Abstract
The cerebral arteries are innervated by afferent fibers from the trigeminal ganglia. Varicella-zoster virus (VZV) frequently resides in the trigeminal ganglion. Reports of arterial ischemic stroke due to VZV cerebral vasculopathy in adults after herpes zoster have been described for decades. Reports of arterial ischemic stroke due to post-varicella cerebral arteriopathy in children have also been described for decades. One rationale for this review has been post-licensure studies that have shown an apparent protective effect from stroke in both adults who have received live zoster vaccine and children who have received live varicella vaccine. In this review, we define common features between stroke following varicella in children and stroke following herpes zoster in adults. The trigeminal ganglion and to a lesser extent the superior cervical ganglion are central to the stroke pathogenesis pathway because afferent fibers from these two ganglia provide the circuitry by which the virus can travel to the anterior and posterior circulations of the brain. Based on studies in pseudorabies virus (PRV) models, it is likely that VZV is carried to the cerebral arteries on a kinesin motor via gE, gI and the homolog of PRV US9. The gE product is an essential VZV protein.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Varicella-zoster virus
- Herpes simplex virus
- Latency
- Herpes zoster ophthalmicus
- Congenital varicella
- Arteriopathy
- Vasculopathy
- Meningoencephalitis
- Varicella vaccine
- Zoster vaccine
1 Introduction
Reports of arterial ischemic stroke due to a cerebral arteriopathy after herpes zoster (shingles) have been described for at least 7 decades in the English-language medical literature (Cope and Jones 1954). Interest in this association has been reignited by ever more convincing evidence that vaccination with the live attenuated zoster vaccine (Zostavax) reduces the likelihood of acute ischemic stroke in the vaccine recipients. For example, a recent retrospective analysis of more than 1.6 million US Medicare beneficiaries who had received Zostavax documented a lower incidence of stroke than found in a control group (Yang et al. 2021). An earlier smaller study analyzed 265,568 adults (50–79 years of age) and concluded that Zostavax reduced the likelihood of stroke by 50% (Klaric et al. 2019). However, a third study that analyzed 87,405 adults (66 years or older) failed to find a protective effect of Zostavax (Yang et al. 2020). The latter study, however, did document a substantial risk of stroke following herpes zoster. In the pediatric literature, varicella-zoster virus (VZV) has also been linked to arteriopathic stroke in childhood (Elkind et al. 2016). A cerebral arteriopathy identified in pediatric stroke patients within 6 months of chickenpox is called “post-varicella arteriopathy.” Because of the importance of this question about whether stroke occurs after herpes zoster and, therefore, whether vaccination against herpes zoster can prevent stroke, we have reviewed the data about neuropathogenesis of stroke that follows varicella-zoster virus (VZV) infection, including primary infection varicella (chickenpox), and the reactivation from latency, herpes zoster (shingles), (Weller 1983).
After carrying out an extensive literature review, we propose in this review that the pathogenesis of arteriopathic stroke after herpes zoster in an adult parallels that of arteriopathic stroke after varicella in children. In both situations, VZV particles present in neuronal ganglia travel via afferent neuronal branches to cerebral arteries, leading to a focal vasculitis and arterial ischemic stroke. This hypothesis is summarized in Fig. 1. To arrive at this conclusion, we include important experimental data about neurological disease caused by a nonhuman herpesvirus closely related to VZV: pseudorabies virus (PRV), a herpesvirus of swine that can also infect humans in close contact with PRV-infected pigs (He et al. 2019).
2 Varicella-Zoster Virus and the Family of Herpesviruses
There are nine human herpesviruses. All share a common structure: an icosahedral capsid surrounded by a tegument surrounded by an envelope containing the viral glycoproteins (Fig. 2). Three belong to the subfamily of alpha herpesviruses; besides VZV, the other two are herpes simplex virus (HSV) types 1 and 2 (Davison 2010). VZV is more closely related genetically to the porcine PRV than to either HSV1 or HSV2. Like the other herpesviruses, the ancestral VZV is at least 70 million years old and has coevolved over millennia as an infectious agent within primates and simians and finally humans (McGeoch and Davison 1999; Grose 2012; Gray et al. 1992). The double-stranded DNA genome has given the virus an inherent stability not found in a virus with a RNA genome, such as the coronavirus SARS-CoV-2 which appears to have arisen from a bat coronavirus in modern times (Zhou et al. 2020). VZV is the only human herpesvirus where the primary infection in childhood (varicella and chickenpox) and the reactivation from the neuronal site of latency in adulthood (herpes zoster and shingles) have different names for the two medical diseases caused by the same virus.
Electron micrographs of varicella-zoster viral particles. a The electron micrograph illustrates prototypical virions assembled in a skin vesicle. The envelope of each virion is pseudocolored blue, and the capsid is pseudocolored orange. b This electron micrograph illustrates viral particles assembled in cultured cells. In cell culture, a majority of virions are aberrant in appearance. Complete virions are designated with orange arrows and light particles with blue arrows. c Complete virions are viral particles with a capsid containing a DNA genome surrounded by a tegument and an outer envelope. Light particles are envelopes that lack an internal capsid
3 The Pathway from Skin to Ganglia: Neuronal Transport of Wild-Type VZV
Varicella (chickenpox) is characterized by its typical exanthem. The skin vesicles are the final site of virion assembly. Prototypical viral particles are formed in the skin (Fig. 2). Viruses that are released within infected skin tissues subsequently enter the termini of sensory nerves and autonomic nerves and are transported retrograde to ganglia such as the dorsal root ganglia, the trigeminal ganglia and the superior cervical ganglia. The viral genomes are delivered to nuclei within the neuronal cell bodies, where they persist as double-stranded DNA episomes in a latent state. When a viral genome reactivates years later, viral particles are assembled and transported anterograde from the trigeminal ganglia and superior cervical ganglia to multiple destinations. VZ virions have been observed by both immunofluorescence and electron microscopy in a trigeminal ganglion removed from a deceased human patient (Esiri and Tomlinson 1972). However, lack of experimental animal models has limited studies of VZV neuronal transport (Steain et al. 2010). Many more studies have been carried out with PRV in various rodent neuronal models (Card and Enquist 2014).
When a PRV particle enters a neuron by a process of fusion, portions of the outer envelope are lost. In turn, the remaining viral capsid with a few remaining tegument proteins, including UL36, UL37 and US3 (VZV open reading frames 22, 21 and 66, respectively), binds to the retrograde molecular motor dynein and the viral particle is transported along microtubules to the nuclear membrane. Therein, the viral genome enters latency. Upon reactivation, progeny capsids are assembled in the nuclei. The capsids exit the nuclei and enter a virion assembly compartment near the Golgi apparatus. The nascent virions are housed within cytoplasmic vacuoles. The three viral proteins gE, gI and US9 in the outer wall of the vacuole form a complex that attaches to kinesin molecular motors (also known as KIFs) (Scherer et al. 2020). The gE glycoprotein is an essential intermediary between US9 and the kinesin motor (Kratchmarov et al. 2013). After formation of the gE/gI/US9 complex, several arginine residues in the US9 protein form the binding site of the gE/gI/US9 complex to the kinesin motor (Diefenbach et al. 2016). In turn, the transport vesicle containing the virions is carried along microtubules by a kinesin molecular motor in an anterograde direction. Subsequently, the virions exit the afferent neuron and enter the outermost or adventitial layer of the cerebral arteries. Homologs of the three PRV proteins are found in the VZ virion (open reading frames 68/gE, 67/gI and 65/US9) (Fig. 3). The properties of the VZV gE glycoprotein and the VZV gE/gI complex have been characterized although similar neuronal studies have not been performed (Grose 2002). The VZV gE product is the predominant viral glycoprotein and also an essential viral protein (Mo et al. 2002; Grose 2002).
4 Known Reservoirs: Latent Wild-Type VZV in the Ganglia of Head and Neck
The most complete epidemiological analysis of herpes zoster was carried out among the residents in the town of Cirencester, England, in the 16-year period of 1947–1962 (Hope-Simpson 1965). The physician Hope-Simpson personally recorded 192 cases of herpes zoster in this community, including 8 instances of a second episode and one instance of a third episode. Hope-Simpson carefully recorded the dermatomal distribution of his cases of herpes zoster and established that the common dermatomal locations of zoster corresponded with sites of more abundant varicella rash on the body when the person had varicella as a child. Since the varicella rash often begins on the upper face, the trigeminal ganglion is a frequent site of latent VZV. See photograph of child’s face with varicella rash in Fig. 1, as an example. Hope-Simpson found that around 15% of herpes zoster was herpes zoster ophthalmicus (HZO); he also found a predilection for herpes zoster to occur in dermatomes T3 through T10, another known location for a more florid varicella exanthem. However, there often is little rash on the feet and toes, presumably because the adaptive immune response has appeared before the rash has spread to this location. Thus, the least common site of herpes zoster in an adult is the large toe (Tannous and Grose 2011). Based on his large dataset of observations, he concluded that VZV was carried to dorsal root ganglia via the sensory fibers in the skin.
In molecular investigations of latent VZV in autopsy samples of elderly people known to have a positive varicella serology, 87% of the patients had VZV DNA in their trigeminal ganglia (Mahalingam et al. 1990). In another autopsy study, latent VZV was also found in the geniculate ganglion, the sympathetic superior cervical ganglion and in the parasympathetic ganglia, including the ciliary, otic, pterygopalatine and submandibular ganglia (Richter et al. 2009). As expected, latent VZV DNA was detected more commonly in the lower thoracic DRG, although some cervical, lumbar and sacral DRG also contained latent VZV (Mahalingam et al. 1992). Further, ganglia at different locations within the same person contained latent VZV DNA.
5 Alternative Pathway to Ganglia: Transport of Wild-Type VZV During a Viremia
Several observational studies in humans and animals have suggested that VZV can be carried via lymphocytes to the ganglia during a viremia (Zerboni and Arvin 2011). Essentially everyone who had wild-type varicella has a viremia (Grose 1981). Although less well known, around 50% of children who have varicella vaccination have a viremia with the varicella vaccine virus (Ozaki et al. 1994). Virus can be carried to the trigeminal ganglion via an artery to trigeminal nerve ganglion that arises from the extracranial segment of middle meningeal artery (Qureshi 2017). In turn, the middle meningeal artery is a branch of the internal maxillary artery and the internal maxillary artery is one of the terminal branches of the external carotid artery. The arterial blood supply to the superior cervical ganglion is also a branch of the external carotid artery, namely the ascending pharyngeal artery (Tubbs et al. 2002).
6 Pathway from Ganglia to Cerebral Arteries: Innervation of the Circle of Willis
The pathogenesis of arterial ischemic stroke caused by an infectious agent requires that the pathogen be carried to the cerebral arteries. For herpesviruses such as VZV, the conduit is a nerve fiber. Evidence regarding the innervation of the cerebral arteries comes predominantly from experiments in an array of animal models. The most well-studied pathway is centered on the trigeminal ganglion (cranial nerve V). Afferent fibers from the trigeminal ganglion travel to the circle of Willis and its numerous arterial extensions (Fig. 4). One early study to define innervation pathways was carried out by damaging the ophthalmic and maxillary branches of the ophthalmic nerves in monkeys and observing the sites of Wallerian degeneration with the internal carotid artery, the middle cerebral artery and the basilar artery (Simons and Ruskell 1988). This study confirmed an even earlier investigation using neuronal tracing with wheat germ agglutinin conjugated with horseradish peroxidase (WGA-HRP) in the rat model. When WGA-HRP was injected into the trigeminal ganglion, labeled nerve fibers were found in arteries of both the anterior circulation (the internal carotid, middle cerebral, anterior cerebral and posterior communicating arteries) as well as the posterior circulation (posterior cerebral, superior cerebellar and basilar arteries) (Arbab et al. 1986).
Innervation of the cerebral arteries by afferent fibers from the trigeminal ganglion. a During varicella, virus is carried retrograde from vesicles on the face to the ophthalmic division of the trigeminal ganglion, where virus usually enters a latent state. b Trigeminal ganglion. c Upon reactivation, virus can travel via afferent fibers to the anterior cerebral artery (ACA), the middle cerebral artery (MCA), the posterior communicating artery (PCOA), the posterior cerebral artery (PCA), the superior cerebellar artery (SCA) and the basilar artery (BA). The most afferent fibers travel to the MCA and the second most fibers travel to the ACA; far fewer fibers travel to the SCA and the BA
When the tracer was applied to the middle cerebral artery, labeled trigeminal cells were present in the ipsilateral trigeminal ganglion. Similarly, when tracer was applied to the middle meningeal artery, labeled calls were found in the ipsilateral trigeminal ganglion. Another study using a similar approach also found evidence of trigeminal innervation of the middle meningeal artery, a branch of the external carotid artery that supplies the meninges (dura mater) (Mayberg et al. 1984). A different group used a retrograde axonal tracer called True Blue to study the innervation of the middle cerebral artery in the rat. They confirmed that the True Blue tracer was carried to the trigeminal ganglion from the middle cerebral artery (Edvinsson et al. 1989).
Even though the most studied pathway is the trigeminal ganglion, the superior cervical ganglion also appears to innervate cerebral arteries, particularly, but not exclusively, the posterior circulation. Both the sensory trigeminal ganglion and the sympathetic superior cervical ganglion harbor latent VZV. Similar tracing techniques were used to study innervation by the superior cervical ganglion. When WGA-HRP was injected into the basilar arteries of cats, the greatest number of labeled cells were found in the superior cervical ganglion (Keller et al. 1985).When the reverse experiment was performed by injection of WGA-HRP into the superior cervical ganglion, labeled nerve fibers were found in the anterior communicating, anterior cerebral, posterior communicating, superior cerebellar and rostral basilar arteries. A similar study was performed in the dog after resection of the superior cervical ganglion and observing Wallerian degeneration. Degeneration of nerve fiber was documented in both the anterior circulation (anterior communicating, anterior cerebral, middle cerebral, posterior communicating arteries) and posterior circulation (posterior cerebral, superior cerebellar and basilar arteries) (Sato et al. 1980).
Collectively, these animal experiments suggest the anterior circulation may be innervated by the sensory trigeminal ganglion and the posterior circulation by the sympathetic superior cervical ganglion. Hence, either could potentially serve as a conduit for VZV to cause a cerebral arteritis.
7 Clinical Evidence that Herpes Zoster Leads to Anterior Cerebral Circulation Involvement and Stroke
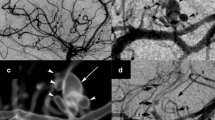
Delayed contralateral hemiparesis is the best-characterized condition of arteritis caused by VZV following HZO. An early description included a 54-year-old woman with right HZO followed 5 weeks later by left hemiparesis (Cope and Jones 1954). She had had varicella as a child (Fig. 5). A subsequent similar case report provided more documentation by arteriography and electroencephalography (Gilbert 1974). This patient was a 73-year-old man who developed left hemiparesis around 3 weeks after right HZO. A right carotid arteriogram showed severe segmental narrowing of the right carotid siphon. A third informative case occurred in a 7-year-old child (Walker et al. 1973). This child had right hemiplegia 6 months after left HZO. During his evaluation for hemiplegia, an arteriogram of the left carotid artery revealed several segmental constrictions in the left middle cerebral artery and one additional constriction in the left anterior cerebral artery. Once the association was established in the literature between HZO and subsequent contralateral hemiplegia, physicians recognized that cases of delayed contralateral hemiparesis also occurred after varicella in children. One report described a 6-year-old girl who developed left hemiparesis 6 weeks after a bout of varicella (Kamholz and Tremblay 1985). Her computer tomography, which showed a hypodensity in the right basal ganglia and several small areas of enhancement in the right centrum semiovale; and arteriography showed stenosis of both the middle and anterior cerebral arteries on the right side and normal arteries on the left side.
Association between herpes zoster ophthalmicus and contralateral hemiplegia. a Varicella was a common childhood infection. b During this infection, virus was carried via sensory neurons to the trigeminal ganglion. c Upon reactivation in adulthood, virus was carried anterograde to the eye to cause herpes zoster ophthalmicus. d The first recognition of an association between herpes zoster and subsequent stroke was found in the syndrome of contralateral hemiplegia following herpes zoster ophthalmicus
Another case report documented fatal disseminated herpes zoster in a 67-year-old woman with cancer (McKelvie et al. 2002). During an autopsy examination of the brain, the authors observed mononuclear cell infiltration of the meninges and vasculitis of the leptomeningeal vessels; the CSF was positive for VZV DNA. The same authors also reviewed an additional nine prior cases reports of VZV encephalitis, all in immunodeficient patients, in which the investigators had documented a necrotizing vasculitis in the brain. The VZV etiology of the vasculitis had been confirmed by immunohistochemistry, in situ hybridization or PCR testing in the nine prior cases.
In one case report, a 78-year-old woman presented with left HZO (Melanson et al. 1996). One month later, she developed right hemiplegia and speech difficulty. Cranial computed tomography revealed a small infarct in the left basal ganglia. Subsequent magnetic resonance angiography showed stenosis in both the left anterior cerebral artery and the left middle cerebral artery. When molecular virology studies were performed, VZV DNA was detected in the CSF as well as in extracts from the left anterior cerebral artery, middle cerebral artery and posterior cerebral artery.
The pathogenesis of an infarcted basal ganglia is based on experiments that showed the innervation of the Circle of Willis. The basal ganglia that are the caudate nucleus, putamen and globus pallidus are supplied by perforating branches from the anterior cerebral artery, the middle cerebral artery and the posterior cerebral artery (Djulejic et al. 2016). Therefore, a vasculitis induced by virus infection of the cerebral arteries would lead to an associated vasculitis in the perforating branches of those arteries with subsequent stroke.
8 Clinical Evidence that Herpes Zoster Leads to Cerebral Posterior Circulation Involvement and Subsequent Stroke
In a case report, a 20-year-old man with Hodgkin’s disease being treated with both irradiation and chemotherapy developed right HZO, followed by dissemination and death (Linnemann and Alvira 1980). VZV infection was documented by immunofluorescence assays of the skin lesion. Examination of the brain during autopsy showed infarcts scattered in the pons, medulla oblongata and the upper cervical spinal cord. The areas of infarction correlated with locations of branches of the basilar artery; in turn, observation of the basilar artery showed an infiltration of histiocytes and mononuclear cells. In turn, nerves within the granulomatous portions of the basilar artery were infiltrated with macrophages. Finally, examination of the damaged arteries by electron microscopy revealed viral particles resembling VZV.
Herpes zoster of the maxillary division of the trigeminal ganglion can lead to a stroke in the occipital lobe. In a case report, a 68-year-old man was first examined because of left homonymous hemianopic scotoma (Powers 1986). He also experienced left arm numbness. He had a history of herpes zoster of the face extending from the tragus of the right ear to the nose 4 months earlier. Results from a computed tomogram with contrast showed a dense homogenous lesion in the medial right occipital lobe, consistent with an infarction in the prior 2–3 weeks. A right vertebral arteriogram showed bead-like segmental narrowing in the right posterior cerebral artery with complete occlusion distally. Bilateral carotid arteriograms were normal. This unusual case may also provide insight into why stroke of the occipital lobe is less common (Marinkovic et al. 1987). This case is important also in that the clinic-pathological correlation confirms laboratory studies showing that the posterior cerebral artery receives innervation from both the ophthalmic and the maxillary branches of the trigeminal ganglion (Simons and Ruskell 1988). In a review of 184 cases of HZO, 158 were located in the ophthalmic branch, 16 were in the maxillary branch and only 10 were in the mandibular branch (Thomas and Howard 1972).
9 Epidemiology of Stroke Following Wild-Type Herpes Zoster in Adults
The association of stroke following herpes zoster has been reported from several countries (Gilden et al. 2003; Amlie-Lefond and Gilden 2016; Sundstrom et al. 2015). The most important epidemiology study to support an association between HZO and subsequent stroke was carried out in Taiwan (Lin et al. 2010). The investigators retrospectively analyzed data from the Taiwan National Health Insurance Research Database. The study cohort included 658 patients with HZO in the years 2003 and 2004; the control cohort included 1974 matched patients. The investigators used survival analysis techniques to calculate the 1-year stroke-free survival rate, and the Cox proportional hazard regression to calculate the adjusted 1-year stroke-free survival rate after consideration of the confounding factors. They observed a stroke diagnosis in 8.1% of HZO patients and 1.7% of control patients during a 1-year follow-up period. HZO patients had a 4.5 fold higher risk of stroke than the control group. In the HZO group, the vast majority of patients were between 50 and 79 years old; 336 were female, and 322 were male.
Another epidemiology study was carried out in Denmark (Sreenivasan et al. 2013). Using the Danish Civil Registration System and the Danish National Register of Medicinal Product Statistics, the authors identified all individuals in Denmark who had taken an antiviral medicine for treatment of herpes zoster between 1995 and 2008. The population included 4,620,980 people with stroke outcomes in 230,341. Among 117,926 people who had taken a course of medication for treatment of herpes zoster, 4876 later had a stroke. Using Poisson regression techniques, they found that the treatment (zoster) group had a 127% increased risk of stroke in the weeks after taking antiviral treatment compared to individuals with no antiviral treatment. The increased risk was most apparent in the people under 40 years old. The Danish investigators were not able to distinguish people with HZO from those who had herpes zoster with no location specified.
Three other studies in England, Sweden and the USA found a less robust association between herpes zoster and subsequent stroke and were also limited by the inability to distinguish HZO from herpes zoster in other locations. In a British study, the authors analyzed a retrospective cohort of 106,601 cases of herpes zoster with a control population of 213,202 people (Breuer et al. 2014). The investigators observed an increased risk of stroke following herpes zoster only in the population group that was younger than 40 years old, a result somewhat similar to the Danish study. Further, in a Swedish register-based cohort study among 1.5 million people, the authors observed an increased risk of stroke in the year after herpes zoster, especially in people under 40 years of age (Sundstrom et al. 2015). In the study conducted in the USA, the study group with herpes zoster was only 4862 patients, while the control group was 19,433 patients (Yawn et al. 2016). They discovered a modestly increased risk of stroke for the herpes zoster group in the first 3 months after the zoster infection but not later.
A case–control study conducted in Iran appeared to support the premise that HZO is associated with subsequent stroke more than herpes zoster at any other location (Hosamirudsari et al. 2018). The investigators analyzed 105 cases of stroke and discovered that 16 had a past history of HZO, while 9 patients had a past history of either thoracic or lumbar herpes zoster. Almost all HZO patients were in the age group between 60 and 80 years. Among the patients in the control group, there was only one case of herpes zoster. These differences were highly significant. Most of the cases of HZO had occurred in the month preceding the stroke.
Together, these studies from five different countries provide evidence of a meaningful association between ischemic stroke and prior HZO, presumably caused by cerebral vasculitis secondary to reactivation of herpes zoster in the trigeminal ganglion and transport of virus to the middle cerebral artery (Grose 2018).
10 Epidemiology of Stroke Following Varicella (Chickenpox) in Children
The association of stroke following varicella in childhood is well documented and accepted as a rare complication (Ciccone et al. 2010; Miravet et al. 2007). In an older Canadian study, the risk of stroke after varicella was estimated to be 1:15,000 (Askalan et al. 2001). A more recent study from Denmark, where children are not routinely vaccinated against VZV, utilized the Danish National Patient Register to identify 191 children with a diagnostic code for arterial ischemic stroke over a 5-year period (2010–2015) (Helmuth et al. 2018). Retrospective medical record review revealed varicella documented within the 12 months preceding the stroke in 15 cases (19%). Overall, the annual incidence of arterial ischemic stroke in their population was 1.3 per 100,000 children (aged 29 days to 16 years); they estimated an annual incidence of post-varicella stroke of 0.2 per 100,000 children. Based on frequency of varicella in their population, they estimated the risk of stroke after varicella to be 1:26,000.
To further define features of varicella-related stroke in children, we reviewed over 60 case reports of stroke following varicella. These reports span the decades before varicella vaccination was commonly administered, so all cases are considered to be wild-type varicella virus (Losurdo et al. 2006; Eda et al. 1983; Liu and Holmes 1990; Caekebeke et al. 1990; Bodensteiner et al. 1992; Ichiyama et al. 1990; Shuper et al. 1990; Tucciarone et al. 1992; Yilmaz et al. 1998; Hausler et al. 1998; Silverstein and Brunberg 1995; Hayman et al. 2001; Okanishi et al. 2009; Rougeot et al. 2006; Danchaivijitr et al. 2006; Ueno et al. 2002; Kimura et al. 2002; Gomez-Gosalvez et al. 2003; Beleza et al. 2008; Hayes et al. 2007; Lopez Medina et al. 2002; West et al. 2006; Tiah et al. 2004; Guillot et al. 2005; Morino et al. 2009; Yaramis et al. 2009; Singhal et al. 2001; Aydin et al. 2006; Cadavid et al. 1999; Leis and Butler 1987; Kramer et al. 1999; Darling et al. 1995; Wieting et al. 1997; Frank et al. 1989; Spiegel et al. 2010; Walker et al. 1973; Hansen et al. 2006; Nguyen et al. 1994; Alehan et al. 2002; Hattori et al. 2000; Moriuchi and Rodriguez 2000; Caruso et al. 2001; Francois et al. 1996). We were particularly interested in the interval between varicella and subsequent stroke. As seen in Fig. 6, the most common interval is one month, followed by two months and three months. Imaging findings suggest that both entities more commonly involve the anterior circulation than the posterior circulation. The most common findings on parenchymal brain imaging were infarcts in the basal ganglia and internal capsule (89%), infarcts in the cerebral lobes (33%), infarcts in the white matter (17%) or infarcts in the thalamus (6%). Correspondingly, most common abnormal findings by angiography were found in the middle cerebral artery (70%), anterior cerebral artery (19%), internal carotid artery (15%), posterior cerebral artery (2%) and basilar artery (2%).
Based on PRV models of neuronal infection (Granstedt et al. 2013; Brittle et al. 2004), we hypothesize that in these children with stroke, virus entering the trigeminal ganglion does not establish latency. Instead, as has already been found in PRV infection of ganglia, some viruses undergo a replication cycle followed a few days later by transport anterograde from the trigeminal ganglia to other sites of innervation. This model would account for the 1–3 months delay between varicella and stroke, similar to the 1–3 months interval between herpes zoster and stroke (Grose and Enquist 2020). Thus, there are many common features between stroke following varicella and stroke following herpes zoster (Fig. 1).
Finally, we include one report of children in Switzerland. Posterior arterial stroke represents 16% of all childhood arterial ischemic strokes that occur in Switzerland (Fink et al. 2019). Most infarcts in descending order were located in the cerebellum, thalamus, pons and medulla. The most commonly affected artery was the posterior cerebral artery followed by the posterior inferior cerebellar artery. One of their 43 cases was a 7-year-old boy who had a posterior arterial ischemic stroke one month after a varicella infection. Magnetic resonance angiography revealed reduced flow through the distal basilar artery and the left vertebral artery. This high percentage of posterior arterial stroke was not observed in a large study of childhood stroke carried out mainly in the USA and Canada (Elkind et al. 2016).
11 Stroke in a Child with Congenital Varicella Infection
Varicella infection in a pregnant woman is occasionally followed by in utero VZV infection. As noted above, almost all people with varicella have a viremia. In a pregnant woman, there may be transplacental transfer of virus with subsequent viremia in the fetus (Grose 1994). The subsequent infection of fetal tissues leads to the most sequelae if the viremia occurs during the first trimester, while lesser fetal damage occurs after infection in the second and third trimesters (Table 1). As noted in Table 1, congenital varicella infection has features of herpes zoster.
Although there are many case reports of congenital varicella syndrome after gestational varicella, there is only one case report of congenital varicella syndrome after herpes zoster in the pregnant woman (West et al. 2006). The woman has herpes zoster at 36 weeks of gestation with an abdominal rash. Like varicella, zoster is also accompanied by a viremia (Satyaprakash et al. 2009). At birth, the baby was noted to have a zoster-like vesicular rash over the right side of the scalp. The rash was treated with acyclovir. At 2.5 years of age, the child presented with a left hemiplegia. Magnetic resonance imaging of her brain showed acute and chronic ischemic changes in the right hemisphere. Cranial angiography showed right cerebral hemisphere small vessel vasculopathy, with arterial stenosis and compensatory poststenotic dilation and neovascularization of perforating arteries. The vesicular rash over the right scalp has recurred prior to the appearance of hemiplegia. Examination of the cerebrospinal fluid revealed a positive test for VZV DNA. This case is important because children with congenital varicella syndrome lack a robust adaptive immune response to VZV (Grose 1989); therefore, VZV reactivation is more common, including anterograde spread to the cerebral vasculature. This child’s neurological disease also closely resembles the cases of HZO and contralateral hemiplegia described in the elderly.
12 Evidence for Vaccine-Type VZV in Neuronal Ganglia
The varicella vaccine virus is a live attenuated virus (Takahashi 2004). The vaccine virus grows much more slowly than wild-type virus in human skin (Moffat et al. 1998; Grose 1996). Nevertheless, vaccine virus can enter neurons and establish latency, similar to wild-type virus. Studies in cell culture have established that the vaccine virus can establish latency at a similar frequency to wild-type virus, but vaccine virus reactivates less frequently than wild-type virus (Sadaoka et al. 2016). Studies in the severe combined immunodeficient mouse model of VZV infection, using fetal dorsal root ganglia xenografts, have demonstrated that vaccine virus can infect the dorsal root ganglia in a similar manner to wild-type virus (Zerboni et al. 2005). Corroborating the findings from laboratory models, an autopsy study of 10 children (deceased of unrelated causes at 1 to 10 years of age) examined neuronal ganglia for VZV. The authors detected wild-type varicella DNA in the dorsal root ganglia of 6 children, 3 of whom had VZV DNA in the trigeminal ganglia (Gershon et al. 2012). Of great importance, vaccine-type DNA was detected in lumbar and thoracic dorsal root ganglia of one child who was 1.7 years old at time of death. This child did not have wild-type VZV DNA in any ganglia. The thoracolumbar location of latent vaccine DNA is likely due to the fact the varicella vaccination at age one-year would have been administered in the thigh.
Another important case report documented that varicella vaccine virus can establish latency in the trigeminal ganglion, even after administration of vaccine in the thigh (Detty et al. 2020). This case involves HZO in a child who was vaccinated in the thigh. The virus DNA found in the vesicles on the face was typed and found to be vaccine lineage. This case suggests that vaccine virus, after administration in the thigh, can replicate in the thigh, then enter the blood stream (viremia), after which the virus can be carried to the trigeminal artery and then into the trigeminal ganglion. In other words, there is a chance that any vaccinated child will have latent vaccine DNA in the trigeminal ganglion.
13 Meningitis Caused by Varicella Vaccine Virus
Further evidence that vaccine virus can establish latency in the trigeminal ganglia is the extremely rare but serious adverse event of meningitis caused by reactivation of vaccine virus (Heusel and Grose 2020; Moodley et al. 2019). The likely route of vaccine virus spread to the meninges is via the afferent fibers of the trigeminal ganglion that travel along the middle meningeal artery to the dura (Horien and Grose 2012). To date, there have been 12 documented cases of varicella vaccine meningitis in children and adolescents (Heusel and Grose 2020). The majority of the children were immunocompetent. The bouts of meningitis were usually preceded by herpes zoster (Ramachandran et al. 2020). The herpes zoster rash was usually located in a cervical or lumbar dermatome, not HZO. Therefore, the virus travelled to the meninges by one of 2 routes: (i) via afferents from the trigeminal ganglion after a reactivation without a facial rash (zoster sine herpete) or (ii) via a viremia that occurred at the time of herpes zoster.
The implications of vaccine-type VZV latency in the trigeminal ganglia on childhood stroke risk remain unclear. Very few childhood strokes have been reported in the month after the varicella vaccination (Wirrell et al. 2004). In general, while childhood infections transiently increase risk of stroke, routine childhood vaccinations appear protective (Fullerton et al. 2015). Three studies of large cohorts of children who received the varicella vaccine (3.2 million US children, 1.2 million Taiwanese children and 0.3 million Canadian children) found no association between vaccine status and ischemic stroke (Donahue et al. 2009).
14 Mechanisms of VZV Arteriopathy
While an array of evidence suggests that VZV can be carried to the cerebral artery via nerve fibers, particularly those from the trigeminal ganglion, the exact mechanisms by which VZV causes an arteriopathy remain less clear. The afore-mentioned reports of cases of stroke after both zoster (particularly HZO) and varicella describe a focal or multifocal cerebral arteriopathy that most often involves the intracranial internal carotid artery or its proximal branches (middle cerebral artery, anterior cerebral artery, posterior communicating artery). The arteriopathy is characterized by irregularity or stenosis, often with a “beaded” or “banded” appearance on angiography. This arteriopathy has been called “VZV vasculopathy” in adult literature and “post-varicella arteriopathy” in pediatric literature (Gilden et al. 2009; Wintermark et al. 2017; Fullerton et al. 2018; Nagel et al. 2017). Post-varicella arteriopathy is considered a subtype of “focal cerebral arteriopathy of childhood,” the most common cause of arterial ischemic stroke in an otherwise healthy child (Wintermark et al. 2017; Fullerton et al. 2018). Focal cerebral arteriopathy and post-varicella arteriopathy have the same imaging appearance and natural history as a monophasic arteriopathy; the singular distinction is the clinical history of varicella (chicken pox) within the preceding 12 months (Lanthier et al. 2005). However, herpesvirus serologies from a prospective cohort of childhood arterial ischemic stroke suggest a broader role for VZV and herpes simplex virus as triggers for focal cerebral arteriopathy as well as other types of childhood stroke, even without a clinical history of varicella (Elkind et al. 2016; Grose 2016).
The mechanism of VZV arteriopathy could be related to direct viral invasion of endothelial cells within the arterial wall, or a para- or post-infectious inflammatory process causing invasion of the arterial wall by inflammatory cells (i.e., vasculitis). Histopathology is rarely available for post-varicella arteriopathy (most children survive), and existing reports are inconsistent: one identified VZV antigen in the affected arterial wall, while another did not (Berger et al. 2000; Hayman et al. 2001). The poor understanding of the underlying mechanisms leads to uncertainty regarding how this arteriopathy should be managed. Pediatric neurologists have increasingly treated focal cerebral arteriopathy, including post-varicella arteriopathy, with high-dose intravenous corticosteroids, although evidence in support of efficacy remains limited to retrospective cohort studies (Steinlin et al. 2017; Elbers et al. 2016). Concurrent treatment with an antiviral agent also remains controversial. A Swiss/Australian cohort study found no difference in outcomes when corticosteroids were given with or without acyclovir (Steinlin et al. 2017). However, they did conclude that the addition of corticosteroid treatment may provide benefit over antithrombotic treatment alone for improved outcome in children with focal cerebral arteriopathy.
15 Commonality of Stroke Following Varicella in Children to Stroke after Herpes Zoster in Adults
It is clear from the medical literature that stroke occasionally follows herpes zoster (shingles) in adults. The recent publication of a study of 1.6 million Zostavax vaccinees, showing a decrease in stroke in people immunized against herpes zoster, supports the concept that herpes zoster may predispose to stroke. Likewise, it is clear from the pediatric literature that stroke occasionally follows wild-type varicella (chickenpox) in children. The main goal of this review is to establish that stroke following varicella has a similar pattern of neuropathogenesis to stroke following herpes zoster (Fig. 1). The trigeminal ganglion and to a lesser extent, the superior cervical ganglion, are central to the stroke pathogenesis pathways after both herpes zoster and varicella, because afferent fibers from these two ganglia provide the circuitry by which the virus can travel to the anterior and posterior circulations of the brain. In this review, we present stroke cases of remarkable similarity after VZV infection at any age, from a 2-year-old child with hemiplegia after congenital varicella infection to older adults with hemiplegia after HZO. Based on the above review, we also suggest that some cases of arterial ischemic stroke are preceded by VZV reactivation in the trigeminal ganglion without a rash (zoster sine herpete). Therefore, future studies to define the percentage of strokes that are associated with prior herpes zoster or varicella will require more sensitive VZV detection technology and more sophisticated neuroimaging on the affected patients than has generally been used in prior clinical stroke studies.
References
Alehan FK, Boyvat F, Baskin E, Derbent M, Ozbek N (2002) Focal cerebral vasculitis and stroke after chickenpox. Eur J Paediatr Neurol 6:331–333
Amlie-Lefond C, Gilden D (2016) Varicella Zoster virus: a common cause of stroke in children and adults. J Stroke Cerebrovasc Dis 25:1561–1569
Arbab MA, Wiklund L, Svendgaard NA (1986) Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience 19:695–708
Askalan R, Laughlin S, Mayank S, Chan A, Macgregor D, Andrew M, Curtis R, Meaney B, Deveber G (2001) Chickenpox and stroke in childhood: a study of frequency and causation. Stroke 32:1257–1262
Aydin K, Sert A, Guzes EA, Kiresi DA (2006) Acute childhood hemiplegia associated with chickenpox and elevated anticardiolipin antibody. J Child Neurol 21:890–893
Beleza P, Fernandes J, Afonso A, Silva H, Jordao MJ (2008) Transient ischemic attacks in a child with post-varicella arteriopathy and MTHFR homozigotic mutation C677T. Arq Neuropsiquiatr 66:256–258
Berger TM, Caduff JH, Gebbers JO (2000) Fatal varicella-zoster virus antigen-positive giant cell arteritis of the central nervous system. Pediatr Infect Dis J 19:653–656
Bodensteiner JB, Hille MR, Riggs JE (1992) Clinical features of vascular thrombosis following varicella. Am J Dis Child 146:100–102
Breuer J, Pacou M, Gauthier A, Brown MM (2014) Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology 82:206–212
Brittle EE, Reynolds AE, Enquist LW (2004) Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol 78:12951–12963
Cadavid D, Pearl PL, Dubovsky EC, Angiolillo A, Vezina LG (1999) Stroke after zoster ophthalmicus in a 12-year-old girl with protein C deficiency. Neurology 53:1128–1129
Caekebeke JF, Peters AC, Vandvik B, Brouwer OF, de Bakker HM (1990) Cerebral vasculopathy associated with primary varicella infection. Arch Neurol 47:1033–1035
Card JP, Enquist LW (2014) Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci 68:1.5.1–1.5.39
Caruso JM, Tung GA, Brown WD (2001) Central nervous system and renal vasculitis associated with primary varicella infection in a child. Pediatrics 107:E9
Ciccone S, Faggioli R, Calzolari F, Sartori S, Calderone M, Borgna-Pignatti C (2010) Stroke after varicella-zoster infection: report of a case and review of the literature. Pediatr Infect Dis J 29:864–867
Cope S, Jones AT (1954) Hemiplegia complicating ophthalmic zoster. Lancet 267:898–899
Danchaivijitr N, Miravet E, Saunders DE, Cox T, Ganesan V (2006) Post-varicella intracranial haemorrhage in a child. Dev Med Child Neurol 48:139–142
Darling CF, Larsen MB, Byrd SE, Radkowski MA, Palka PS, Allen ED (1995) MR and CT imaging patterns in post-varicella encephalitis. Pediatr Radiol 25:241–244
Davison AJ (2010) Herpesvirus systematics. Vet Microbiol 143:52–69
Detty SQ, Peebles JK, Guerrieri JM, Seroogy CM, Struck MC, Arkin LM, Henderson SL (2020) Vaccine-strain Herpes Zoster ophthalmicus in a 14-month-old boy prompting an immunodeficiency workup: case report and review of vaccine-strain Herpes Zoster. Pediatr Infect Dis J 39:e25–e27
Diefenbach RJ, Davis A, Miranda-Saksena M, Fernandez MA, Kelly BJ, Jones CA, Lavail JH, Xue J, Lai J, Cunningham AL (2016) The basic domain of Herpes simplex virus 1 pUS9 recruits kinesin-1 To facilitate Egress from neurons. J Virol 90:2102–2111
Djulejic V, Marinkovic S, Georgievski B, Stijak L, Aksic M, Puskas L, Milic I (2016) Clinical significance of blood supply to the internal capsule and basal ganglia. J Clin Neurosci 25:19–26
Donahue JG, Kieke BA, Yih WK, Berger NR, Mccauley JS, Baggs J, Zangwill KM, Baxter R, Eriksen EM, Glanz JM, Hambidge SJ, Klein NP, Lewis EM, Marcy SM, Naleway AL, Nordin JD, Ray P, Belongia EA, Vaccine Safety Datalink Team (2009) Varicella vaccination and ischemic stroke in children: is there an association? Pediatrics 123:e228-e234
Eda I, Takashima S, Takeshita K (1983) Acute hemiplegia with lacunar infarct after varicella infection in childhood. Brain Dev 5:494–499
Edvinsson L, Hara H, Uddman R (1989) Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: colocalization with different peptides. J Cereb Blood Flow Metab 9:212–218
Elbers J, Armstrong D, Yau I, Benseler S (2016) Vascular imaging outcomes of childhood primary angiitis of the central nervous system. Pediatr Neurol 63:53–59
Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, Deveber GA, Fullerton HJ, Investigators* V (2016) Herpesvirus infections and childhood arterial ischemic stroke: results of the VIPS study. Circulation 133:732–741
Esiri MM, Tomlinson AH (1972) Herpes zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci 15:35–48
Fink M, Slavova N, Grunt S, Perret E, Regenyi M, Steinlin M, Bigi S (2019) Posterior arterial ischemic stroke in childhood. Stroke 50:2329–2335
Francois P, Bost C, Pavese P, Bost M (1996) Herpes zoster ophthalmicus with delayed contralateral hemiplegia. Pediatr Infect Dis J 15:471–472
Frank Y, Lim W, Kahn E, Farmer P, Gorey M, Pahwa S (1989) Multiple ischemic infarcts in a child with AIDS, varicella zoster infection, and cerebral vasculitis. Pediatr Neurol 5:64–67
Fullerton HJ, Hills NK, Elkind MS, Dowling MM, Wintermark M, Glaser CA, Tan M, Rivkin MJ, Titomanlio L, Barkovich AJ, Deveber GA, Investigators V (2015) Infection, vaccination, and childhood arterial ischemic stroke: results of the VIPS study. Neurology 85:1459–1466
Fullerton HJ, Stence N, Hills NK, Jiang B, Amlie-Lefond C, Bernard TJ, Friedman NR, Ichord R, Mackay MT, Rafay MF, Chabrier S, Steinlin M, Elkind MSV, Deveber GA, Wintermark M, Investigators V (2018) Focal cerebral arteriopathy of childhood: novel severity score and natural history. Stroke 49:2590–2596
Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M (2012) Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc 123:17–33; discussion 33–5
Gilbert GJ (1974) Herpes zoster ophthalmicus and delayed contralateral hemiparesis. Relationship of the syndrome to central nervous system granulomatous angiitis. JAMA 229:302–304
Gilden D, Cohrs RJ, Mahalingam R, Nagel MA (2009) Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 8:731–740
Gilden DH, Cohrs RJ, Mahalingam R (2003) Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol 16:243–258
Gomez-Gosalvez FA, Menor-Serrano F, Escriva-Tomas P, Clemente-Yago F, Sala-Sanchez AG, Rubio-Soriano A, Perez-Rueda C, Carbonell-Nadal J (2003) Ischemic stroke secondary to post varicella angiopathy in a 3 year old male. Rev Neurol 36:930–932
Granstedt AE, Brunton BW, Enquist LW (2013) Imaging the transport dynamics of single alphaherpesvirus particles in intact peripheral nervous system explants from infected mice. mBio 4:e00358–13
Gray WL, Pumphrey CY, Ruyechan WT, Fletcher TM (1992) The simian varicella virus and varicella zoster virus genomes are similar in size and structure. Virology 186:562–572
Grose C (1981) Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735–737
Grose C (1989) Congenital varicella-zoster virus infection and the failure to establish virus-specific cell-mediated immunity. Mol Biol Med 6:453–462
Grose C (1994) Congenital infections caused by varicella zoster virus and herpes simplex virus. Semin Pediatr Neurol 1:43–49
Grose C (1996) Pathogenesis of infection with varicella vaccine. Infect Dis Clin North Am 10:489–505
Grose C (2002) The predominant varicella-zoster virus gE and gI glycoprotein complex. In: Holzenburg A, Bogner E (eds) Structure-function relationships of human pathogenic viruses. Kluwer Academic Press, New York
Grose C (2012) Pangaea and the out-of-Africa model of Varicella-Zoster virus evolution and phylogeography. J Virol 86:9558–9565
Grose C (2016) Biological plausibility of a link between arterial ischemic stroke and infection with varicella-zoster virus or herpes simplex virus. Circulation 133:695–697
Grose C (2018) Heightened risk of ischemic stroke after recent herpes zoster ophthalmicus. J Med Virol 90:1283–1284
Grose C, Enquist LW (2020) The round trip model for severe herpes zoster caused by live attenuated varicella vaccine virus. J Med Virol 92:938–940
Guillot M, el Hachem C, Amiour M, Harchaoui S, Bessiere A, Lasjaunias P, Mafoufi N, Tardieu M (2005) Varicella, acute postinfectious arteriopathy and cerebral arterial thrombosis in childhood: a unique clinical and etiologic framework to be fully acknowledged. Arch Pediatr 12(Suppl 1):S58-60
Hansen LM, Sloth-Fjordside L, Dunkhase-Heinl U (2006) Acute hemiparesis after chickenpox. Ugeskr Laeger 168:2261–2262
Hattori H, Higuchi Y, Tsuji M (2000) Recurrent strokes after varicella. Ann Neurol 47:136
Hausler MG, Ramaekers VT, Reul J, Meilicke R, Heimann G (1998) Early and late onset manifestations of cerebral vasculitis related to varicella zoster. Neuropediatrics 29:202–207
Hayes B, Baker L, Alhajeri A, Ryan S, Lynch B (2007) Ischaemic stroke in children secondary to post varicella angiopathy. Ir Med J 100:332–333
Hayman M, Hendson G, Poskitt KJ, Connolly MB (2001) Postvaricella angiopathy: report of a case with pathologic correlation. Pediatr Neurol 24:387–389
He W, Auclert LZ, Zhai X, Wong G, Zhang C, Zhu H, Xing G, Wang S, He W, Li K, Wang L, Han GZ, Veit M, Zhou J, Su S (2019) Interspecies transmission, genetic diversity, and evolutionary dynamics of pseudorabies virus. J Infect Dis 219:1705–1715
Helmuth IG, Molbak K, Uldall PV, Poulsen A (2018) Post-varicella arterial ischemic stroke in Denmark 2010–2016. Pediatr Neurol 80:42–50
Heusel EH, Grose C (2020) Twelve children with varicella vaccine meningitis: neuropathogenesis of reactivated live attenuated varicella vaccine virus. Viruses 12
Hope-Simpson RE (1965) The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 58:9–20
Horien C, Grose C (2012) Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol 19:124–129
Hosamirudsari H, Rashed P, Afsari F, Akbarpour S, Bagherzadeh A (2018) Correlation between herpes zoster and stroke-a case-control study. J Med Virol 90:1370–1374
Ichiyama T, Houdou S, Kisa T, Ohno K, Takeshita K (1990) Varicella with delayed hemiplegia. Pediatr Neurol 6:279–281
Kamholz J, Tremblay G (1985) Chickenpox with delayed contralateral hemiparesis caused by cerebral angiitis. Ann Neurol 18:358–360
Keller JT, Beduk A, Saunders MC (1985) Origin of fibers innervating the basilar artery of the cat. Neurosci Lett 58:263–268
Kimura M, Hasegawa Y, Sejima H, Inoue M, Yamaguchi S (2002) Serial magnetic resonance angiography in cerebral infarction after varicella infection. Psychiatry Clin Neurosci 56:585–588
Klaric JS, Beltran TA, McClenathan BM (2019) An association between herpes zoster vaccination and stroke reduction among elderly individuals. Mil Med 184:126–132
Kramer LA, Villar-Cordova C, Wheless JW, Slopis J, Yeakley J (1999) Magnetic resonance angiography of primary varicella vasculitis: report of two cases. J Magn Reson Imaging 9:491–496
Kratchmarov R, Kramer T, Greco TM, Taylor MP, Ch’Ng TH, Cristea IM, Enquist LW (2013) Glycoproteins gE and gI are required for efficient KIF1A-dependent anterograde axonal transport of alphaherpesvirus particles in neurons. J Virol 87:9431–9440
Lanthier S, Armstrong D, Domi T, Deveber G (2005) Post-varicella arteriopathy of childhood: natural history of vascular stenosis. Neurology 64:660–663
Leis AA, Butler IJ (1987) Infantile herpes zoster ophthalmicus and acute hemiparesis following intrauterine chickenpox. Neurology 37:1537–1538
Lin HC, Chien CW, Ho JD (2010) Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology 74:792–797
Linnemann CC, Alvira MM (1980) Pathogenesis of varicella-zoster angiitis in the CNS. Arch Neurol 37:239–240
Liu GT, Holmes GL (1990) Varicella with delayed contralateral hemiparesis detected by MRI. Pediatr Neurol 6:131–134
Lopez Medina JA, Martinez Algar JL, Pastor Pons E, Azcon Gonzalez De Aguilar P, Gualda Canton J, Roldan Aparicio S (2002) [Ischemic stroke after varicella infection] An Esp Pediatr 57:174–176
Losurdo G, Giacchino R, Castagnola E, Gattorno M, Costabel S, Rossi A, Amato S, di Pietro P, Molinari AC (2006) Cerebrovascular disease and varicella in children. Brain Dev 28:366–370
Mahalingam R, Wellish M, Wolf W, Dueland AN, Cohrs R, Vafai A, Gilden D (1990) Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med 323:627–631
Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH (1992) Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol 31:444–448
Marinkovic SV, Milisavljevic MM, Lolic-Draganic V, Kovacevic MS (1987) Distribution of the occipital branches of the posterior cerebral artery correlation with occipital lobe infarcts. Stroke 18:728–732
Mayberg MR, Zervas NT, Moskowitz MA (1984) Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol 223:46–56
Mcgeoch DJ, Davison AJ (1999) The molecular evolutionary history of the herpesviruses. In: Domingo E, Webster R, Holland J (eds) Origin and evolution of viruses. Academic Press, New York
McKelvie PA, Collins S, Thyagarajan D, Trost N, Sheorey H, Byrne E (2002) Meningoencephalomyelitis with vasculitis due to varicella zoster virus: a case report and review of the literature. Pathology 34:88–93
Melanson M, Chalk C, Georgevich L, Fett K, Lapierre Y, Duong H, Richardson J, Marineau C, Rouleau GA (1996) Varicella-zoster virus DNA in CSF and arteries in delayed contralateral hemiplegia: evidence for viral invasion of cerebral arteries. Neurology 47:569–570
Miravet E, Danchaivijitr N, Basu H, Saunders DE, Ganesan V (2007) Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol 49:417–422
Mo C, Lee J, Sommer M, Grose C, Arvin AM (2002) The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176–186
Moffat JF, Zerboni L, Kinchington PR, Grose C, Kaneshima H, Arvin AM (1998) Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol 72:965–974
Moodley A, Swanson J, Grose C, Bonthius DJ (2019) Severe herpes zoster following varicella vaccination in immunocompetent young children. J Child Neurol 34:184–188
Morino M, Yamano H, Sasaki N (2009) Role of varicella virus and anticardiolipin antibodies in the development of stroke in a patient with down syndrome associated with Moyamoya syndrome. Pediatr Int 51:300–302
Moriuchi H, Rodriguez W (2000) Role of varicella-zoster virus in stroke syndromes. Pediatr Infect Dis J 19:648–653
Nagel MA, Jones D, Wyborny A (2017) Varicella zoster virus vasculopathy: the expanding clinical spectrum and pathogenesis. J Neuroimmunol 308:112–117
Nguyen P, Reynaud J, Pouzol P, Munzer M, Richard O, Francois P (1994) Varicella and thrombotic complications associated with transient protein C and protein S deficiencies in children. Eur J Pediatr 153:646–649
Okanishi T, Kondo A, Inoue T, Maegaki Y, Ohno K, Togari H (2009) Bilateral middle cerebral artery infarctions following mild varicella infection: a case report. Brain Dev 31:86–89
Ozaki T, Masuda S, Asano Y, Kondo K, Namazue J, Yamanishi K (1994) Investigation of varicella-zoster virus DNA by the polymerase chain reaction in healthy children with varicella vaccination. J Med Virol 42:47–51
Powers JM (1986) Herpes zoster Maxillaris with delayed occipital infarction. J Clin Neuroophthalmol 6:113–115
Qureshi AI (2017) Artery of trigeminal nerve ganglion. J Vasc Interv Neurol 9:57–58
Ramachandran V, Elliott SC, Rogers KL, Cohrs RJ, Weinberger M, Jackson W, Carpenter JE, Grose C, Bonthius DJ (2020) Varicella Vaccine meningitis as a complication of herpes zoster in twice-immunized immunocompetent adolescents. J Child Neurol 883073820938597
Richter ER, Dias JK, Gilbert JE, Atherton SS (2009) Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 200:1901–1906
Rougeot C, Boissier C, Chabrier S (2006) Post-varicella arteriopathy: benefits of using serial transcranial Doppler examinations. Eur J Paediatr Neurol 10:152–153
Sadaoka T, Depledge DP, Rajbhandari L, Venkatesan A, Breuer J, Cohen JI (2016) In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc Natl Acad Sci USA 113:E2403–E2412
Sato T, Sato S, Suzuki J (1980) Correlation with superior cervical sympathetic ganglion and sympathetic nerve innervation of intracranial artery-electron microscopical studies. Brain Res 188:33–41
Satyaprakash AK, Tremaine AM, Stelter AA, Creed R, Ravanfar P, Mendoza N, Mehta SK, Rady PL, Pierson DL, Tyring SK (2009) Viremia in acute herpes zoster. J Infect Dis 200:26–32
Scherer J, Hogue IB, Yaffe ZA, Tanneti NS, Winer BY, Vershinin M, Enquist LW (2020) A kinesin-3 recruitment complex facilitates axonal sorting of enveloped alpha herpesvirus capsids. PLoS Pathog 16:e1007985
Shuper A, Vining EP, Freeman JM (1990) Central nervous system vasculitis after chickenpox–cause or coincidence? Arch Dis Child 65:1245–1248
Silverstein FS, Brunberg JA (1995) Postvaricella basal ganglia infarction in children. AJNR Am J Neuroradiol 16:449–452
Simons T, Ruskell GL (1988) Distribution and termination of trigeminal nerves to the cerebral arteries in monkeys. J Anat 159:57–71
Singhal AB, Singhal BS, Ursekar MA, Koroshetz WJ (2001) Serial MR angiography and contrast-enhanced MRI in chickenpox-associated stroke. Neurology 56:815–817
Spiegel R, Miron D, Lumelsky D, Horovitz Y (2010) Severe meningoencephalitis due to late reactivation of Varicella-Zoster virus in an immunocompetent child. J Child Neurol 25:87–90
Sreenivasan N, Basit S, Wohlfahrt J, Pasternak B, Munch TN, Nielsen LP, Melbye M (2013) The short- and long-term risk of stroke after herpes zoster—a nationwide population-based cohort study. PLoS One 8:e69156
Steain M, Slobedman B, Abendroth A (2010) Experimental models to study varicella-zoster virus infection of neurons. Curr Top Microbiol Immunol 342:211–228
Steinlin M, Bigi S, Stojanovski B, Gajera J, Regenyi M, El-Koussy M, Mackay MT, Swiss Neuropediatric Stroke R (2017) Focal cerebral arteriopathy: do steroids improve outcome? Stroke 48:2375–2382
Sundstrom K, Weibull CE, Soderberg-Lofdal K, Bergstrom T, Sparen P, Arnheim-Dahlstrom L (2015) Incidence of herpes zoster and associated events including stroke–a population-based cohort study. BMC Infect Dis 15:488
Takahashi M (2004) Effectiveness of live varicella vaccine. Expert Opin Biol Ther 4:199–216
Tannous R, Grose C (2011) Calculation of the anterograde velocity of varicella-zoster virions in a human sciatic nerve during shingles. J Infect Dis 203:324–326
Thomas JE, Howard FM (1972) Segmental zoster paresis–a disease profile. Neurology 22:459–466
Tiah AL, Phelan E, McMenamin J, Webb D (2004) Childhood stroke following varicella infection. Ir Med J 97:120–121
Tubbs RS, Salter G, Wellons JC, Oakes WJ (2002) Blood supply of the human cervical sympathetic chain and ganglia. Eur J Morphol 40:283–288
Tucciarone L, Ballati G, Chiaramida N, Frangella E, Diamanti A (1992) Cerebral infarction in a child. A case report. Padiatr Padol 27:101–104
Ueno M, Oka A, Koeda T, Okamoto R, Takeshita K (2002) Unilateral occlusion of the middle cerebral artery after varicella-zoster virus infection. Brain Dev 24:106–108
Walker RJ, El-Gammal T, Allen MB (1973) Cranial arteritis associated with herpes zoster. Case report with angiographic findings. Radiology 107:109–110
Weller TH (1983) Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med 309:1434–1440
West SL, Newton RW, Baildam EM, Turner AJ, Arkwright PD (2006) Recurrent hemiplegia associated with cerebral vasculopathy following third trimester maternal herpes zoster infection. Dev Med Child Neurol 48:991–993
Wieting JM, Dykstra DD, Ruggiero MP, Robbins GB, Galusha K (1997) Central nervous system ischemia after varicella infection and desmopressin therapy for enuresis. J Am Osteopath Assoc 97:293–295
Wintermark M, Hills NK, Deveber GA, Barkovich AJ, Bernard TJ, Friedman NR, Mackay MT, Kirton A, Zhu G, Leiva-Salinas C, Hou Q, Fullerton HJ, Investigators V (2017) Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: results of the VIPS study. AJNR Am J Neuroradiol 38:2172–2179
Wirrell E, Hill MD, Jadavji T, Kirton A, Barlow K (2004) Stroke after varicella vaccination. J Pediatr 145:845–847
Yang Q, Chang A, Tong X, Merritt R (2021) Herpes zoster vaccine live and risk of stroke among medicare beneficiaries: a population-based cohort study. Stroke 52:1712–1721
Yang Q, George MG, Chang A, Tong X, Merritt R, Hong Y (2020) Effect of herpes zoster vaccine and antiviral treatment on risk of ischemic stroke. Neurology 95:e708–e717
Yaramis A, Herguner S, Kara B, Tatli B, Tuzun U, Ozmen M (2009) Cerebral vasculitis and obsessive-compulsive disorder following varicella infection in childhood. Turk J Pediatr 51:72–75
Yawn BP, Wollan PC, Nagel MA, Gilden D (2016) Risk of stroke and myocardial infarction after herpes zoster in older adults in a US community population. Mayo Clin Proc 91:33–44
Yilmaz K, Caliskan M, Akdeniz C, Aydinli N, Karabocuoglu M, Uzel N (1998) Acute childhood hemiplegia associated with chickenpox. Pediatr Neurol 18:256–261
Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM (2005) Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci U S A 102:6490–6495
Zerboni L, Arvin AM (2011) Investigation of varicella-zoster virus neurotropism and neurovirulence using SCID mouse-human DRG xenografts. J Neurovirol 17:570–577
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
Acknowledgements
We thank Lynn Enquist (Princeton University) for comments about his studies on the neuropathogenesis of pseudorabies virus.
Grant support. CG is supported by NIH grant AI153817; HJF is supported by NIH grant NS104094.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Grose, C., Shaban, A., Fullerton, H.J. (2021). Common Features Between Stroke Following Varicella in Children and Stroke Following Herpes Zoster in Adults. In: Arvin, A.M., Moffat, J.F., Abendroth, A., Oliver, S.L. (eds) Varicella-zoster Virus. Current Topics in Microbiology and Immunology, vol 438. Springer, Cham. https://doi.org/10.1007/82_2021_236
Download citation
DOI: https://doi.org/10.1007/82_2021_236
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15304-4
Online ISBN: 978-3-031-15305-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)