Abstract
Epigenetics is considered as a dynamic interface between the genome and the environment and encompasses different mechanisms that regulate chromatin dynamics and gene expression by DNA methylation, histone post-translational modifications, histone variants, non-coding RNAs, genome topology, among others. The molecular bases of ageing are multi factorial, and the change in the patterns of epigenetic marks is emerging as a hallmark of this physiological process in several tissues. Research in epigenetics has led to the identification of non-invasive biomarkers and therapeutic strategies in age-associated diseases. Furthermore, epigenetic modifications are reversible and might be preventable, making them attractive therapeutic targets to assure a healthy-ageing process. In the present chapter, we aim to provide an overview about the current research focused on the participation of epigenetic mechanisms in ageing health and disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AMPK:
-
AMP-activated protein kinase
- circRNA:
-
Circular RNA
- CTCF:
-
CCCTC-binding factor
- DNMT:
-
DNA methyltransferase
- ES:
-
Estrogen receptor
- EWAS:
-
Epigenome-wide association study
- EZH2:
-
Enhancer of zeste 2 polycomb repressive complex 2 subunit
- H2BK20:
-
Lysine 20 of histone 2B
- H3K18Ac:
-
Histone 3 acetylated at lysine 18
- H3K27me:
-
Histone 3 methylated at lysine 27
- H3K4me:
-
Histone 3 methylated at lysine 4
- H3K9Ac:
-
Histone 3 acetylated at lysine 9
- H3K9me:
-
Histone 3 methylated at lysine 9
- H4K12:
-
Lysine 12 of histone 4
- H4K16:
-
Lysine 16 of histone 4
- H4K20me:
-
Histone 4 methylated at lysine 20
- HAT:
-
Histone acetyl transferases
- HDAC:
-
Histone deacetylases
- HGS:
-
Hutchinson-Gilford syndrome
- HOTAIR:
-
HOX antisense intergenic RNA
- HP1:
-
Heterochromatin protein 1
- KMT1/Suv39:
-
Histone lysine methyltransferase family encoded by the EHM1 gene
- LADs:
-
Lamina-associated domains
- lncRNA:
-
Long non-coding RNA
- miRNA:
-
MicroRNAs
- ncRNA:
-
Non-coding RNA
- NL:
-
Nuclear lamina
- NOS2:
-
Nitric oxide synthase
- PD:
-
Parkinson’s disease
- PRC2:
-
Polycomb repressive complex 2
- PTGS2:
-
Cyclooxygenase 2
- PTM:
-
Post-translational modification
- RA:
-
Rheumatoid arthritis
- RBBP4:
-
RB Binding Protein 4
- RISC:
-
RNA-induced silencing complex
- ROS:
-
Reactive oxygen species
- SAHF:
-
Senescence-associated heterochromatic foci
- SALNR:
-
Senescence-associated lncRNA
- SMCs:
-
Smooth muscle cells
- T2D:
-
Type 2 diabetes
- TADs:
-
Topologically associated domains
- TERF2:
-
Telomeric repeat binding factor 2
- TOR:
-
Target of rapamycin
- WHO:
-
World Health Organization
- XIST:
-
X-inactive-specific transcript
References
Brennan-Olsen SL, Cook S, Leech MT, Bowe SJ, Kowal P, Naidoo N, et al. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet Disord. 2017;18(1):271.
Andersen-Ranberg K, Christensen K, Jeune B, Skytthe A, Vasegaard L, Vaupel JW. Declining physical abilities with age: a cross-sectional study of older twins and centenarians in Denmark. Age Ageing. 1999;28(4):373–7.
Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, et al. The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychol Sci. 2014;25(1):103–12.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194.
Mc Auley MT, Guimera AM, Hodgson D, Mcdonald N, Mooney KM, Morgan AE, et al. Modelling the molecular mechanisms of aging. Biosci Rep. 2017;37(1):BSR20160177.
Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13(2):97–109.
Molina-Serrano D, Kyriakou D, Kirmizis A. Histone modifications as an intersection between diet and longevity. Front Gen. 2019;10:192.
Milagro FI, Mansego ML, De Miguel C, Martínez JA. Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med. 2013;34(4):782–812.
Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Ann N Y Acad Sci. 2002;981:61–81.
Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14(3):204–20.
Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21.
Xiao FH, Wang HT, Kong QP. Dynamic DNA methylation during aging: a “prophet” of age-related outcomes. Front Genet. 2019;10:107.
Wilson VL, Jones PA. DNA methylation decreases in aging but not in immortal cells. Science. 1983;220(4601):1055–7.
Jintaridth P, Mutirangura A. Distinctive patterns of age-dependent hypomethylation in interspersed repetitive sequences. Physiol Genomics. 2010;41(2):194–200.
Bollati V, Tarantini L, Baccarelli A, Schwartz J, Wright R, Litonjua A, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130(4):234–9.
Xiao F-H, Kong Q-P, Perry B, He Y-H. Progress on the role of DNA methylation in aging and longevity. Brief Funct Genomics. 2016;15(6):454–9.
Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14(6):924–32.
Ishimi Y, Kojima M, Takeuchi F, Miyamoto T, Yamada MA, Hanaoka F. Changes in chromatin structure during aging of human skin fibroblasts. Exp Cell Res. 1987;169(2):458–67.
Dell’Orco R. Research WW-B and biophysical, 1982 U. Micrococcal nuclease and DNase I digestion of DNA from aging human diploid cells. Biochem Biophys Res Commun. 1982;107(1):117–22.
Gentilini D, Garagnani P, Pisoni S, Bacalini MG, Calzari L, Mari D, et al. Stochastic epigenetic mutations (DNA methylation) increase exponentially in human aging and correlate with X chromosome inactivation skewing in females. Aging (Albany NY). 2015;7(8):568–78.
Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci. 2012;109(26):10522–7.
Bacalini MG, Boattini A, Gentilini D, Giampieri E, Pirazzini C, Giuliani C, et al. A meta-analysis on age-associated changes in blood DNA methylation: results from an original analysis pipeline for Infinium 450k data. Aging (Albany NY). 2015;7(2):97–109.
Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20(3):332–40.
Reynolds LM, Taylor JR, Ding J, Lohman K, Johnson C, Siscovick D, et al. Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat Commun. 2014;5:5366.
Mcclay JL, Aberg KA, Clark SL, Nerella S, Kumar G, Xie LY, et al. A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum Mol Genet. 2014;23(5):1175–85.
Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12(4):413–25.
Bell JT, Tsai PC, Yang TP, Pidsley R, Nisbet J, Glass D, et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012;8(4):e1002629.
Bocker MT, Hellwig I, Breiling A, Eckstein V, Ho AD, Lyko F. Genome-wide promoter DNA methylation dynamics of human hematopoietic progenitor cells during differentiation and aging. Blood. 2011;117(19):e182–9.
Longo DL, Traynor BJ, Cookson MR, Singleton AB, Hernandez DG, Gibbs JR, et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 2011;20(6):1164–72.
Grönniger E, Weber B, Heil O, Peters N, Stäb F, Wenck H, et al. Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genet. 2010;6(5):6.
Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, et al. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010;20(4):434–9.
Day K, Waite LL, Thalacker-Mercer A, West A, Bamman MM, Brooks JD, et al. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biol. 2013;14(9):R102.
Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CPG island context. PLoS Genet. 2009;5(8):e1000602.
Slieker RC, Relton CL, Gaunt TR, Slagboom PE, Heijmans BT. Age-related DNA methylation changes are tissue-specific with ELOVL2 promoter methylation as exception. Epigenetics Chromatin. 2018;11(1):25.
Griñán-Ferré C, Izquierdo V, Otero E, Puigoriol-Illamola D, Corpas R, Sanfeliu C, et al. Environmental enrichment improves cognitive deficits, AD hallmarks and epigenetic alterations presented in 5xFAD mouse model. Front Cell Neurosci. 2018;12:224.
Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol. 2015 Jan;13:7.
Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18(4):447–76.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115.
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda SV, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–67.
Bekaert B, Kamalandua A, Zapico SC, Van De Voorde W, Decorte R. Improved age determination of blood and teeth samples using a selected set of DNA methylation markers. Epigenetics. 2015;10(10):922–30.
Purohit G, Mukherjee AK, Sharma S, Chowdhury S. Extratelomeric binding of the telomere binding protein TRF2 at the PCGF3 promoter is G-quadruplex motif-dependent. Biochemistry. 2018;57(16):2317–24.
Mu W, Starmer J, Yee D, Magnuson T. EZH2 variants differentially regulate polycomb repressive complex 2 in histone methylation and cell differentiation. Epigenetics Chromatin. 2018;11(1):71.
Wang X, Goodrich KJ, Gooding AR, Naeem H, Archer S, Paucek RD, et al. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol Cell. 2017;65(6):1056–1067.e5.
Malousi A, Andreou AZ, Georgiou E, Tzimagiorgis G, Kovatsi L, Kouidou S. Age-dependent methylation in epigenetic clock CpGs is associated with G-quadruplex, co-transcriptionally formed RNA structures and tentative splice sites. Epigenetics. 2018;13(8):808–21.
Fransquet PD, Wrigglesworth J, Woods RL, Ernst ME, Ryan J. The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clin Epigenetics. 2019;11(1):62.
El-Maarri O, Becker T, Junen J, Manzoor SS, Diaz-Lacava A, Schwaab R, et al. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum Genet. 2007;122(5):505–14.
Peterson VS. Sex matters. Int Fem J Polit. 2014;16:389–409.
Singmann P, Shem-Tov D, Wahl S, Grallert H, Fiorito G, Shin SY, et al. Characterization of whole-genome autosomal differences of DNA methylation between men and women. Epigenetics Chromatin. 2015;8(1):43.
Cotton AM, Price EM, Jones MJ, Balaton BP, Kobor MS, Brown CJ. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum Mol Genet. 2015;24(6):1528–39.
Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019;195:172–85.
Khurana I, Kaspi A, Ziemann M, Block T, Connor T, Spolding B, et al. DNA methylation regulates hypothalamic gene expression linking parental diet during pregnancy to the offspring’s risk of obesity in Psammomys obesus. Int J Obes. 2016;40(7):1079–88.
Kaspi A, Khurana I, Ziemann M, Connor T, Spolding B, Zimmet P, et al. Diet during pregnancy is implicated in the regulation of hypothalamic RNA methylation and risk of obesity in offspring. Mol Nutr Food Res. 2018;62(14):e1800134.
Ramaswamy A, Ioshikhes I. Dynamics of modeled oligonucleosomes and the role of histone variant proteins in nucleosome organization. Adv Protein Chem Struct Biol. 2013;90:119–49.
Cutter AR, Hayes JJ. A brief review of nucleosome structure. FEBS Lett. 2015;589:2914–22.
Song S, Johnson FB. Epigenetic mechanisms impacting aging: a focus on histone levels and telomeres. Genes (Basel). 2018;9(4):E201.
Adams PD, Ivanov A, Pawlikowski J, Manoharan I, Van TJ, Nelson DM, et al. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202(1):129–43.
O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17(10):1218–25.
Hauer MH, Seeber A, Singh V, Thierry R, Sack R, Amitai A, et al. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat Struct Mol Biol. 2017;24(2):99–107.
Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science (80). 2006;312(5776):1059–63.
Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci. 2006;103(23):8703–8.
Ucar D, Márquez EJ, Chung C-H, Marches R, Rossi RJ, Uyar A, et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J Exp Med. 2017;214(10):3123–44.
Talbert PB, Henikoff S. Histone variants on the move: substrates for chromatin dynamics. Nat Rev Mol Cell Biol. 2017;18(2):115–26.
Rogakou EP, Sekeri-Pataryas KE. Histone variants of H2A and H3 families are regulated during in vitro aging in the same manner as during differentiation. Exp Gerontol. 1999;34(6):741–54.
Correia-Melo C, Jurk D, Passos JF. Robust multiparametric assessment of cellular senescence. Methods Mol Biol. 2013;965:409–19.
Maze I, Wenderski W, Noh K-M, Bagot RC, Tzavaras N, Purushothaman I, et al. Critical role of histone turnover in neuronal transcription and plasticity. Neuron. 2015;87(1):77–94.
Contrepois K, Coudereau C, Benayoun BA, Schuler N, Roux PF, Bischof O, et al. Histone variant H2A. J accumulates in senescent cells and promotes inflammatory gene expression. Nat Commun. 2017;8:14995.
Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–80.
Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121–41.
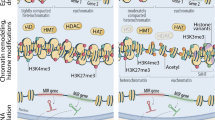
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921.
Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354–7.
Benevolenskaya EV. Histone H3K4 demethylases are essential in development and differentiation. Biochem Cell Biol. 2007;85(4):435–43.
Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8(4):307–18.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37.
Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459(7248):802–7.
Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466(7304):383–7.
Li L, Greer C, Eisenman RN, Secombe J. Essential functions of the histone demethylase Lid. PLoS Genet. 2010;6(11):e1001221.
Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ. Polycomb repressive complex 2 and trithorax modulate drosophila longevity and stress resistance. Proc Natl Acad Sci. 2010;107(1):169–74.
Maures TJ, Greer EL, Hauswirth AG, Brunet A. The H3K27 demethylase UTX-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell. 2011;10(6):980–90.
Ryder DJ, Judge SM, Beharry AW, Farnsworth CL, Silva JC, Judge AR. Identification of the acetylation and ubiquitin-modified proteome during the progression of skeletal muscle atrophy. PLoS One. 2015;10(8):e0136247.
Rodova M, Lu Q, Li Y, Woodbury BG, Crist JD, Gardner BM, et al. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J Bone Miner Res. 2011;26(8):1974–86.
Rao RA, Ketkar AA, Kedia N, Krishnamoorthy VK, Lakshmanan V, Kumar P, et al. KMT1 family methyltransferases regulate heterochromatin–nuclear periphery tethering via histone and non-histone protein methylation. EMBO Rep. 2019;20(5):e43260.
Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–16.
Chandra T, Kirschner K, Thuret J-Y, Pope BD, Ryba T, Newman S, et al. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol Cell. 2012;47(2):203–14.
Zhang W, Li J, Suzuki K, Qu J, Wang P, Zhou J, et al. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015;348(6239):1160–3.
Succoio M, Comegna M, D’Ambrosio C, Scaloni A, Cimino F, Faraonio R. Proteomic analysis reveals novel common genes modulated in both replicative and stress-induced senescence. J Proteomics. 2015;128:18–29.
Kurz DJ, Payeli S, Greutert H, Briand Schumacher S, Lüscher TF, Tanner FC. Epigenetic regulation of tissue factor inducibility in endothelial cell senescence. Mech Ageing Dev. 2014;140:1–9.
Ahuja G, Bartsch D, Yao W, Geissen S, Frank S, Aguirre A, et al. Loss of genomic integrity induced by lysosphingolipid imbalance drives ageing in the heart. EMBO Rep. 2019;20(4):e47407.
Pavlopoulos E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, et al. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med. 2013;5(200):200ra115.
Engel N, Mahlknecht U. Aging and anti-aging: unexpected side effects of everyday medication through sirtuin1 modulation. Int J Mol Med. 2008;21(2):223–32.
Cho S-H, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J Neurosci. 2015;35(2):807–18.
Mohamad Nasir NF, Zainuddin A, Shamsuddin S. Emerging roles of sirtuin 6 in Alzheimer’s disease. J Mol Neurosci. 2018;64(2):157–61.
Narayan P, Dragunow M. Alzheimer’s disease and histone code alterations. Adv Exp Med Biol. 2017;978:321–36.
Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28(10):1057–68.
Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76(12):3446–50.
Lin RK, Wang YC. Dysregulated transcriptional and post-translational control of DNA methyltransferases in cancer. Cell Biosci. 2014;4(1):46.
Hałasa M, Wawruszak A, Przybyszewska A, Jaruga A, Guz M, Kałafut J, et al. H3K18Ac as a marker of cancer progression and potential target of anti-cancer therapy. Cells. 2019;8(5):485.
Rai TS, Cole JJ, Nelson DM, Dikovskaya D, Faller WJ, Vizioli MG, et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of Neoplasia. Genes Dev. 2014;28(24):2712–25.
Pérez RF, Tejedor JR, Bayón GF, Fernández AF, Fraga MF. Distinct chromatin signatures of DNA hypomethylation in aging and cancer. Aging Cell. 2018;17(3):e12744.
Chen D, Kerr C. The epigenetics of stem cell aging comes of age. Trends Cell Biol. 2019;29(7):563–8.
Fernández AF, Bayón GF, Urdinguio RG, Toraño EG, García MG, Carella A, et al. H3K4me1 marks DNA regions hypomethylated during aging in human stem and differentiated cells. Genome Res. 2015;25(1):27–40.
Chen Z, Chang WY, Etheridge A, Strickfaden H, Jin Z, Palidwor G, et al. Reprogramming progeria fibroblasts re-establishes a normal epigenetic landscape. Aging Cell. 2017;16(4):870–87.
Van der Kraan PM, Van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr Cartil. 2012;20(3):223–32.
El Mansouri FE, Chabane N, Zayed N, Kapoor M, Benderdour M, Martel-Pelletier J, et al. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011;63(1):168–79.
Iliopoulos D, Malizos KN, Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis. 2007;66(12):1616–21.
Portal-Núñez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1–10.
Kim K, Park YS, Im GI. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J Bone Miner Res. 2013;28(5):1050–60.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Danka Mohammed CP, Park JS, Nam HG, Kim K. MicroRNAs in brain aging. Mech Ageing Dev. 2017;168:3–9.
Lin X, Zhan J-K, Wang Y-J, Tan P, Chen Y-Y, Deng H-Q, et al. Function, role, and clinical application of microRNAs in vascular aging. Biomed Res Int. 2016;2016:6021394.
Victoria B, Nunez Lopez YO, Masternak MM. MicroRNAs and the metabolic hallmarks of aging. Mol Cell Endocrinol. 2017;455:131–47.
Iwakawa H, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015;25(11):651–65.
Gomez-Verjan JC, Vazquez-Martinez ER, Rivero-Segura NA, Medina-Campos RH. The RNA world of human ageing. Hum Genet. 2018;137(11–12):865–79.
Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–77.
Kumar S, Vijayan M, Bhatti JS, Reddy PH. MicroRNAs as peripheral biomarkers in aging and age-related diseases. Prog Mol Biol Transl Sci. 2017;146:47–94.
Smith-Vikos T, Liu Z, Parsons C, Gorospe M, Ferrucci L, Gill TM, et al. A serum miRNA profile of human longevity: findings from the Baltimore Longitudinal Study of Aging (BLSA). Aging (Albany NY). 2016;8(11):2971–87.
Olivieri F, Albertini MC, Orciani M, Ceka A, Cricca M, Procopio AD, et al. DNA damage response (DDR) and senescence: shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015;6(34):35509–21.
Rani A, O’Shea A, Ianov L, Cohen RA, Woods AJ, Foster TC. miRNA in circulating microvesicles as biomarkers for age-related cognitive decline. Front Aging Neurosci. 2017;9:323.
Hekmatimoghaddam S, Dehghani Firoozabadi A, Zare-Khormizi MR, Pourrajab F. Sirt1 and Parp1 as epigenome safeguards and microRNAs as SASP-associated signals, in cellular senescence and aging. Ageing Res Rev. 2017;40:120–41.
Huh CJ, Zhang B, Victor MB, Dahiya S, Batista LF, Horvath S, et al. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife. 2016;5:e18648.
Gudmundsson H, Gudbjartsson DF, Kong A, Gudbjartsson H, Frigge M, Gulcher JR, et al. Inheritance of human longevity in Iceland. Eur J Hum Genet. 2000;8(10):743–9.
ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N, et al. Genome-wide miRNA signatures of human longevity. Aging Cell. 2012;11(4):607–16.
Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB, Evans MK. microRNA expression patterns reveal differential expression of target genes with age. PLoS One. 2010;5(5):e10724. Blagosklonny MV, editor
Gombar S, Jung H, Dong F, Calder B, Atzmon G, Barzilai N, et al. Comprehensive microRNA profiling in B-cells of human centenarians by massively parallel sequencing. BMC Genomics. 2012;13(1):353.
Serna E, Gambini J, Borras C, Abdelaziz KM, Belenguer A, Sanchis P, et al. Centenarians, but not octogenarians, up-regulate the expression of microRNAs. Sci Rep. 2012;2(1):961.
Balzano F, Deiana M, Dei Giudici S, Oggiano A, Pasella S, Pinna S, et al. MicroRNA expression analysis of centenarians and rheumatoid arthritis patients reveals a common expression pattern. Int J Med Sci. 2017;14(7):622–8.
Borrás C, Serna E, Gambini J, Inglés M, Vina J. Centenarians maintain miRNA biogenesis pathway while it is impaired in octogenarians. Mech Ageing Dev. 2017;168:54–7.
Crocco P, Montesanto A, Passarino G, Rose G. Polymorphisms falling within putative miRNA target sites in the 3′UTR region of SIRT2 and DRD2 genes are correlated with human longevity. J Gerontol Ser A Biol Sci Med Sci. 2016;71(5):586–92.
Lafferty-Whyte K, Cairney CJ, Jamieson NB, Oien KA, Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792(4):341–52.
Noren Hooten N, Fitzpatrick M, Wood WH, De S, Ejiogu N, Zhang Y, et al. Age-related changes in microRNA levels in serum. Aging (Albany NY). 2013;5(10):725–40.
Pourrajab F, Vakili Zarch A, Hekmatimoghaddam S, Zare-Khormizi MR. The master switchers in the aging of cardiovascular system, reverse senescence by microRNA signatures; as highly conserved molecules. Prog Biophys Mol Biol. 2015;119(2):111–28.
Rippo MR, Olivieri F, Monsurrò V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–63.
Chan SY, Zhang Y-Y, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–84.
Lauri A, Pompilio G, Capogrossi MC. The mitochondrial genome in aging and senescence. Ageing Res Rev. 2014;18:1–15.
Bandiera S, Rüberg S, Girard M, Cagnard N, Hanein S, Chrétien D, et al. Nuclear outsourcing of RNA interference components to human mitochondria. Pfeffer S, editor. PLoS One. 2011;6(6):e20746.
Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–10.
Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299(1):E110–6.
Guo Y, Li P, Gao L, Zhang J, Yang Z, Bledsoe G, et al. Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell. 2017;16(4):837–46.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4.
Capri M, Olivieri F, Lanzarini C, Remondini D, Borelli V, Lazzarini R, et al. Identification of miR-31-5p, miR-141-3p, miR-200c-3p, and GLT1 as human liver aging markers sensitive to donor-recipient age-mismatch in transplants. Aging Cell. 2017;16(2):262–72.
Toutfaire M, Bauwens E, Debacq-Chainiaux F. The impact of cellular senescence in skin ageing: a notion of mosaic and therapeutic strategies. Biochem Pharmacol. 2017;142:1–12.
Reddy PH, Williams J, Smith F, Bhatti JS, Kumar S, Vijayan M, et al. MicroRNAs, aging, cellular senescence, and Alzheimer’s disease. Prog Mol Biol Transl Sci. 2017;146:127–71.
Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol. 2012;235(2):491–6.
Tan L, Yu J-T, Liu Q-Y, Tan M-S, Zhang W, Hu N, et al. Circulating miR-125b as a biomarker of Alzheimer’s disease. J Neurol Sci. 2014;336(1–2):52–6.
Hadar A, Milanesi E, Walczak M, Puzianowska-Kuźnicka M, Kuźnicki J, Squassina A, et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s disease. Sci Rep. 2018;8(1):8465.
Barnes PJ. Senescence in COPD and its comorbidities. Annu Rev Physiol. 2017;79(1):517–39.
Brown DM, Goljanek-Whysall K. microRNAs: modulators of the underlying pathophysiology of sarcopenia? Ageing Res Rev. 2015;24(Pt B):263–73.
Marini F, Cianferotti L, Brandi M. Epigenetic mechanisms in bone biology and osteoporosis: can they drive therapeutic choices? Int J Mol Sci. 2016;17(8):1329.
Choi SW, Lee JY, Kang K-S. miRNAs in stem cell aging and age-related disease. Mech Ageing Dev. 2017;168:20–9.
Okada M, Kim HW, Matsu-ura K, Wang Y-G, Xu M, Ashraf M. Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells. 2016;34(1):148–59.
Neault M, Couteau F, Bonneau É, De Guire V, Mallette FA. Molecular regulation of cellular senescence by microRNAs: implications in cancer and age-related diseases. Int Rev Cell Mol Biol. 2017;334:27–98.
Saeidimehr S, Ebrahimi A, Saki N, Goodarzi P, Rahim F. MicroRNA-based linkage between aging and cancer: from Epigenetics View Point. Cell J. 2016;18(2):117–26.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89.
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46.
Jain S, Thakkar N, Chhatai J, Pal Bhadra M, Bhadra U. Long non-coding RNA: functional agent for disease traits. RNA Biol. 2017;14(5):522–35.
Wu C-L, Wang Y, Jin B, Chen H, Xie B-S, Mao Z-B. Senescence-associated long non-coding RNA (SALNR) delays oncogene-induced senescence through NF90 regulation. J Biol Chem. 2015;290(50):30175–92.
Abdelmohsen K, Panda A, Kang M-J, Xu J, Selimyan R, Yoon J-H, et al. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell. 2013;12(5):890–900.
Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, Srinivasan R, et al. Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res. 2008;68(16):6797–802.
Abdelmohsen K, Panda AC, De S, Grammatikakis I, Kim J, Ding J, et al. Circular RNAs in monkey muscle: age-dependent changes. Aging (Albany NY). 2015;7(11):903–10.
Yoon J-H, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939.
Grammatikakis I, Panda AC, Abdelmohsen K, Gorospe M. Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY). 2014;6(12):992–1009.
Luo Q, Chen Y. Long noncoding RNAs and Alzheimer’s disease. Clin Interv Aging. 2016;11:867–72.
Chen L-L, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–8.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626–41.
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. Preiss T, editor. PLoS One. 2012;7(2):e30733.
Cortés-López M, Miura P. Emerging functions of circular RNAs. Yale J Biol Med. 2016;89(4):527–37.
Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806.
Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(9):e1003777.
Han Y-N, Xia S-Q, Zhang Y-Y, Zheng J-H, Li W. Circular RNAs: a novel type of biomarker and genetic tools in cancer. Oncotarget. 2017;8(38):64551–63.
Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, et al. The emerging landscape of circular RNA in life processes. RNA Biol. 2017;14(8):992–9.
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–58.
Panda AC, Grammatikakis I, Kim KM, De S, Martindale JL, Munk R, et al. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res. 2017;45(7):4021–35.
Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429.
Cheng J, Huang J, Yuan S, Zhou S, Yan W, Shen W, et al. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. Yu Y, editor. PLoS One. 2017;12(6):e0177888.
Knupp D, Miura P. CircRNA accumulation: a new hallmark of aging? Mech Ageing Dev. 2018;173:71–9.
Gruner H, Cortés-López M, Cooper DA, Bauer M, Miura P. CircRNA accumulation in the aging mouse brain. Sci Rep. 2016;6(1):38907.
Piwecka M, Glažar P, Hernandez-Miranda LR, Memczak S, Wolf SA, Rybak-Wolf A, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science (80). 2017;357(6357):eaam8526.
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–85.
Lukiw WJ, Circular RNA. (circRNA) in Alzheimer’s disease (AD). Front Genet. 2013;4:307.
Ashapkin VV, Kutueva LI, Kurchashova SY, Kireev II. Are there common mechanisms between the Hutchinson-Gilford progeria syndrome and natural aging? Front Genet. 2019;10:455.
Nmezi B, Xu J, Fu R, Armiger TJ, Rodriguez-Bey G, Powell JS, et al. Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina. Proc Natl Acad Sci. 2019;116(10):4307–15.
Crasto S, Di Pasquale E. Induced pluripotent stem cells to study mechanisms of laminopathies: focus on epigenetics. Front Cell Dev Biol. 2018;6:172.
Bertrand AT, Chikhaoui K, Yaou RB, Bonne G. Clinical and genetic heterogeneity in laminopathies. Biochem Soc Trans. 2011;39(6):1687–92.
Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42(1):301–34.
Doksani Y, Wu JY, de Lange T, Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013;155(2):345–56.
Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev. 2009;130(6):377–83.
Chojnowski A, Ong PF, Wong ES, Lim JS, Mutalif RA, Navasankari R, et al. Progerin reduces LAP2α-telomere association in hutchinson-gilford progeria. Elife. 2015;4:1–21.
Chandra T, Ewels PA, Schoenfelder S, Furlan-Magaril M, Wingett SW, Kirschner K, et al. Global reorganization of the nuclear landscape in senescent cells. Cell Rep. 2015;10(4):471–83.
Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, et al. Formation of macroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8(1):19–30.
Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc Natl Acad Sci U S A. 2010;107(32):14280–5.
Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol. 2009;11(10):1261–7.
Cho I, Tsai P-F, Lake RJ, Basheer A, Fan H-Y. ATP-dependent chromatin remodeling by Cockayne syndrome protein B and NAP1-like histone chaperones is required for efficient transcription-coupled DNA repair. PLoS Genet. 2013;9(4):e1003407.
Sikora E. Rejuvenation of senescent cells-The road to postponing human aging and age-related disease? Exp Gerontol. 2013;48(7):661–6.
Obeid R, Schadt A, Dillmann U, Kostopoulos P, Fassbender K, Herrmann W. Methylation status and neurodegenerative markers in Parkinson disease. Clin Chem. 2009;55(10):1852–60.
Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging (Albany NY). 2015;7(12):1130–42.
Kontopoulos E, Parvin JD, Feany MB. α-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15(20):3012–23.
Lee J-H, Ryu H. Epigenetic modification is linked to Alzheimer’s disease: is it a maker or a marker? BMB Rep. 2010;43(10):649–55.
Silva PNO, Gigek CO, Leal MF, Bertolucci PHF, De Labio RW, Payão SLM, et al. Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer’s disease. J Alzheimer’s Dis. 2008;13(2):173–6.
Bennett DA, Yu L, Yang J, Srivastava GP, Aubin C, De Jager PL. Epigenomics of Alzheimer’s disease. Transl Res. 2015;165(1):200–20.
Furuya TK, Da Silva PNO, Payão SLM, Rasmussen LT, De Labio RW, Bertolucci PHF, et al. SORL1 and SIRT1 mRNA expression and promoter methylation levels in aging and Alzheimer’s Disease. Neurochem Int. 2012;61(7):973–5.
Borodinova AA, Kuznetsova MA, Alekseeva VS, Balaban PM. Histone acetylation determines transcription of atypical protein kinases in rat neurons. Sci Rep. 2019;9(1):4332.
Zhang K, Schrag M, Crofton A, Trivedi R, Vinters H, Kirsch W. Targeted proteomics for quantification of histone acetylation in Alzheimer’s disease. Proteomics. 2012;12(8):1261–8.
Gensous N, Bacalini MG, Pirazzini C, Marasco E, Giuliani C, Ravaioli F, et al. The epigenetic landscape of age-related diseases: the geroscience perspective. Biogerontology. 2017;18(4):549–59.
Escoubas CC, Silva-García CG, Mair WB. Deregulation of CRTCs in aging and age-related disease risk. Trends Genet. 2017;33(5):303–21.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Monroy-Jaramillo, N., Vázquez-Martínez, E.R. (2020). Epigenetics and Ageing. In: Gomez-Verjan, J., Rivero-Segura, N. (eds) Clinical Genetics and Genomics of Aging. Springer, Cham. https://doi.org/10.1007/978-3-030-40955-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-40955-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40954-8
Online ISBN: 978-3-030-40955-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)