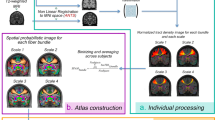
Abstract
We present an automated approach to the problem of connectivity-based partitioning of brain structures using diffusion imaging. White-matter fibres connect different areas of the brain, allowing them to interact with each other. Diffusion-tensor MRI measures the orientation of white-matter fibres in vivo, allowing us to perform connectivity-based partitioning non-invasively. Our new approach leverages atlas-based segmentation to automate anatomical labeling of the cortex. White-matter connectivities are inferred using a probabilistic tractography algorithm that models crossing pathways explicitly. The method is demonstrated with the partitioning of the corpus callosum of eight healthy subjects.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Pierpaoli, C., Jezzard, P., Basser, P.J., Barnett, A., Chiro, G.D.: Diffusion tensor imaging of the human brain. Radiology 201, 637–648 (1996)
Mori, S., van Zijl, P.C.M.: Fiber tracking: principles and strategies – a technical review. NMR in Biomed. 15, 468–480 (2002)
Huang, H., Zhang, J., Jiang, H., Wakana, S., Poetscher, L., Miller, M.I., van Zijl, P.C.M., Hillis, A.E., Wytike, R., Mori, S.: DTI tractography based parcellation of white matter: Application to the mid-sagittal morphology of corpus callosum. NeuroImage (2004), doi:10.1016/j.neuroimage.2005.01.019
Gee, J.C., Zhang, H., Dubb, A., Avants, B.A., Yushkevich, P.A., Duda, J.T.: Anatomy-based visualizations of diffusion tensor images of brain white matter. In: Welckert, J., Hagen, H. (eds.) Visualization and Image Processing of Tensor Fields. Springer, Heidelberg (in Press)
Johansen-Berg, H., Behrens, T.E., Sillery, E., Ciccarelli, O., Thompson, A.J., Smith, S.M., Matthews, P.M.: Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Medical Image Analysis 15, 31–39 (2005)
Behrens, T.E., Johansen-Berg, H., Woolrich, M.W., Smith, S.M., Wheeler-Kingshott, C.A., Boulby, P.A., Barker, G.J., Sillery, E.L., Sheehan, K., Ciccarelli, O., Thompson, A.J., Brady, J.M., Matthews, P.M.: Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757 (2003)
Basser, P.J., Mattiello, J., Le Bihan, D.: MR diffusion tensor specstroscopy and imaging. Biophys J 66, 259–267 (1994)
Basser, P.J., Pierpaoli, C.: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. JMR B111, 209–219 (1996)
Alexander, D.C.: An introduction to computational diffusion MRI: the diffusion tensor and beyond. In: Weichert, J., Hagen, H. (eds.) Visualization and Image Processing of Tensor Fields. Springer, Heidelberg (2005)
Stieltjes, B., Kaufmann, W.E., van Zijl, P.C.M., Fredericksen, K., Pearlson, G.D., Solaiyappan, M., Mori, S.: Diffusion tensor imaging and axonal tracking in the human brainstem. NeuroImage 14, 723–735 (2001)
Parker, G.J.M., Wheeler-Kingshott, C.A.M., Haroon, H.A.: A framework for a streamline-based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. JMRI 18, 242–254 (2003)
Dubb, A., Yushkevich, P.A., Xie, Z., Gur, R.C., Gur, R.E., Gee, J.C.: Regional structural characterization of the brain of schizophrenia patients. In: Barillot, C., Haynor, D.R., Hellier, P. (eds.) MICCAI 2004. LNCS, vol. 3217, pp. 688–695. Springer, Heidelberg (2004)
Avants, B.A., Schoenemann, P.T., Gee, J.C.: Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. MIA (in press)
Parker, G.J.M., Alexander, D.C.: Probabilistic monte-carlo based mapping of cerebral connections utilising whole-brain crossing fibre information. In: Proc. IPMI, pp. 684–695 (2003)
Alexander, D.C., Barker, G.J., Arridge, S.R.: Detection and modeling of non-gaussian apparent diffusion coefficient profiles in human brain data. MRM 48, 331–340 (2002)
Cook, P.A., Alexander, D.C., Parker, G.J.M.: Modelling noise-induced fibre-orientation error in diffusion-tensor MRI. In: Proc. ISBI, pp. 332–336 (2004)
Mardia, K.V., Jupp, P.E.: Directional Statistics. Wiley, Chichester (2000)
Virgilio, G.D., Clarke, S.: Direct interhemispheric visual input to human speech areas. HMP 5, 347–354 (1997)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2005 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Cook, P.A. et al. (2005). An Automated Approach to Connectivity-Based Partitioning of Brain Structures. In: Duncan, J.S., Gerig, G. (eds) Medical Image Computing and Computer-Assisted Intervention – MICCAI 2005. MICCAI 2005. Lecture Notes in Computer Science, vol 3749. Springer, Berlin, Heidelberg. https://doi.org/10.1007/11566465_21
Download citation
DOI: https://doi.org/10.1007/11566465_21
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-29327-9
Online ISBN: 978-3-540-32094-4
eBook Packages: Computer ScienceComputer Science (R0)