Abstract
Rapid and non-invasive analytical method for quality control is a priority for the food industry, hence the objective of this study is to use Raman spectroscopy (RS) to identify phytic acid in RKB. Phytic Acid was extracted from treated ground dry RKB, while standard concentration of PA was prepared from a PA solution 50% w/w. Extracted samples and standard solutions were analyzed using the RS. The laser light beams focused on the PA samples generated molecular bond vibrations which resulted to inelastic scattering of photons in the light beam. The result showed that P-O-C, and P=O which are bonds identified with PA emitted light intensities at wavelength 858.13, and 1198.04 nm, respectively. Correlation (0.93) was established between results obtained for the standard RAMAN spectroscopy method and UV spectrophotometric method. Canned BL treatments showed increased concentration with blanched samples with lowest concentration (0.1175 g/mL) for P-O-C. Thus, the correlation established is indicative that the RS has potential applications in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. Introduction
Phytic acid, myo-inositol 1, 2, 3, 4, 5, 6-hexakisphosphate (IP6) (Figure 1) is a principal storage form of phosphorous compound existing naturally in plants. Phytic acid (PA) is indigestible by humans and monogastric animals due to the lack of the enzyme phytase, which dephosphorylates the PA compound. It has been identified as an anti-nutritional factor [1], [2].
Legumes contain significant amount of PA, which exist intrinsically as phytate in the anionic form in plants. It exists as a complex salt of cations (calcium, magnesium and potassium) in matured seeds, and is usually bound to macronutrients such as proteins and starches. Raw whole and cooked red kidney beans contain about 9.56 and 8.09 mmol/kg, respectively [3], [4] reported phytic content of 4.4, 24.3, 57, 39 mg/g, in carrots, hard wheat, hard wheat brand and fermented hard wheat brand bread, respectively.
The Ca and Mg complex salt form of PA and also referred as phytin [5]. Phytin is usually stored in the germ of corn, the aleurone layer and outer bran of wheat and rice, and globoids in legumes and oil seeds [6], [7], [5]. In effect, PA inhibits the bioavailability of minerals by forming insoluble complexes with mono-di and trivalent cations, thus impedes the hydrolytic enzymes such as protease and amylase.
Soaking of legumes in water prior to canning is a critical step in reducing the PA concentration [8], [9]. Tempering is a hydration processing without direct submergence in water. It is a method that subjects specimens to a specific environmental condition for a specific period of time to induce chemical and morphological changes. Thapa, Juelich and Kassama [10] reported an equivalent hydration coefficient for conventional soaking to tempering red kidney beans at high relative humidity pretreatments.
The AOAC method 986.11 [11] is one of the traditional spectrophotometry methods for quantifying IP6 by means of determining inorganic phosphorous. The limitation of traditional method is the capacity to distinguish between IP6 and inositol pentakisphosphate, IP5 hence systematically overestimates IP6 content [3]. Currently high performance liquid chromatograph (HPLC) is one of the common methods used to determine PA in red kidney beans. Chen [3] estimated PA and IP5 in varieties of beans and nuts to be between 0.1 to 0.2 μg/100 μL (1.5 to 3.4 μM) with HPLC, Dost and Tokul [4] reported 10 to 125 μg/mL in wheat and wheat products. However, there is increase interest in the rapid detection and quantification of organic chemical compounds in foods without complex sample derivatization and preparation protocol.
Surface-enhanced Raman scattering is a novel vibrational spectroscopy for rapid analysis of organic and biochemical compounds within wide range different complex components [12], [13]. The Raman scattering signals of targeted molecules can be largely enhanced through adsorption of analyte molecules onto nanostructured metal surface [12], [14], [15]. Raman spectroscopies quantify antioxidants in vegetables oils, microbial contaminants in foods and in acetylated food proteins [15], [16]. Hampton, Demoin and Glaser [17] and De Gelder, de Gussem, Vandenabeele and Moens [18] reported the P-O-C and P=O stretch has been identified at wavenumbers ranging from 787 – 1088 cm-1 and 1140 – 1320cm-1 using Raman spectroscopy. However, limited studies have been conducted on Raman based detection of PA in beans and other legumes. Therefore the objective of the study is to evaluate the feasibility of Raman spectroscopy as an alternate approach to quantify PA in red kidney beans.
II. Material and methods
A. Dry Wholesome RKB Kernels
Dry red kidney beans (RKB) were obtained in the courtesy of the Chippewa Valley Beans, Co., Inc. Menomonie, Wisconsin. The RKB kernels were passed through stereoscopic microscope with a digital camera (Model DC5-420th, Cole Palmer, Vernon hills, Illinois) to identify wholesome kernels from cracked or fissured kernels. About 500 g of RKB kernels were tempered in the Environmental chamber (EC) (ESPEC Model BTL 433-4 CFT, Hudsonville, Michigan).
B. Extraction of PA in Red Kidney Beans
The treatment RKB samples were freeze dried using a Freezone freeze dryer (Model 195, Labconco Corporation, Kansas City, MO) and ground with the Thomas-Willey Laboratory mill (Model 4, Arthur H. Thomas Company, Philadelphia, PA), the ground samples were passed through US Standard Testing Sieve Size 20 (Tyler Equivalent 20 Mesh). Phytic acid was extracted from 1g of dry ground samples by adding 0.66 M of hydrochloric acid (HCL) in an Erlenmeyer flask (250 mL). The flasks were placed on a Laboratory Shaker (Eberbach Orbital-60 CV-AC, Eberbach Corporation, Ann Arbor, MI) and shake overnight. The extracts were transferred into microfuge tubes (1.5 mL) and centrifuged at 13,000 rpm for 10 min with the Fisher Scientific Marathon Centrifuges (Model 16km, Fisher Scientific, USA). Supernatant were carefully separated and was neutralized with 0.5 mL of 0.75 M of NaOH solution.
C. Enzymatic Assay of PA
Neutralized solutions of the PA extracts were used in the enzymatic de-phosphorylation reaction assay using Magazine kits [19]. The total phosphorous and free phosphorous concentrations were determined using the colorimetric method. Phytic acid content was computed [19] from the result obtained from the relative absorbance determined with a UV-VIS Spectrophotometer (UVmini-1240, Shimadzu Corporation, Kyoto, Japan).
D. Preparation of Standard Solutions
A stock standard solution of PA (50% solution in water with a concentration of 1.1 mol/mL) purchased from Fisher Scientific, USA was prepared in HCL and stored at 4°C until used. A working standard solution concentrations (0.1, 0.2, 0.3, 0.4, 0.5 mol/L) were prepared by diluting with the stock solution with HCL. Concentration of PA in standard solution was determined using a UV-VIS Spectrophotometer (UVmini-1240, Shimadzu Corporation, Kyoto, Japan).
E. Surface Raman Spectroscopy Determination of Phytic
A Dilor Confocal Laser Raman imaging microscope system (Model Labram, Horiba Jobin Yvon, France) equipped with an Olympus BX40 with 20x microscope objective lens was used to determine the PA content on the standard solutions and the extracts samples solutions. The objective lens was set at 10x with an acquisition time of 20 s at a grating rate of 1800 g/mm. Sample solutions were pipetted into cuvettes and loaded on the stage preceding equipment calibration. The screen was the adjusted to ensure that the laser light beam passes through the solution prior to data acquisition, and the light intensity was subsequently plotted against the wavenumber.
F. Canning of Red Kidney Beans
Wholesome RKB kernels were used for canning after the tempering process and were soaked and blanched (BL) as method used by Thapa [20], and some treatments were not-blanched (UBL). The RKB samples (160 g) were canned (No. 300) in 175 mL of brine solution [12]. The cans were sealed with the Dixie Automatic Can Sealer (Model 23-500, Dixie Canner Co, Athens, GA). Cooking and sterilization was done in All American Electric Pressure Steam sterilizer (Model no 25X, Wisconsin Aluminum Soundry Co. Inc, Manitowoc, WI) at a temperature and pressure of 116°C (240°F) for 40 min and the cans were rapidly cooled after cooking. Raw and non-cooked RKB samples are referred as not-canned (UC).
G. Experimental Design
The experimental treatments applied in this study were conventional soaked beans (SB), tempered no soaking (T25), tempered and soaked for 1 h (TS1) and 3 h (TS3) and the control (WB). Each treatment was canned as described above and phytic acid content was determined before and after canning. All the experiments were conducted in three replications.
III. Results and discussion
The effects of the different pretreatment on the PA strength in RKB were determined using Raman vibrational spectroscopy to detect the chemical structure of interest (myo-inositol hexaphosphate). Phytic acid, myo-inositol hexaphosphate (IP6) is a ring of 6-carbon atoms (myo-inositol or cyclo hexane) connected by single bonds and has 6-phosphate groups attached [21], [22], [23]. Barrientos, Scott and Murthy [23] reported that phytase hydrolysis results to sequential reduction of phosphate ester from PA, hence, structural modification of IP6 to IP5 and gradually to IP. Hydrolysis of phosphate esters is indicative of the weakening of the P-O-C and P=O bond, which attaches the phosphate group to the inositol. Molecular bond vibration frequency was used to identify the P-O-C and P=O. The molecular bond Raman spectral frequency of the P-O-C and P=O detected in this experiment and their respective harmonic wave number is shown in Fig. 2.
A. Phytic acid in standard solutions
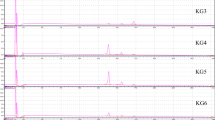
The Raman vibrational spectra of phytic acid (C6H18O24P6: inositol hexakisphosphate) standard solutions (0, 0.1, 0.2, 0.3, 0.4, 0.5 mol/mL) was used as the baseline for quantifying PA content in the treatments as shown in Fig. 2. Hydrochloric acid (HCl) was used for the extraction of phytic acid in the RKB samples, hence also used as the blank (Fig. 2). The Raman vibration spectra peaks for the samples were overlaid on the HCl and standard solutions spectra for comparison of the peaks. The Raman vibration spectra peaks of the samples coincided with the peaks of the standard solutions but not the blank, thus indicate the harmonization of the specific compounds that vibrates at the same frequency. The identification of the intense spectra is not repeated in HCl spectrum. These regions marked the point of interest, and were selected as the region of PA molecular bond vibration (Fig. 2).
The Raman vibration peaks of the P-O-C and P=O stretches were identified at the following spectra regions 787 – 1088 cm-1 and 1140 – 1320 cm-1, respectively, this was corroborated by the studies of Hampton, Demoin and Glaser [17]; Yang et al. [24]; and De Gelder, de Gussem, Vandenabeele and Moens [18]. The spectra of all the solutions displayed peaks coinciding to the P-O-C and P=O stretches. The highest intensity was observed at the frequency of 858.13 cm-1 for the P-O-C stretches. The higher frequency (1198.04 cm-1) region of the spectrum shows the least contribution based on the localized vibrations within phytic acid observed at P=O stretch shown in Fig. 3. Hampton, Demoin and Glaser [17] reported a medium strength for P=O stretch at this range in Raman spectroscopy. The frequency intensity for standard solutions obtained at the selected P-O-C and P=O stretches indicated a linear correlation with the concentration as shown in Table 1.
Table 2 shows the relationship between the UV-Vis and the Raman vibrational spectroscopic data of the standard solutions. A correlation (R2 = 0.93) was established between the intensities of the P-O-C stretch of the standard solution by Raman spectroscopy and standard UV-Vis spectroscopy as shown in Fig. 4. The reliability of the correlation ensures the applications of Raman spectroscopy technique used in analyzing the PA strength of RKB and future uses in other food matrix.
The Raman peaks obtained from the blank and the standard solution at 0.5 mol/mL was compared to the peaks obtained for each of the treatments as shown in Fig. 5. Although, the frequency of the entire 5 band shifted by ca. 40 cm-1 observed for the P-O-C stretches in contrast to the standard solution spectrum, but the bond conformation was observed at 902.02 cm-1. The band shift could be the result of the pretreatment that may have affected the molecular bond structure. However, the peak falls within the vibrational frequency region as reported in many studies by Hampton, Demoin and Glaser [17]; Yang et al. [24]; and De Gelder, de Gussem, Vandenabeele and Moens [18]. Isbrandt and Oertel [25] also reported a 10 cm-1 band shift for myo-Inositol Hexakisphosphate. Thus, frequency shift was not observed for the P=O band shown in Fig. 5.
The selected frequencies of uncooked RKB samples subjected to different treatments and their spectra intensities are tabulated in Table 3. The intensity values of the treatment spectrums observed for each wave number did not vary significantly (p > 0.05). The vibration peaks emitted are closely knitted due to the breakdown of PA, hence the bonds stretch deteriorate as the concentration of PA decreases (Fig. 5).
The lowest intensity values were observed in the T25 treatments while the WB treatment registered the highest value at both wavelengths (902.02 and 1198.04 Cm-1). Thus tempering at 25°C seems to weaken the structural bond linking the phosphate group to the inositol and the other treatments also showed the same characteristic feature compared to the WB treatment. The result obtained from the phytic quantification using the method developed by Megazyme [19] corroborated the values obtained by Raman spectroscopy, although no significant (p > 0.05) changes were observed between the control and all treated values as shown in Table 4.
Thapa [20] reported (12.4 to 23.6 mg/g) phytic acid concentrations in dry RKB, these values are much less than the values (25 to 29 g/100g) obtained in this research shown in Table 4. The results of Thapa, [20] were based on tempering at 45°C for 12 hours, which is lower than the value obtained for 25°C tempering for 12 hours in this study, the variation could be attributed to the temperature differences.
B. Phytic acid concentration in canned red kidney beans
The correlation of Raman vibration peaks for the P-O-C and P=O stretch to concentration of phytic acid was used to determine the phytic acid concentration in canned RKB as shown in Tables 5 and 6. Whole bean uncooked beans for UC and UBL treatments showed decreased concentration of PA for the P-O-C and P=O stretch. The other treatments had higher concentrations compared to the WB. All canned BL treatments showed increased concentration with TS3 BL having the lowest concentration (0.1175 g/mL) for P-O-C. The variation is probably due to the bond strength and liberation of phosphorous during the pretreatment. Tables 5 and 6 shows the data of tempering (T25) without blanching reduced the Raman spectral intensity when compared with the WB, thus shows an indication of reduced PA concentration of 0.074 g/mL for P-O-C and P=C. However, an increased Raman spectral intensity was observed on blanched (T25) treatment and the relative PA concentration (0.1281 g/mL for P-O-C) increased compared to WB. Increased intensity was equally observed after the immersion of tempered samples in water (TS1 and TS3). Bond hydration as a result of diffusion might have some effect on the P-O-C and P=O structure. Tempering at high RH environment tend to hydrate without immersing in water have similar effect as soaking in reducing PA strength.
Megazyme [19] indicated in their studies the ease of de-phosphorylate IP6 to IPs because of the less resistance observed in the region IP2 to IP. Reduction of PA during soaking is proven as a result of partial diffusion of hydrolyzed phosphate group into soaking water [8], [9]. Phytic acid strength was impaired in all tempered samples as indicated in the results obtained. The increase intensity of Raman Spectra and the corresponding concentrations after immersing in water for the UC, UBL, and BL samples indicates high resistant to de-phosphorylation hence could be classified in the IP2 to IP region due to the high vibration intensity of the P-O-C and P=O bonds.
IV. Conclusion
The correlation of Raman vibrational spectra peaked for the P-O-C and P=O stretch to concentration of phytic acid content in RKB was established. The hydration pretreatment have significant (p < 0.05) effect on the P-O-C and P=O structure and hence contribute to the final concentration of phytic acids in RKB. The frequency of the entire 5 band shifted by ca. 40 cm-1 for the P-O-C stretches in contrast to the standard solution spectrum, thus the bond conformation was observed at 902.02 cm-1. All canned BL treatments showed increased concentration with TS3 BL having the lowest concentration (0.1175 g/mL) for P-O-C. The results indicated that the use of Raman molecular vibrational spectroscopy to determine chemical constituents of food have potential applications in the food industry.
References
L. Oatway, T Vasanthan, and J. Helm. Phytic acid. Food Reviews International, Vol. 17, pp. 419-431, 2001.
G. M. Akond, H. Crawford, J. Berthold, I. T. Zahiru, and K. Hossain. (Zn, Fe, Ca and Mg) and anti-nutrient (phytic acid) constituent in common bean. American Journal of Food Technology, Vol. 6, pp. 235-243, 2010.
Q. Chen. Determination of phytic acid and inositol pentakisphosphates in foods by high performance ion chromatography. Journal of Agricultural and Food Chemistry, Vol. 52, no. 15, pp. 4604-4613, 2004.
K. Dost, and O. Tokul. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anlytica Chimica Acta, Vol. 558, pp. 22-27, 2006.
R. Angel, N. M. Tamim, T. J. Applegate, A. S, Dhandu, and L. E. Ellestad. Phytic Acid Chemistry: Influence on Phytin-Phosphorus Availability and Phytase Efficacy. The Journal of Applied Poultry Research, Vol. 11, no. 4, pp. 471-480, 2002.
V. Ravindran, W. L. Bryden, and E. T. Kornegay. Phytates: occurrence, bioavailability and implications in poultry nutrition. Avian and Poultry Biology.Reviews. Vol. 6, pp. 125-143, 1995.
B. L. O’Dell. and A. R. Boland. Complexation of phytate with protein and cations in corn germ and oil seed meals. Journal of Agricultural Food Chemistry, Vol. 24, no. 4, pp.804-808, 1976.
C. Hotz, and R. S. Gibson. Traditional food processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. The Journal of Nutrition, Vol. 137, no. 4, pp.1097-1100, 2007.
K. S. Queiroz, A. C. De Oliveira, E. Helbig, S. M. Reis, and F. Carraro. Soaking the common bean in a domestic preparation reduced the contents of raffinose-type oligosaccharides but did not interfere with nutritive value. Journal of Nutritional Science and Vitaminology, Vol. 48, no. 4, pp. 283-289, 2002.
N. Thapa, A. C. Juelich, and L. S. Kassama. (2009). High Humidity Tempering Effect on Soaking Times on Retort Canning Quality of Red Kidney Bean (Phaseolus vulgaris). In Proceedings of annual NCUR, WI, pp.
AOAC. Method 986.11 phytate in foods, anion exchange method. In Official Method Analysis, Vol. 32, pp. 57-58, 2000.
Y. Pan, L. Keqiang, Y. Fan, L. Chunying, L. Pei, B. A. Rasco, and Y. Huang. Determination of Tert-Butylhydroquinone in vegetable oils using surface-enhanced Raman spectroscopy. Journal of Food Science, Vol. 79, no. 7, pp. 1225-1231, 2014.
Y. Fan, L. Lai, B. A. Rasco, and Y. Huang. Analysis of phomet residue in apples with surface enhanced Raman spectroscopy. Food Control, Vol. 37, pp. 153-157, 2014.
P. L. Stiles, J. A. Dieringer, N. C. Shah, and R. P. Van Duyne. Surface-Enhanced Raman Spectroscopy. Annual Review of Analytical Chemistry. Vol. 1, pp. 601-26, 2008.
E. C. Y. Li-Chan. The applications of Raman spectroscopy in food science. Trends in Food Science & Technology, Vol. 7, no. 11, pp. 361-370, 1996.
Y. Z. Hao, C. Y. Ma, S. N. Yuen, and D. L. Phillips. Study of Acetylated Food Proteins by Raman Spectroscopy. Journal of Food Science, Vol. 69, no. 3, pp. 206-213, 2004.
C. Hampton, D. Demoin, and R. E. Glaser. (2010). Vibrational spectroscopy tutorial: sulfur and phosphorus. Available from http://faculty.missouri.edu/~glaserr/8160f10/A03_Silver.pdf.
J. De Gelder, K. De Gussem, P. Vandenabeele, and L. Moens. Reference database of Raman spectra of biological molecules. Journal of Raman Spectroscopy, Vol. 38, no. 9, pp. 1133-1147, 2007.
Megazyme (2011). Phytic acid (phytate)/total phosphorus: measured as phosphorus released by phytase and alkaline phosphatase; assay procedure. Available at http://secure.megazyme.com/files/BOOKLET/K-PHYT_1212_DATA.pdf.
N. Thapa. Effect of Tempering and Other Processing Treatments on the Anti-nutritional factors and a Canning Quality Attribute of Dark Red Kidney Beans. Master Thesis, Dept. of Food and Nutrition, University of Wisconsin-Stout, Menomonie, WI, 2012.
C. E. Ophardt. (2003). Organic molecule geometry. Virtual Chembook: Elmhurst College. Available from http://www.elmhurst.edu/~chm/vchembook/209rings.html.
B. L. Turner, A. E. Richardson and E. J. Mullaney. (2007). Inositol Phosphates: Linking Agriculture and the Environment. Available from http://books.google.com/books
L. Barrientos, J. J. Scott and P. P. Murthy, P.P. Specificity of hydrolysis of phytic acid by alkaline phytase from lily pollen. Plant Physiology, Vol. 106, no. 4, pp. 1489-1495, 1994.
F. Yang, J. Feng, Y. L. Liu, Y. Yang, Z. R. Zhang, G. L. Shen, and R. Q. Yu. Electrochemical and Surface Enhanced Raman Scattering Spectroelectrochemical Study of Phytic Acid on the Silver Electrode. The Journal of Physical Chemistry B, Vol. 108, no. 45, pp. 17412-17417, 2004.
L. R. Isbrandt and R. P. Oertel. Conformational states of myo-Inositol Hexakis(phosphate) in aqueous solution. A13C NMR, 31P NMR, and Raman Spectroscopic investigation. Journal of the American Chemical Society, Vol. 109, no. 9, pp. 3144-3148, 1980.
Acknowledgment (Heading 5)
This work was supported by the USDA National Institute of Food and Agriculture, [Evans-Allen]- Project Title: Enzymatic Biosynthesis of Anti-nutritional Factors by Solid State Fermentation Couple with Retort Canning Process of Red Kidney Beans (Phaseolus vulgaris L.) [Accession number: 228270]. The authors also acknowledged Dr. Steven Babalola and Dr. Claudiu Muntele of the AAMU Irradiation Center, for their support and assistance with the Raman Technique development.
Author information
Authors and Affiliations
Additional information
Author’s Profile

Ms. Temitope Opeyemi Sanusi had her elementary education at the Nigerian National Petroleum Corporation (NNPC) Staff School located in Mosimi, Sagamu, Nigeria and graduated in 1990. She earned her high school certificate from the Queens’ School located in Apata, Ibadan, Nigeria in 1997. She earned her B. Tech degree from Ladoke Akintola University of Technology located in Ogbomoso, Nigeria in 2005. She obtained her Master of Science degree in Food Science and Technology at Alabama A&M University in 2014.
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
A. Authors and Affiliations
1) Lamin S. Kassama (Assistant professor) and Temitope O. Sanusi (Graduate Student), Depratment of Food and Animal Science. Alabama Agricultural and Mechanical University, 4900 Meridian Street, Normal Alabama 35762.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kassama, L., Sanusi, T. Characterization of Phytic acid in Tempered Canned Red kidney beans (Phaseolus vulgaris) using Raman Spectroscopy. GSTF J Agric Eng 2, 1 (2016). https://doi.org/10.7603/s40872-015-0001-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40872-015-0001-8