clinical symptoms and signs associated with the development of ACS are swelling, pain on passive stretch, pain out of proportion to the injury, paraesthesia and paresis/paralysis. The diagnostic performance characteristics of these symptoms and signs are found in Table 5.4.
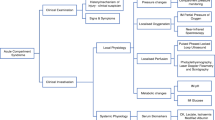
Swelling is almost a universally seen sign with all the causes of ACS and is very subjective. Despite pain being an important early symptom of ACS in the awake and alert patient [15], it is common after most injuries, is very subjective/patient dependent and is not universally present in all cases of ACS [88]. Pain assessment is also not possible when regional anaesthesia has been used or in the unconscious patient [13, 14, 18]. Pain has a low sensitivity and a large false-negative/missed cases rate reported in the literature [5, 6, 15, 33, 89]. Paraesthesia or reduced sensation is now established as a late sign of ACS [8] with a very low sensitivity and a rate of false negatives [15]. This rate of false negatives excludes paraesthesia as an accurate diagnostic indicator. Paralysis of the muscles within compartment is also a very late sign of ACS and is indicative of irreversible damage to the soft tissues within the compartment. It is associated with a poor outcome [30, 31, 38, 49, 90, 91] and has the worst combined sensitivity and specificity in the literature [15]. Vascular assessment is not an early clinical sign of ACS, with absent peripheral pulses, pallor and reduced capillary refill time all associated with either an acute vascular injury that needs an urgent angiogram/intervention or possibly an established ACS where an amputation is very possible [4]. Importantly, it is also not possible to rule out ACS due to strong distal pulses.
Some studies have tried to directly compare the use of clinical assessment alone with compartment pressure monitoring. In a study from our centre, we reported on 25 patients with a tibial shaft fracture that developed ACS [6]. There were 13 patients who underwent compartment pressure monitoring and 12 patients who had clinical assessment alone. There was a significant delayed time from presentation to fasciotomy for the non-monitored group (16 hour difference; p < 0.05), with also a significantly increased rate of late sequelae (91% vs. 0%; p < 0.01) and delay to union (8 week delay; p < 0.05) [6].
A further study reported on 218 patients that included 109 consecutive tibial shaft fractures that had continuous compartment pressure monitoring and retrospectively compared them with 109 control patients that underwent clinical assessment only [72]. The authors reported comparable rates of fasciotomy (15.6% vs. 14.7%). However, there was no significant difference in either patient outcome or time to fasciotomy [72]. One potential criticism of this study is that the control group had clinical examination performed hourly, which could be argued to be inconsistent with routine clinical practice.
Harris et al. are the only authors, to our knowledge, to have carried out a prospective randomised trial [71]. Their study included 200 consecutive tibial shaft fractures and randomised patients to clinical assessment alone (n = 100) or compartment pressure monitoring (n = 100). All five cases of ACS in the study were in the clinical assessment group. The authors chose a primary outcome of late ACS sequelae at the six-month assessment. Complications that were reported included sensory loss, muscle weakness, contractures and toe clawing, and fracture non-union. There was no significant difference in overall complication rates found between groups (27% vs. 29%). A potential criticism of this study was that the indication for fasciotomy was clinical assessment, with monitoring only employed at the discretion of the treating surgeon [71].
Diagnostic Performance Characteristics (Table 5.4)
The diagnostic performance characteristics of continuous invasive compartment pressure monitoring and those of clinical symptoms/signs are found in Table 5.4. Our centre has reported on a series of 850 adult patients with an acute tibial shaft fracture using a slit catheter technique in the anterior compartment of the leg and a diagnostic pressure threshold differential (ΔP) of less than 30 mmHg for more than 2 hours as indication for fasciotomy [87]. We reported high diagnostic performance characteristics, with 11 false-positive cases and 9 false-negative cases. In order to attain comparable characteristics to these, Ulmer et al. found in their systematic review of clinical assessment that three clinical signs are needed, with the third being paralysis – a sign associated with irreversible damage to the muscle [15]. Symptoms and signs in isolation were also found to perform poorly and are known to be better at ruling out rather than confirming the diagnosis (Table 5.4).
Limitations and Pitfalls
ACS continues to be a catastrophic complication and is associated with significant patient morbidity and high litigation costs [11, 92]. A review from Canada over a 10-year period reported that 77% of plaintiffs had permanent disability and 55% of cases had a judgement for the plaintiff or an unfavourable decision for the physician, with the primary clinical issue a delay or failure to diagnose ACS [92]. Despite all this evidence highlighting the issues with a delay in the diagnosis, there remains an extraordinary lack of consistency in the clinical assessment of the condition [93, 94].
A key limitation of the literature on ACS is how we define the time of onset of acute compartment syndrome (e.g. when the diagnosis was made), as well as the time to fasciotomy. In the acute trauma clinical setting, authors have suggested that the time to fasciotomy is best determined as the point from admission as this is the most likely easily definable moment in the patient journey [4, 32, 51]. The obvious exception to this is crush syndrome, as the nature of the diagnosis is associated with a prolonged period of compression that makes it almost impossible to determine the exact time of onset.
The current data is also deficient in good quality prospective mid-term and long-term outcome data on the efficacy of compartment pressure monitoring, as well as the outcome of fasciotomy and ACS. There is also very little literature reporting on the various diagnostic performance characteristics for the pressure measurement techniques available, nor for the diagnostic protocols associated with these. Much of the data in the literature relates to adults and the lower leg. More data is needed on ACS in adolescent patients, as well as for other areas of the body. This would potentially then allow us to establish the indications, thresholds and protocols for using pressure monitoring in these patient groups. In children, given the normally lower diastolic pressure in this patient group, the mean arterial pressure (MAP) might be a preferred option when calculating the differential pressure [95].
Finally, one of the key problems with the current literature on the diagnosis of ACS is the absence of an agreed gold-standard reference. Given the incidence is known to be below 30% [21,22,23], routine statistical methods are not likely rigorous enough. Alternative methods such as latent class analysis and Bayes theorem are required to accurately calculate the diagnostic performance characteristics of the various methods used.
Future Directions
Given the superior published diagnostic performance characteristics of continuous pressure monitoring when compared to clinical symptoms and signs, a clinical diagnosis alone of ACS we feel should not be the gold standard. Continuous pressure monitoring is of benefit in all patients at risk of developing ACS, and universally clear and accepted clinical guidelines are needed to allow the early diagnosis in all units managing acute trauma patients. This would, most probably, result in the single biggest advance in the management of the condition. Clearly, the ultimate goal would be a sufficiently powered large multicentre prospective randomised controlled trial of the clinical signs of ACS versus continuous pressure monitoring. However, the ‘Hawthorn effect’ comes into play here due to the probability of modifying what is normal day-to-day clinical practice, due to the predictable improvement in the frequency and rigour of the clinical assessment for such a trial.
The role of non-invasive compartment pressure measurements and those measuring blood flow continue to be investigated in the literature [96]. The potential advantages are without question, but the utilisation of these techniques is thus far not been sufficiently validated in the literature. Near-infrared spectroscopy utilises a probe placed on the skin to determine the degree of oxygenated haemoglobin in the muscle tissues [97,98,99,100]. It has been shown to correlate well with tissue pressures from experimental data [97], as well as in healthy human volunteers [98]. The role of ultrasound scanning to detect waveforms associated with displacement of the fascia by the arterial pulse continues to be unclear. There has been investigations trying to correlate compartment pressure readings of greater than 30 mmHg with fascial displacement in healthy volunteers, with the reported sensitivity 77% and specificity 93% [101]. The clear limitation of this technique is the likely reduction in sensitivity for the hypotensive patient.
Methods to prevent or reduce the effects of ACS are also potential areas for future work. Research has already started on methods to reduce the compartment pressure with the administration of intravenously hypertonic fluids [102], but these have never been successful clinically. Nevertheless, an experiment on human subjects using tissue ultrafiltration to remove fluid from the compartment has been shown to reduce compartment pressure [103, 104]. Whether this technique can be useful clinically remains to be seen. There is also work on the potential role of antioxidants on the outcome of ACS with some promising findings reported [105], with extension into human studies the next step.
Take-Home Message
-
Pain is documented as the index sign associated with the development of acute compartment syndrome. However, clinical symptoms and signs in isolation are reported to have inadequate diagnostic performance characteristics, with the sensitivity ranging from 13% to 54% for each in the literature.
-
Continuous invasive intra-compartmental pressure monitoring has been reported to have superior diagnostic performance characteristics with a high estimated sensitivity (94%) and specificity (98%) for the diagnosis of ACS when using a slit catheter technique and a differential pressure threshold of 30 mmHg for more than 2 hours.
-
Continuous pressure monitoring should be utilised as a diagnostic adjunct in all patients at risk of developing ACS, with youth the key risk factor and tibial diaphyseal fractures the most common precipitating injury identified in the literature.
-
Patients and surgeons need to acknowledge that when using compartment pressure monitoring for diagnosing ACS, the risk should inevitably lean towards an unnecessary fasciotomy (false positive) rather than a missed ACS (false negative).
-
Future non-invasive techniques of calculating tissue perfusion via blood flow or pH remain areas of future research, along with interventions that can potentially reduce the effects of ACS.
References
Gelberman RH, Zakaib GS, Mubarak SJ, Hargens AR, Akeson WH. Decompression of forearm compartment syndromes. Clin Orthop Relat Res. 1978;134:225–9.
Holden CE. The pathology and prevention of Volkmann’s ischaemic contracture. J Bone Joint Surg Br. 1979;61-B(3):296–300.
Finkelstein JA, Hunter GA, Hu RW. Lower limb compartment syndrome: course after delayed fasciotomy. J Trauma. 1996;40(3):342–4.
McQueen MM. Acute compartment syndrome. In: Bucholz RW, Court-Brown CM, Heckman JD, Tornetta III P, editors. Rockwood and Green’s fractures in adults. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 689–708.
Matsen FA III, Clawson DK. The deep posterior compartmental syndrome of the leg. J Bone Joint Surg Am. 1975;57(1):34–9.
McQueen MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78(1):95–8.
Mullett H, Al-Abed K, Prasad CV, O’Sullivan M. Outcome of compartment syndrome following intramedullary nailing of tibial diaphyseal fractures. Injury. 2001;32(5):411–3.
Rorabeck CH, Macnab L. Anterior tibial-compartment syndrome complicating fractures of the shaft of the tibia. J Bone Joint Surg Am. 1976;58(4):549–50.
Sheridan GW, Matsen FA III. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112–5.
Schmidt AH. The impact of compartment syndrome on hospital length of stay and charges among adult patients admitted with a fracture of the tibia. J Orthop Trauma. 2011;25(6):355–7.
Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86-A(4):864–8.
Matsen FA III, Stephens L, Jette JL, Warme WJ, Posner KL. Lessons regarding the safety of orthopaedic patient care: an analysis of four hundred and sixty-four closed malpractice claims. J Bone Joint Surg Am. 2013;95(4):e201–8.
Mubarak SJ, Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282–4.
Harrington P, Bunola J, Jennings AJ, Bush DJ, Smith RM. Acute compartment syndrome masked by intravenous morphine from a patient-controlled analgesia pump. Injury. 2000;31(5):387–9.
Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma. 2002;16(8):572–7.
Mithofer K, Lhowe DW, Vrahas MS, Altman DT, Altman GT. Clinical spectrum of acute compartment syndrome of the thigh and its relation to associated injuries. Clin Orthop Relat Res. 2004;425:223–9.
Richards H, Langston A, Kulkarni R, Downes EM. Does patient controlled analgesia delay the diagnosis of compartment syndrome following intramedullary nailing of the tibia? Injury. 2004;35(3):296–8.
Davis ET, Harris A, Keene D, Porter K, Manji M. The use of regional anaesthesia in patients at risk of acute compartment syndrome. Injury. 2006;37(2):128–33.
Mar GJ, Barrington MJ, McGuirk BR. Acute compartment syndrome of the lower limb and the effect of postoperative analgesia on diagnosis. Br J Anaesth. 2009;102(1):3–11.
Roberts CS, Gorczyca JT, Ring D, Pugh KJ. Diagnosis and treatment of less common compartment syndromes of the upper and lower extremities: current evidence and best practices. Instr Course Lect. 2011;60:43–50.
McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82(2):200–3.
Park S, Ahn J, Gee AO, Kuntz AF, Esterhai JL. Compartment syndrome in tibial fractures. J Orthop Trauma. 2009;23(7):514–8.
O’Toole RV, Whitney A, Merchant N, Hui E, Higgins J, Kim TT, et al. Variation in diagnosis of compartment syndrome by surgeons treating tibial shaft fractures. J Trauma. 2009;67(4):735–41.
Collinge CA, Attum B, Lebus GF, Tornetta P III, Obremskey W, Ahn J, et al. Acute compartment syndrome: an expert survey of orthopaedic trauma association members. J Orthop Trauma. 2018;32(5):e181–4.
Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36(3):535–43.
Simpson NS, Jupiter JB. Delayed onset of forearm compartment syndrome: a complication of distal radius fracture in young adults. J Orthop Trauma. 1995;9(5):411–8.
Morin RJ, Swan KG, Tan V. Acute forearm compartment syndrome secondary to local arterial injury after penetrating trauma. J Trauma. 2009;66(4):989–93.
Duckworth AD, McQueen MM. The diagnosis of acute compartment syndrome: a critical analysis review. JBJS Rev. 2017;5(12):e1.
McQueen MM, Duckworth AD, Aitken SA, Sharma RA, Court-Brown CM. Predictors of compartment syndrome after Tibial fracture. J Orthop Trauma. 2015;29(10):451–5.
Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93–7.
Bradley EL III. The anterior tibial compartment syndrome. Surg Gynecol Obstet. 1973;136(2):289–97.
Hope MJ, McQueen MM. Acute compartment syndrome in the absence of fracture. J Orthop Trauma. 2004;18(4):220–4.
Eaton RG, Green WT. Volkmann’s ischemia. A volar compartment syndrome of the forearm. Clin Orthop Relat Res. 1975;113:58–64.
Mubarak S, Owen CA. Compartmental syndrome and its relation to the crush syndrome: a spectrum of disease. A review of 11 cases of prolonged limb compression. Clin Orthop Relat Res. 1975;113:81–9.
Reis ND, Michaelson M. Crush injury to the lower limbs. Treatment of the local injury. J Bone Joint Surg Am. 1986;68(3):414–8.
Gelberman RH, Garfin SR, Hergenroeder PT, Mubarak SJ, Menon J. Compartment syndromes of the forearm: diagnosis and treatment. Clin Orthop Relat Res. 1981;161:252–61.
Geary N. Late surgical decompression for compartment syndrome of the forearm. J Bone Joint Surg Br. 1984;66(5):745–8.
Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392–400.
Mithofer K, Lhowe DW, Altman GT. Delayed presentation of acute compartment syndrome after contusion of the thigh. J Orthop Trauma. 2002;16(6):436–8.
Frink M, Hildebrand F, Krettek C, Brand J, Hankemeier S. Compartment syndrome of the lower leg and foot. Clin Orthop Relat Res. 2010;468(4):940–50.
Tischenko GJ, Goodman SB. Compartment syndrome after intramedullary nailing of the tibia. J Bone Joint Surg Am. 1990;72(1):41–4.
Moed BR, Strom DE. Compartment syndrome after closed intramedullary nailing of the tibia: a canine model and report of two cases. J Orthop Trauma. 1991;5(1):71–7.
Koval KJ, Clapper MF, Brumback RJ, Ellison PS Jr, Poka A, Bathon GH, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184–9.
Williams J, Gibbons M, Trundle H, Murray D, Worlock P. Complications of nailing in closed tibial fractures. J Orthop Trauma. 1995;9(6):476–81.
Tornetta P III, French BG. Compartment pressures during nonreamed tibial nailing without traction. J Orthop Trauma. 1997;11(1):24–7.
McQueen MM, Christie J, Court-Brown CM. Compartment pressures after intramedullary nailing of the tibia. J Bone Joint Surg Br. 1990;72(3):395–7.
Allmon C, Greenwell P, Paryavi E, Dubina A, O’Toole RV. Radiographic predictors of compartment syndrome occurring after Tibial fracture. J Orthop Trauma. 2016;30(7):387–91.
Ziran BH, Becher SJ. Radiographic predictors of compartment syndrome in tibial plateau fractures. J Orthop Trauma. 2013;27(11):612–5.
DeLee JC, Stiehl JB. Open tibia fracture with compartment syndrome. Clin Orthop Relat Res. 1981;(160):175–84.
Blick SS, Brumback RJ, Poka A, Burgess AR, Ebraheim NA. Compartment syndrome in open tibial fractures. J Bone Joint Surg Am. 1986;68(9):1348–53.
McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99–104.
Prasarn ML, Ouellette EA. Acute compartment syndrome of the upper extremity. J Am Acad Orthop Surg. 2011;19(1):49–58.
Kosir R, Moore FA, Selby JH, Cocanour CS, Kozar RA, Gonzalez EA, et al. Acute lower extremity compartment syndrome (ALECS) screening protocol in critically ill trauma patients. J Trauma. 2007;63(2):268–75.
Matsen FA III, Mayo KA, Sheridan GW, Krugmire RB Jr. Monitoring of intramuscular pressure. Surgery. 1976;79(6):702–9.
Matsen FA III, Winquist RA, Krugmire RB Jr. Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am. 1980;62(2):286–91.
Whitesides TE Jr, Haney TC, Harada H, Holmes HE, Morimoto K. A simple method for tissue pressure determination. Arch Surg. 1975;110(11):1311–3.
Mubarak SJ, Hargens AR, Owen CA, Garetto LP, Akeson WH. The wick catheter technique for measurement of intramuscular pressure. A new research and clinical tool. J Bone Joint Surg Am. 1976;58(7):1016–20.
Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH. Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60(8):1091–5.
Rorabeck CH, Castle GS, Hardie R, Logan J. Compartmental pressure measurements: an experimental investigation using the slit catheter. J Trauma. 1981;21(6):446–9.
Moed BR, Thorderson PK. Measurement of intracompartmental pressure: a comparison of the slit catheter, side-ported needle, and simple needle. J Bone Joint Surg Am. 1993;75(2):231–5.
Shakespeare DT, Henderson NJ, Clough G. The slit catheter: a comparison with the wick catheter in the measurement of compartment pressure. Injury. 1982;13(5):404–8.
Duckworth AD, McQueen MM. Continuous intracompartmental pressure monitoring for acute compartment syndrome. JBJS Essent Surg Tech. 2014;3(3):e13.
McDermott AG, Marble AE, Yabsley RH, Phillips MB. Monitoring dynamic anterior compartment pressures during exercise. A new technique using the STIC catheter. Am J Sports Med. 1982;10(2):83–9.
McDermott AG, Marble AE, Yabsley RH. Monitoring acute compartment pressures with the S.T.I.C. catheter. Clin Orthop Relat Res. 1984;190:192–8.
Willy C, Gerngross H, Sterk J. Measurement of intracompartmental pressure with use of a new electronic transducer-tipped catheter system. J Bone Joint Surg Am. 1999;81(2):158–68.
Large TM, Agel J, Holtzman DJ, Benirschke SK, Krieg JC. Interobserver variability in the measurement of lower leg compartment pressures. J Orthop Trauma. 2015;29(7):316–21.
White TO, Howell GE, Will EM, Court-Brown CM, McQueen MM. Elevated intramuscular compartment pressures do not influence outcome after tibial fracture. J Trauma. 2003;55(6):1133–8.
Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285–92.
Matava MJ, Whitesides TE Jr, Seiler JG III, Hewan-Lowe K, Hutton WC. Determination of the compartment pressure threshold of muscle ischemia in a canine model. J Trauma. 1994;37(1):50–8.
Saikia KC, Bhattacharya TD, Agarwala V. Anterior compartment pressure measurement in closed fractures of leg. Indian J Orthop. 2008;42(2):217–21.
Harris IA, Kadir A, Donald G. Continuous compartment pressure monitoring for tibia fractures: does it influence outcome? J Trauma. 2006;60(6):1330–5.
Al-Dadah OQ, Darrah C, Cooper A, Donell ST, Patel AD. Continuous compartment pressure monitoring vs. clinical monitoring in tibial diaphyseal fractures. Injury. 2008;39(10):1204–9.
Hargens AR, Akeson WH, Mubarak SJ, Owen CA, Evans KL, Garetto LP, et al. Fluid balance within the canine anterolateral compartment and its relationship to compartment syndromes. J Bone Joint Surg Am. 1978;60(4):499–505.
Halpern AA, Greene R, Nichols T, Burton DS. Compartment syndrome of the interosseous muscles: early recognition and treatment. Clin Orthop Relat Res. 1979;(140):23–5.
Allen MJ, Stirling AJ, Crawshaw CV, Barnes MR. Intracompartmental pressure monitoring of leg injuries. An aid to management. J Bone Joint Surg Br. 1985;67(1):53–7.
Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43–51.
Heppenstall RB, Sapega AA, Scott R, Shenton D, Park YS, Maris J, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;226:138–55.
Heckman MM, Whitesides TE Jr, Grewe SR, Judd RL, Miller M, Lawrence JH III. Histologic determination of the ischemic threshold of muscle in the canine compartment syndrome model. J Orthop Trauma. 1993;7(3):199–210.
Brooker AF Jr, Pezeshki C. Tissue pressure to evaluate compartmental syndrome. J Trauma. 1979;19(9):689–91.
Ozkayin N, Aktuglu K. Absolute compartment pressure versus differential pressure for the diagnosis of compartment syndrome in tibial fractures. Int Orthop. 2005;29(6):396–401.
Janzing HM, Broos PL. Routine monitoring of compartment pressure in patients with tibial fractures: beware of overtreatment! Injury. 2001;32(5):415–21.
Hargens AR, Romine JS, Sipe JC, Evans KL, Mubarak SJ, Akeson WH. Peripheral nerve-conduction block by high muscle-compartment pressure. J Bone Joint Surg Am. 1979;61(2):192–200.
Heppenstall RB, Sapega AA, Izant T, Fallon R, Shenton D, Park YS, et al. Compartment syndrome: a quantitative study of high-energy phosphorus compounds using 31P-magnetic resonance spectroscopy. J Trauma. 1989;29(8):1113–9.
Whitney A, O’Toole RV, Hui E, Sciadini MF, Pollak AN, Manson TT, et al. Do one-time intracompartmental pressure measurements have a high false-positive rate in diagnosing compartment syndrome? J Trauma Acute Care Surg. 2014;76(2):479–83.
Kakar S, Firoozabadi R, McKean J, Tornetta P III. Diastolic blood pressure in patients with tibia fractures under anaesthesia: implications for the diagnosis of compartment syndrome. J Orthop Trauma. 2007;21(2):99–103.
Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92(2):361–7.
McQueen MM, Duckworth AD, Aitken SA. Court-Brown CM. The estimated sensitivity and specificity of compartment pressure monitoring for acute compartment syndrome. J Bone Joint Surg Am. 2013;95(8):673–7.
Badhe S, Baiju D, Elliot R, Rowles J, Calthorpe D. The ‘silent’ compartment syndrome. Injury. 2009;40(2):220–2.
Wright JG, Bogoch ER, Hastings DE. The ‘occult’ compartment syndrome. J Trauma. 1989;29(1):133–4.
Duckworth AD, Mitchell SE, Molyneux SG, White TO, Court-Brown CM, McQueen MM. Acute compartment syndrome of the forearm. J Bone Joint Surg Am. 2012;94(10):e63.
Willis RB, Rorabeck CH. Treatment of compartment syndrome in children. Orthop Clin North Am. 1990;21(2):401–12.
Shadgan B, Menon M, Sanders D, Berry G, Martin C Jr, Duffy P, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53(5):329–34.
Wall CJ, Richardson MD, Lowe AJ, Brand C, Lynch J, de Steiger RN. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77(9):733–7.
Williams PR, Russell ID, Mintowt-Czyz WJ. Compartment pressure monitoring--current UK orthopaedic practice. Injury. 1998;29(3):229–32.
Mars M, Hadley GP. Raised compartmental pressure in children: a basis for management. Injury. 1998;29(3):183–5.
Shadgan B, Menon M, O’Brien PJ, Reid WD. Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma. 2008;22(8):581–7.
Arbabi S, Brundage SI, Gentilello LM. Near-infrared spectroscopy: a potential method for continuous, transcutaneous monitoring for compartmental syndrome in critically injured patients. J Trauma. 1999;47(5):829–33.
Gentilello LM, Sanzone A, Wang L, Liu PY, Robinson L. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51(1):1–8, discussion.
Shuler MS, Reisman WM, Cole AL, Whitesides TE Jr, Moore TJ. Near-infrared spectroscopy in acute compartment syndrome: case report. Injury. 2011;11
Shuler MS, Roskosky M, Kinsey T, Glaser D, Reisman W, Ogburn C, et al. Continual near-infrared spectroscopy monitoring in the injured lower limb and acute compartment syndrome. Bone Joint J. 2018;100-B(6):787–97.
Lynch JE, Lynch JK, Cole SL, Carter JA, Hargens AR. Noninvasive monitoring of elevated intramuscular pressure in a model compartment syndrome via quantitative fascial motion. J Orthop Res. 2009;27(4):489–94.
Better OS, Zinman C, Reis DN, Har-Shai Y, Rubinstein I, Abassi Z. Hypertonic mannitol ameliorates intracompartmental tamponade in model compartment syndrome in the dog. Nephron. 1991;58(3):344–6.
Odland R, Schmidt AH, Hunter B, Kidder L, Bechtold JE, Linzie BM, et al. Use of tissue ultrafiltration for treatment of compartment syndrome: a pilot study using porcine hindlimbs. J Orthop Trauma. 2005;19(4):267–75.
Odland RM, Schmidt AH. Compartment syndrome ultrafiltration catheters: report of a clinical pilot study of a novel method for managing patients at risk of compartment syndrome. J Orthop Trauma. 2011;25(6):358–65.
Kearns SR, Daly AF, Sheehan K, Murray P, Kelly C, Bouchier-Hayes D. Oral vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004;86(6):906–11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Duckworth, A.D., Court-Brown, C.M., McQueen, M.M. (2019). Pressure Measurement: Surrogate of Ischaemia. In: Mauffrey, C., Hak, D., Martin III, M. (eds) Compartment Syndrome. Springer, Cham. https://doi.org/10.1007/978-3-030-22331-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-22331-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22330-4
Online ISBN: 978-3-030-22331-1
eBook Packages: MedicineMedicine (R0)