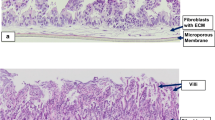
Abstract
The intestine is a complex organ formed of different types of cell distributed in different layers of tissue. To minimize animal experiments, for decades, researchers have been trying to develop in vitro/ex vivo systems able to mimic the cellular diversity naturally found in the gut. Such models not only help our understanding of the gut physiology but also of intestinal toxicity. This review describes the different systems used to evaluate the effects of drugs/contaminants on intestinal functions and compares their advantages and limitations. The comparison showed that the organotypic model is the best available model to perform intestinal toxicity studies, including on human tissues.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ajandouz EH, Berdah S, Moutardier V et al (2016) Hydrolytic Fate of 3/15-acetyldeoxynivalenol in humans: specific deacetylation by the small intestine and liver revealed using in vitro and ex vivo approaches. Toxins 8:E232. https://doi.org/10.3390/toxins8080232
Akbari P, Braber S, Gremmels H, Koelink PJ et al (2014) Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J 28:2414–2429. https://doi.org/10.1096/fj.13-238717
Alassane-Kpembi I, Gerez JR, Cossalter A-M et al (2017a) Intestinal toxicity of the type B trichothecene mycotoxin fusarenon-X: whole transcriptome profiling reveals new signaling pathways. Sci Rep 7:7530. https://doi.org/10.1038/s41598-017-07155-2
Alassane-Kpembi I, Puel O, Pinton P et al (2017b) Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch Toxicol 91:2677–2687. https://doi.org/10.1007/s00204-016-1902-9
Artursson P, Palm K, Luthman K (2001) Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 46:27–43. https://doi.org/10.1016/S0169-409X(00)00128-9
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69–70:1–18. https://doi.org/10.1016/j.addr.2014.02.008
Augeron C, Laboisse CL (1984) Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res 44:3961–3969
Autrup H, Barrett LA, Jackson FE et al (1978) Explant culture of human colon. Gastroenterology 74:1248–1257
Autrup H, Essigmann JM, Croy RG et al (1979) Metabolism of aflatoxin B1 and identification of the major aflatoxin B1-DNA adducts formed in cultured human bronchus and colon. Cancer Res 39:694–698
Autrup H, Harris CC, Schwartz RD et al (1980a) Metabolism of 1,2-dimethylhydrazine by cultured human colon. Carcinogenesis 1:375–380
Autrup H, Schwartz RD, Essigmann JM et al (1980b) Metabolism of aflatoxin B1, benzo[a]pyrene, and 1,2-dimethylhydrazine by cultured rat and human colon. Teratog Carcinog Mutagen 1:3–13
Autrup H, Harris CC, Wu SM et al (1984) Activation of chemical carcinogens by cultured human fetal liver, esophagus and stomach. Chem Biol Interact 50(1):15–25. https://doi.org/10.1016/0009-2797(84)90128-5
Bolte G, Beuermann K, Stern M (1997) The cell lines CACO-2, T84, and HT-29: models of enterocytic differenciation and function. J Pediatr Gastroenterol Nutr 24:473
Branka J, Vallette G, Jarry A et al (1997) Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology 112:1887–1894. https://doi.org/10.1053/gast.1997.v112.pm9178681
Browning TH, Trier JS (1969) Organ culture of mucosal biopsies of human small intestine. J Clin Invest 48:1423–1432. https://doi.org/10.1172/JCI106108
Cai T, Qi Y, Jergens A et al (2018) Effects of six common dietary nutrients on murine intestinal organoid growth. PLoS ONE 13:e0191517. https://doi.org/10.1371/journal.pone.0191517
Choi YJ, Seelbach MJ, Pu H, Eum SY et al (2010) Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect 118:976–981. https://doi.org/10.1289/ehp.0901751
Cohen GM, Grafstrom RC et al (1983) Metabolism of benzo(a)pyrene and 1-naphthol in cultured human tumorous and non tumorous colon. Cancer Res 1983 43:1312-5
Danielsen EM, Hansen GH (2017) Intestinal surfactant permeation enhancers and their interaction with enterocyte cell membranes in a mucosal explant system. Tissue Barriers 3:e1361900. https://doi.org/10.1080/21688370.2017.1361900
Del Regno M, Adesso S, Popolo A et al (2015) Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells. Toxicol Appl Pharmacol 285:118–127. https://doi.org/10.1016/j.taap.2015.04.002
Devreese M, Pasmans F, De Backer P, Croubels S (2013) An in vitro model using the IPEC-J2 cell line for efficacy and drug interaction testing of mycotoxin detoxifying agents. Toxicol In Vitro 27:157–163. https://doi.org/10.1016/j.tiv.2012.09.020
Devriese S, Van den Bossche L, Van Welden S et al (2017) T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem Cell Biol 148:85–93. https://doi.org/10.1007/s00418-017-1539-7
Dharmsathaphorn K, McRoberts JA, Mandel KG et al (1984) A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol 246:G204–G208. https://doi.org/10.1152/ajpgi.1984.246.2.G204
Drost J, van Jaarsveld RH, Ponsioen B et al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521:43–47. https://doi.org/10.1038/nature14415
Duizer E, Gilde AJ, Versantvoort CH, Groten JP (1999) Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines. Toxicol Appl Pharmacol 155:117–126. https://doi.org/10.1006/taap.1998.8589
Fatehullah A, Tan SH, Barker N (2016) Organoids as an in vitro model of human development and disease. Nat Cell Biol 18:246–254. https://doi.org/10.1038/ncb3312
Flora AD, Teel LD, Smith MA, Sinclair JF, Melton-Celsa AR, O’Brien AD (2013) Ricin crosses polarized human intestinal cells and intestines of ricin-gavaged mice without evident damage and then disseminates to mouse kidneys. PLoS ONE 8:e69706. https://doi.org/10.1371/journal.pone.0069706
Flynn TJ, Vohra SN (2018) Simultaneous determination of intestinal permeability and potential drug interactions of complex mixtures using Caco-2 cells and high-resolution mass spectrometry: studies with Rauwolfia serpentina extract. Chem Biol Interact 290:37–43. https://doi.org/10.1016/j.cbi.2018.05.006
Fogh J, Fogh JM, Orfeo T (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59:221–226
Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J (ed) Human tumor cells in vitro, 1st edn. pp 115–159
Foulke-Abel J, In J, Kovbasnjuk O et al (2014) Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med Maywood NJ 239:1124–1134. https://doi.org/10.1177/1535370214529398
García GR, Payros D, Pinton P, Dogi CA (2018) Intestinal toxicity of deoxynivalenol is limited by Lactobacillus rhamnosus RC007 in pig jejunum explants. Arch Toxicol 92:983–993. https://doi.org/10.1007/s00204-017-2083-x
Gerbe F, van Es JH, Makrini L et al (2011) Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192:767–780. https://doi.org/10.1083/jcb.201010127
Gerbe F, Sidot E, Smyth DJ et al (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529:226–230. https://doi.org/10.1038/nature16527
Gonneaud A, Jones C, Turgeon N, et al (2016). A SILAC-based method for quantitative proteomic analysis of intestinal organoids. Sci Rep 6:38195. https://doi.org/10.1038/srep38195, 30 Nov 2016.
Grabinger T, Luks L, Kostadinova F et al (2014) Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis 5:e1228. https://doi.org/10.1038/cddis.2014.183
Grenier B, Applegate TJ (2013) Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins 5:396–430. https://doi.org/10.3390/toxins5020396
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Hill DR, Huang S, Tsai YH, Spence JR, Young VB (2017) Real-time measurement of epithelial barrier permeability in human intestinal organoids. J Vis Exp 130:e56960. https://doi.org/10.3791/56960
Hurley BP, McCormick BA (2003) Translating tissue culture results into animal models: the case of Salmonella typhimurium. Trends Microbiol 11:562–569. https://doi.org/10.1016/j.tim.2003.10.002
Hurley BP, Pirzai W, Eaton AD et al (2016) An experimental platform using human intestinal epithelial cell lines to differentiate between hazardous and non-hazardous proteins. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 92:75–87. https://doi.org/10.1016/j.fct.2016.04.003
Hynds RE, Giangreco A (2013) Concise review: the relevance of human stem cell-derived organoid models for epithelial translational medicine. Stem Cells Dayt Ohio 31:417–422. https://doi.org/10.1002/stem.1290
Jensen-Jarolim E, Gajdzik L, Haberl I et al (1998) Hot spices influence permeability of human intestinal epithelial monolayers. J Nutr 128:577–581
Jung P, Sato T, Merlos-Suárez A et al (2011) Isolation and in vitro expansion of human colonic stem cells. Nat Med 17:1225–1227. https://doi.org/10.1038/nm.2470
Kaeffer B, Bénard C, Lahaye M et al (1999) Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med 65:527–531. https://doi.org/10.1055/s-1999-14009
Kaeffer B, Briollais S (1998) Primary culture of colonocytes in rotating bioreactor. In Vitro Cell Dev Biol—Anim 34:622–625. https://doi.org/10.1007/s11626-996-0008-8
Kaminski EJ (1953) A method for the preparation of isolated cells for use in high-resolution autoradiographic studies. Br J Radiol 26:378–380. https://doi.org/10.1259/0007-1285-26-307-378
Karve SS, Pradhan S, Ward DV, Weiss AA (2017) Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS ONE 12:e0178966. https://doi.org/10.1371/journal.pone.0178966
Kobayashi S, Shinohara M, Nagai T, Konishi Y (2013) Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci Biotechnol Biochem 77:2210–2217. https://doi.org/10.1271/bbb.130404
Kolars JC, Benedict P, Schmiedlin-Ren P, Watkins PB (1994) Aflatoxin B1-adduct formation in rat and human small bowel enterocytes. Gastroenterology 106:433–439. https://doi.org/10.1016/0016-5085(94)90602-5
Le TH, Alassane-Kpembi I, Oswald IP, Pinton P (2018) Analysis of the interactions between environmental and food contaminants, cadmium and deoxynivalenol, in different target organs. Sci Total Environ 622–623:841–848. https://doi.org/10.1016/j.scitotenv.2017.12.014
Leslie JL, Huang S, Opp JS et al (2015) Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83:138–145. https://doi.org/10.1128/IAI.02561-14
Loiseau N, Debrauwer L, Sambou T, Bouhet S et al (2007) Fumonisin B1 exposure and its selective effect on porcine jejunal segment: sphingolipids, glycolipids and trans-epithelial passage disturbance. Biochem Pharmacol 74:144–152. https://doi.org/10.1016/j.bcp.2007.03.031
Lu W, Rettenmeier E, Paszek M et al (2017) Crypt Organoid culture as an in vitro model in drug metabolism and cytotoxicity studies. Drug Metab Dispos Biol Fate Chem 45:748–754. https://doi.org/10.1124/dmd.117.075945
Lucioli J, Pinton P et al (2013) The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: interest of ex vivo models as an alternative to in vivo experiments. Toxicon 66:31–36. https://doi.org/10.1016/j.toxicon.2013.01.024
McCracken KW, Catá EM, Crawford CM et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404. https://doi.org/10.1038/nature13863
Neal MD, Sodhi CP, Jia H et al (2012) Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 287:37296–37308. https://doi.org/10.1074/jbc.M112.375881
Okada T, Narai A, Matsunaga S et al (2000) Assessment of the marine toxins by monitoring the integrity of human intestinal Caco-2 cell monolayers. Toxicol Vitro Int J Publ Assoc BIBRA 14:219–226
Park J-H, Choi A-J, Kim S-J et al (2016) AhR activation by 6-formylindolo[3,2-b]carbazole and 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of mouse intestinal epithelial cells. Environ Toxicol Pharmacol 43:44–53. https://doi.org/10.1016/j.etap.2016.02.007
Payros D, Dobrindt U, Martin P, Secher T, Bracarense AP, Boury M, Laffitte J, Pinton P, Oswald E, Oswald IP (2017) the food contaminant deoxynivalenol exacerbates the genotoxicity of gut microbiota. MBio 8:e00007–e00017. https://doi.org/10.1128/mBio.00007-17
Pierron A, Mimoun S, Murate LS et al (2016a) Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch Toxicol 90:2037–2046. https://doi.org/10.1007/s00204-015-1592-8
Pierron A, Mimoun S, Murate LS, Loiseau N (2016b) Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci Rep 6:29105. https://doi.org/10.1038/srep29105
Pinton P, Tsybulskyy D, Lucioli J et al (2012) Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol Sci 130:180–190. https://doi.org/10.1093/toxsci/kfs239
Pinton P, Graziani F, Pujol A, Nicoletti C et al (2015) Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol Nutr Food Res 59:1076–1087. https://doi.org/10.1002/mnfr.201500005
Powell RH, Behnke MS (2017) WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol Open 6:698–705. https://doi.org/10.1242/bio.021717
Quaroni A, Wands J, Trelstad RL, Isselbacher KJ (1979) Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80:248–265
Randall KJ, Turton J, Foster JR (2011) Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol Toxicol 27:267–284. https://doi.org/10.1007/s10565-011-9187-5
Razzuoli E, Mignone G, Lazzara F, Vencia W et al (2018) Impact of cadmium exposure on swine enterocytes. Toxicol Lett 287:92–99. https://doi.org/10.1016/j.toxlet.2018.02.005
Robert H, Payros D, Pinton P, Théodorou V, Mercier-Bonin M, Oswald IP (2017) Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J Toxicol Env Health: Part B: Crit Rev 20:249–275. https://doi.org/10.1080/10937404.2017.1326071
Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245:718–725
Rousset M (1986) The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie 68:1035–1040
Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. https://doi.org/10.1038/nature07935
Sessa M, Balestrieri ML, Ferrari G et al (2014) Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem 147:42–50. https://doi.org/10.1016/j.foodchem.2013.09.088
Siddiqui KM, Chopra DP (1984) Primary and long term epithelial cell cultures from human fetal normal colonic mucosa. In vitro 20:859–868
Sigal M, Rothenberg ME, Logan CY et al (2015) Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology 148(1392–1404):e21. https://doi.org/10.1053/j.gastro.2015.02.049
Simian M, Bissell MJ (2017) Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 216:31–40. https://doi.org/10.1083/jcb.201610056
Smith MC, Gheux A, Coton M, Madec S et al (2018) In vitro co-culture models to evaluate acute cytotoxicity of individual and combined mycotoxin exposures on Caco-2, THP-1 and HepaRG human cell lines. Chem Biol Interact 281:51–59. https://doi.org/10.1016/j.cbi.2017.12.004
Soucek K, Gajduskova P, Brazdova M, Hyzdalova M et al (2010) Fetal colon cell line FHC exhibits tumorigenic phenotype, complex karyotype, and TP53 gene mutation. Cancer Genet Cytogenet 197:107–116. https://doi.org/10.1016/j.cancergencyto.2009.11.009
Sun H, Chow EC, Liu S et al (2008) The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol 4:395–411. https://doi.org/10.1517/17425255.4.4.395
Thompson AA, Dilworth S, Hay RJ (1985) Isolation and culture of colonic epithelial cells in serum-free medium. J Tissue Culture Meth 9:117–122. https://doi.org/10.1007/bf01797782
Trowell OA (1959) The culture of mature organs in a synthetic medium. Exp Cell Res 16:118–147. https://doi.org/10.1016/0014-4827(59)90201-0
VanDussen KL, Marinshaw JM, Shaikh N et al (2015) Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64:911–920. https://doi.org/10.1136/gutjnl-2013-306651
Vázquez M, Devesa V, Vélez D (2015) Characterization of the intestinal absorption of inorganic mercury in Caco-2 cells. Toxicol Vitro Int J Publ Assoc BIBRA 29:93–102. https://doi.org/10.1016/j.tiv.2014.09.013
Walton DG, Acton AB, Stich HF (1984) DNA repair synthesis following exposure to chemical mutagens in primary liver, stomach, and intestinal cells isolated from rainbow trout. Cancer Res 44:1120–1121
Wang Y, DiSalvo M, Gunasekara DB et al (2017) Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol 4(165–182):e7. https://doi.org/10.1016/j.jcmgh.2017.02.011
Wilson TH, Wiseman G (1954) The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol 123:116–125
Yin X, Farin HF, van Es JH et al (2014) Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11:106–112. https://doi.org/10.1038/nmeth.2737
Young M, Reed KR (2016) Organoids as a model for colorectal cancer. Curr Colorectal Cancer Rep 2016(12):281–287
Zhou X, Li Y, Li C (2017) Autophagy plays a positive role in zinc-induced apoptosis in intestinal porcine epithelial cells. Toxicol in vitro 44:392–402. https://doi.org/10.1016/j.tiv.2017.08.006
Acknowledgements
This research was partially supported by the French National Research Agency (projects CaDON ANR-15-CE21-0001, Fumolip ANR-16-CE21-0003, and ExpoMycoPig17-CARN-012-001). The authors thank D. Goodfellow for English editing.
Model | Advantages | Limitations |
|---|---|---|
Primary cells | - Close to reality - Simple model enable easier identification of molecular events involved in toxicity | - Isolation of primary human cells requires access to human tissue - Short lifespan limits their use to very short-term studies - Lack of standardization |
Immortalized cells | - Can be maintained for a long time in culture (allowing long-term exposure experiments) - Simple model enabling easier identification of molecular events involved in toxic mechanisms - Low variability of results obtained with this model | - Not all cell types present in the epithelium can be isolated and cultured - Immortalized cells are transformed |
Tumor cell lines | - Commercially available - Can be maintained for a long time in culture (allowing long-term exposure experiments) - Enable separate evaluation of the effects of toxins on major cell types present in the epithelium when co-cultured (e.g. enterocytes and goblet cells) | - Display genetic alterations - Far from the physiological state |
Explants | - Contain all cell types normally present in the epithelium, in the correct proportions - May also contain other cells types such as immune cells, myofibroblasts, and cells from the enteric nerve system (ENS) (neurons and enteric glial cells) | - Limited survival time (48 h) - Access to apical compartment only when mounted in a Ussing chamber - Require access to human intestinal tissue - Higher variability due to inter-individual differences in response to toxins |
Organoids | - Contain most of the cell types normally present in the epithelium (but with no guarantee of them being in the correct proportion) - Can be maintained for a long time in culture, allowing long-term exposure to toxins - Commercially available (low variability) - Derived from healthy or diseased individuals | - Contain only epithelial cells (absence of immune or ENS cells except if co-cultured with other cells) |
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maresca, M., Pinton, P., Ajandouz, E.H., Menard, S., Ferrier, L., Oswald, I.P. (2018). Overview and Comparison of Intestinal Organotypic Models, Intestinal Cells, and Intestinal Explants Used for Toxicity Studies. In: Bagnoli, F., Rappuoli, R. (eds) Three Dimensional Human Organotypic Models for Biomedical Research. Current Topics in Microbiology and Immunology, vol 430. Springer, Cham. https://doi.org/10.1007/82_2018_142
Download citation
DOI: https://doi.org/10.1007/82_2018_142
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62451-4
Online ISBN: 978-3-030-62452-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)