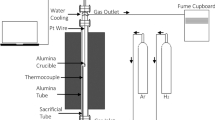
Abstract
The electrical properties of Co(III) in LiCoO2 on Pt working electrode in 1023 K NaCl–CaCl2(1:1) molten salt are studied by cyclic voltammetry and square wave voltammetry. The reduction of Co(III) in LiCoO2 to cobalt is a two-step reduction process, Co(III) → Co(II) → Co. The cobalt of the flakes is directly prepared in 1023 K molten NaCl–CaCl2. The sheet LiCoO2 is used as a cathode, and the graphite is used as an anode. The potential of the preparation of elemental cobalt by LiCoO2 is determined by electrochemical tests. The reduced flakes are analyzed by XRD, EDS, and SEM to confirm that the reduced material is pure metallic cobalt. The production of metallic cobalt by molten chloride salt electrolysis is a green and environmentally friendly metal preparation process.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others

References
Li L, Zhai L, Zhang X et al (2014) Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. Power Sour 262:380–385
Horeh NB, Mousavi SM, Shojaosadati SA (2016) Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillusniger. Power Sources 320:257–266
Li L, Qu W, Zhang X et al (2015) Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. Power Sour 282:544–551
Zou FL (2006) Resources, Market and Application of Cobalt. Carbide 1:176–181
Han YB, Zeng QL (2013) Research on the recycling treatment of waste lithium batteries. China Resour Compr Utilization 31(7):31–32
Xu AD (2005) Main problems in the development of nickel-cobalt industry in China. Chin J Metals 47:14
Chen GZ, Fray DJ, Farthing TW (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361
Whittingham MS, Reviews C (2004) Lithium batteries and cathode materials. Chem Rev 35:4271–4301
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sour 195:939–954
Hu H, Gao Y, Lao Y et al (2018) Yttria-stabilized zirconia aided electrochemical investigation on ferric ions in mixed molten calcium and sodium chlorides. Metall Mater Trans B 49(5):2794–2808
Hu X, Wang J, Li Z et al (2017) MCNTs@ MnO2 nanocomposite cathode integrated with soluble O2-carrier Co-salen in electrolyte for high-performance Li–air batteries. Nano Lett 17(3):2073–2078
Tang DY, Yin HY, Cheng XH et al (2016) Green production of nickel powder by electro-reduction of NiO in molten Na2CO3–K2CO3. Int J Hydrogen Energy 41(41):18699–18705
Tran-Nguyen DH, Jewell D, Fray DJ (2014) Electrochemical preparation of tungsten, tungsten carbide and cemented tungsten carbide. Min Process Extract Metall 123(1):53–60
Kim DH, Bae SE, Park TH et al (2014) Real-time monitoring of metal ion concentration in LiCl–KCl melt using electrochemical techniques. Microchem J 114:261–265
Acknowledgements
The study is financially supported by the National Natural Science Foundation of China (Project No. 51774143, 51674120) and the Hebei Province Graduate Innovation Program (Project No. 2019050).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Wang, J., Liang, J., Li, H., Xu, J. (2020). Reduction Mechanism of Metal Cobalt from Cathode Material of Waste Lithium Cobalt Oxide Battery. In: TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36296-6_120
Download citation
DOI: https://doi.org/10.1007/978-3-030-36296-6_120
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36295-9
Online ISBN: 978-3-030-36296-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)