Abstract
Organotin(IV) compounds have received tremendous attention for their synthesis, characterization and biological activities and their ability to bind with sulfur ligand such as dithiocarbamate ligand. Organotin(IV) dithiocarbamate complexes can be synthesized by the reaction of carbon disulphide with secondary amine. Four new organotin(IV) dithiocarbamate complexes were successfully synthesized by the reactions between di-2-ethylhexyldithiocarbamate, C16H34NCS2 and N-methylbutyldithiocarbamate, C5H12NCS2 with dibutyltin(IV) dichloride and triphenyltin(IV) chloride, RxSnClx (R = Bu, Ph) and (x = 1, 2 or 3). These series of complexes were synthesized using “in situ” insertion method and characterized by determination of melting point, CHNS elemental analysis, infrared spectroscopy (FT-IR) and UV-vis spectroscopy. The melting points of the synthesized compounds were in the range 88.3 - 128.4 °C. The experimental CHNS data of the compounds, mostly exhibit the calculated CHNS values. Using infrared spectra (FT-IR), the formation of organotin(IV) dithiocarbamate complexes were confirmed by the presence of v(C=N+) at 1464 - 1497 cm-1, v(C-S) at 963 - 1025 cm-1 and v(Sn-S) at 412 - 445 cm-1 in all complexes. Besides that, the UV-vis analysis showed the peaks chromophore of N=C within range 259 - 260 nm, nonbonding electron on sulphur and charges transferred between the tin metal and ligand (M-L). Using TGA analysis, complex 1 and 3 show two degradation curve but complex 2 and 4 shows only one degradation curves. The antibacterial activity of all synthesized organotin(IV) dithiocarbamate complexes were then determined towards four types of bacterial strains which were Escherichia coli, Staphylococcus aureus, Salmonella thypi and Bacillus cereus. Penicillin was used as positive control and streptomycin as negative control in order to compare diameter of inhibition zone between both controls and complexes. From antibacterial test, almost all synthesized complexes were found to give positive results. Thus, it can be concluded that the complexes formed do have applications in biological and medical aspects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. Introduction
Organotin(IV) compounds have received tremendous attention for their synthesis, characterization and biological activities and their ability to bind with sulfur ligand such as thione or dithiocarbamate. Organotin compounds contain at least one bond between tin and carbon [1]. Tin compound has an ability to form stable bonds with carbon as well as heteroatoms that have received much attention both in academic and applied research. The first organotin compound that was prepared is in 1894 which is diethyl dichloride by Sir Edward Frankland.
Organotin(IV) compounds of oxygen and nitrogen donor ligands are well known for their biological activity. Moreover, some tin complexes are also known to exhibit anti-bacterial and anti-tumor activity. In the previous report, organotin compounds have a growing importance in medicine. Organotin derivatives have caught much attention during the last two decades for their potential biocidal activities. In recent years, several organotin compounds have been synthesized, some with interesting cytotoxic properties [2].
Dithiocarbamate ligand have formula molecule R2NCS2 - and are the half amide of dithiocarbamic acid [3]. It is also the analogs of carbamate, R2NCO2 -. The possession of the two donor sulphur atoms are directly related to the strong metal binding properties of the dithiocarbamate ligand. The dithiocarbamate (DTC) ligands are well known compounds that bind strongly and selectively to many metal ions [4].
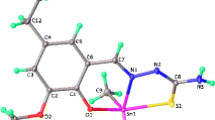
Dithiocarbamate also is the ligand which can stabilize the metal complexes with high oxidation number. Dithiocarbamate ligands readily form chelates with all transition metal ions through its two donor sulfur atom. The dithiocarbamate ligand of organotin(IV) dithiocarbamates may coordinate to tin(IV) atom in three different ways which include monodentate, bidentate and anisobidentate [5]. For the anisobidentate mode the two metal to sulfur bonds are not equivalent.
Recently, the studies on the synthesis and application of organotin(IV) dithiocarbamate complexes have increasing rapidly. Organotin dithiocarbamate complexes have attracted attention around the world due to their biological property. Then, we are aiming to synthesize several organotin complexes by using di-2-ethylhexyldithiocarbamate and N-methylbutyldithiocarbamate as the ligands and will observe their antibacterial property against selected bacterial strains such as S.thypi, E.coli, S.aureus and B.cereus.
II. Materials and method
A. Reagents
All chemicals and solvents were purchased from suppliers as follows: acetone, methanol, N-methylbutylamine and triphenyltin(IV) chloride (Merck); carbon disulphide, chloroform, dimethyl sulfoxide and ethanol (Fischer Scientific); di-2-ethylhexylamine and dibutyltin(IV) dichloride (Acros). All the chemicals were used as supplied without purification.
B. Synthesis of Organotin (IV) Dithiocarbamate Complexes
Four complexes were synthesized using organotin(IV) dithiocarbamate and dithiocarbamate compound to produce new organotin(IV) dithiocarbamate complex via in situ method. In this study, dibutyltin(IV) dichloride and triphenyltin(IV) chloride, were reacted with secondary amine which are di-2-ethylhexylamine and N-methylbutylamine in ethanol and carbon disulphide in ethanol was dropped into the mixture slowly. Temperature for overall reaction was below 2 °C.
C. Physical Measurement
All synthesized complexes were characterized using basic spectroscopy and analytical techniques [6]. Melting point analyzer was used to determine purity and melting point of the complexes. The range for melting point of pure complex is not over 3 °C. Then, next characterization was Series 1112 Flashea CHNS microanalysis to analyze elements which exist in these complexes including carbon (C), hydrogen (H), nitrogen (N) and sulphur (S) which will be compared with theoretical percentage.
Besides, frequency of important functional groups in complexes was recorded by Perkin Elmer 100 FTIR spectrophotometer which KBr pellet was used at low speed in the range of 4000 - 400 cm-1. FT-IR spectrum can be used to show presence of various important functional groups from molecules and atoms interaction with electromagnetic ray. Location for IR absorption peak can be determined which closely related to wave number, v (cm-1) or wavelength, λ (nm). Meanwhile, for ultraviolet visible absorption, it was recorded using Shimadzu UV-vis spectrophotometer. Thermogravimetric analysis also has been done to study the changes in physical properties of the complexes with temperature [7,8,9].
D. Antibacterial Test
Antibacterial activity using Disc Diffussion Test (DDT) was carried out to determine antibacterial behaviour of synthesized organotin(IV) dithiocarbamate against selected bacterial strain by measuring its inhibition zone ( Two types of bacterial strains were used; Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus) and Gram-negative bacteria (Salmonella thypi and Escherichia coli) were used in this test [10].
Organotin(IV) dithiocarbamate complex was dissolved in dimethylsulfoxide (DMSO) at concentration 0.1 mg/ 1000 μL. Then, this solution was dropped onto both sides of specialized paper disc and dried in room condition. Paper disc for positive (penicillin) and negative (streptomycin) control also were provided.
There were four petri dishes used. Each petri dish represented different bacteria. Bacterial layer was fully swabbed onto nutrient agar to make sure cultivation of bacteria is uniform. Four paper discs which contained various organotin(IV) dithiocarbamate were put in one petri dish. Bacteria will not grow if the organotin(IV) dithiocarbamate were antibacterial agent. Each disc was put far from one another to avoid overlap of inhibition zone. All of complete petri dishes were left for 24 hours. In the final procedure, diameter of inhibition zone was measured using ruler in mm unit and was compared with positive and negative control.
III. Results and discussion
A. Synthesis of Organotin(IV) Dithiocarbamate Complexes
In this study, four new organotin(IV) dithiocarbamate complexes were synthesized. In the synthesis of organotin(IV) dithiocarbamate complexes, the method of in-situ was used. The entire complex was successfully synthesized by using this method. These two new complexes were synthesized by adding organotin(IV) chloride [dibutyltin(IV) dichloride, (C4H9)2SnCl2, triphenyltin(IV) chloride, (C6H5)3SnCl] into di-2-ethylhexylamine in ethanol under 2 ºC. The mixture of the compound was synthesized in the ice bath at the temperature of 2 °C to avoid the formation of the dithiocarbamic acid during the reaction process. This mixture was stirred for 1 hour. Then, cold ethanolic carbon disulphide was added dropwisely into the mixture. After 1 hour, this reaction produced white solid precipitate which was organotin(IV) dithiocarbamate complex.
Another two new complexes were synthesized by the same method of the first series by the reaction between organotin(IV) chloride with N-methylbutyldithiocarbamate ligands. The reaction produced white solid precipitate which shows the formation of organotin(IV) dithiocarbamate complexes. The synthesis of each complex has taken time about 2 hours. One hour for the synthesis of dithiocarbamate ligand and another one hour for the formation of complex between the ligand and organotin compound. All complexes were in the form of white precipitate. After filtration and washed with little cold ethanol and dried, white powder of complexes were obtained.
Ethanol was used as the solvent in the synthesized for all organotin(IV) dithiocarbamate complexes. The reason why ethanol was used as the solvent in this study is because all organotin(IV) chloride complexes were soluble in these solvents. The stoichiometric coefficient ratio organotin to ligand in the synthesis of dibutyltin(IV) complexes is 1:2 [11]. The stoichiometric coefficient ratio organotin to ligand for synthesis of triphenyltin(IV) dithiocarbamate is 1:1. This is because there is only one leaving group for triphenyltin(IV) chloride.
The four new organotin(IV) dithiocarbamate complexes that were successfully synthesized are dibutyltin(IV) di-2-ethylhexyldithiocarbamate, Bu2Sn[C16H34NCS2]2, triphenyltin(IV) di-2-ethylhexyldithiocarbamate, Ph3SnC16H34NCS2, dibutyltin(IV) N-methylbutyldithiocarbamate, Bu2Sn[S2CNC5H12]2 and triphenyltin(IV) N-methylbutyldithiocarbamate, Ph3SnS2CNC5H1.
All synthesized complexes were successfully synthesized and characterized to check the purity and structure of the complexes. All synthesized complexes were characterized by using melting point analysis, CHNS elemental analysis, thermogravimetric analysis, infrared spectroscopy (FTIR) and ultraviolet-visible spectroscopy (UV-vis). Besides that, antibacterial test also was done to all synthesized organotin(IV) dithiocarbamate. They were tested against selected gram-positive and gram-negative bacterial strains such as Bacillus cereus, Staphylococcus aureus, Salmonella thypi and Escherichia coli.
Physical properties and analytical data of the organotin(IV) dithiocarbamate complexes are shown in Table I. The percentage yield of the complexes that have been obtained based on Table I, shows that complex 1 and 3 have lower than 50 % of the theoretical yield. The percentage yield of complex 1 is 44 % and complex 3 is 47 %. For complex 2 and 4, they have more than 50 % of the theoretical yield, which are 66 % and 78 %. Based on the result obtained, complex 2 and 4 have been synthesized successfully and as the early conclusion, the in-situ method used, really worked for synthesis of complex 2 and 4.
B. Melting Point Determination
Melting point is a temperature when a solid phase compound changes into liquid phase. This is a method used to check the purity of a compound. A compound can be assumed as pure if it has melting point range less than 3 ºC [11]. From the result, all organotin(IV) dithiocarbamate complexes has melting point range less than 3 ºC. They can be assumed as pure complexes.
C. Elemental Analysis (CHNS)
Elemental analysis has been done to all of the four organotin(IV) dithiocarbamate complexes. CHNS elemental analysis is done to determine the weight percentage of carbon (C), hydrogen (H), nitrogen (N) and sulphur (S) in molecule. This analysis also is useful to check purity of the complex [12]. Actual weight percentage of elements will be compared with the theoretical data.
There are three most important percentage of elements which are the weight percentage of carbon, hydrogen and nitrogen. Data for elemental anaylsis for C, H, N and S are in Table I. From the result, all the values obtained are in the range of accepted thereotical values except for complex 1 which shows large difference range between the actual than theoretical values for carbon. The differences exists probably because of the presence of impurities or solvent in the analyzed compound [13].
D. Determination of Complex solubility
The organotin(IV) dithiocarbamate complexes have been through the solubility test. The solvent that were used are divided into polar and non-polar solvent. For polar solvent, it is divided into two group which are polar protic that have the hydroxyl (OH) group and can form hydrogen bonding and polar aprotic that do not have the OH group and cannot forming any hydrogen bonding.
Polar protic solvent that were used are ethanol, methanol and water. All complexes did not dissolved in water because of the bulkiness and there are many hydrocarbon group attached to this complex. Complex 1 and 2 were soluble in ethanol but for complex 3 and 4, they were partially soluble in ethanol. Complex 2 was soluble in methanol but complex 1, 3 and 4 are partially soluble in methanol. Polar aprotic solvent that were used in the solubility test are acetone and dimethylsulfoxide. All complexes was soluble in acetone and dimethylsulfoxide.
Non polar solvents contain bonds between atoms with similar electronegativities, such as carbon and hydrogen. Bonds between atoms with similar electronegativities will not have partial charges. This absence of charges will make these molecules “non-polar”. For non-polar solvent, chloroform was used in the solubility test. As the result, all organotin(IV) dithiocarbamate complexes have successfully soluble in chloroform.
E. Infrared Spectroscopy Analysis
Infrared spectroscopy (IR) was used to observe the important band that presents in the complex. Potassium bromide pellet was used with the range of wavenumber 4000 – 400 cm-1, by using FTIR Spectophotometer Perkin Elmer 100 model. There are some important vibration that can be observed using IR spectroscopy. There are three important absorption regions present in IR spectrum for organotin dithiocarbamate complex [13] as shown below:
-
i)
Absorption at 1550 – 1450 cm-1 is assigned as vibration for functional group (C=N+)
-
ii)
Absorption at 1050 – 950 cm-1 is assigned as vibration for functional group (C-S)
-
iii)
Absorption at 350 – 450 cm-1 is assigned as vibration for functional group (Sn-S)
Table II shows the important vibrational mode that present in the organotin(IV) dithiocarbamate complex. The peaks form has been classified as strong (s), moderate (m) and weak (w).
Based on the IR spectra of the four organotin(IV) dithiocarbamate complexes, the absorption of complexes 1, 2 and 3 for (C=N+) produced moderate peak at range 1479 - 1464 cm-1. But for the absorption of complex 4 for (C=N+), it produced strong peak at range 1497 cm-1. The dithiocarbamate ligand of organotin(IV) dithiocarbamates may coordinate to tin(IV) atom in three different ways which include monodentate, bidentate and anisobidentate [5]. For the anisobidentate mode the two metals to sulfur bonds are not equivalent.
The delocalization of electron will give different effect to bond of C-S. There are two possibilities will occur, firstly, if a doublet arises around 1000 cm-1, dithiocarbamate ligand is monodentate and if singlet appears at around 1000 cm-1, dithiocarbamate ligand is bidentate. A doublet will be formed when there are two different C-S stretching vibrations [14].
The range of peak for (C-S) value in the synthesized complexes is 1025 - 963 cm-1. The patterns of bands in all IR spectra of all complexes are variable. Complex 1 and 3 produced doublet peaks at C-S range. It shows that their dithiocarbamate ligands are monodentate. However, for complex 2 and 4, only a single peak was produced in the range 1050 - 950 cm-1. So, the mode of coordination for dithiocarbamate ligand in complex 2 and 4 should be bidentate.
The vibration band due to v (Sn-C) stretching usually lies in the range 625 - 475 cm−1 [15]. For complex 1 and 2, it gives a strong peak at 563 cm-1 and 693 cm-1 respectively are the area for v (Sn-C) is located. It can be symmetric and asymmetric.
This vibrational mode of bond is least important, but it can still be used to determine whether the tin is still existing in the complex or not. For complex 3, it does not show the peak of v (Sn-C). But for the absorption of complex 4, it produced weak peak at range 605 - 567 cm-1.
For the vibration of v (Sn-S), complex 1 and 2 shows this kind of mode at the range of 412 cm-1 and 445 cm-1 respectively. Commonly, this type of vibration shows a very weak intensity in the spectrum of infrared. For the absorption of complex 4, it produced a strong peak at the range of 445 cm-1. The presence of the peak in the infrared spectrum can prove the coordination between the ligand and metal bond via the sulphur atom.
F. Ultraviolet visible (UV-vis)Spectroscopy Analysis
Another analysis used in this study is ultraviolet visible (UV-vis) spectroscopy analysis. This analysis was done to observe the electronic transition present in synthesized complexes [14]. Dimethylsulfoxide (DMSO) was used in this analysis because DMSO is a solvent which does not interfere with important absorption bands. The cut-off point of the solvent that be used in the UV-vis spectroscopy analysis is at 265 nm.
All four synthesized organotin(IV) dithiocarbamate complexes were successfully dissolved in DMSO. These complexes must dissolve completely in the solvent because it is important to ensure there is no undissolved component which can block UV-vis ray passing through solution before it can be run in the spectroscopy. The concentrations for the complexes are 1.00 x 10-4 M and 1.00 x 10-3 M and the wavelength range of absorption spectrum was in the range of 200 - 400 nm.
In the organotin(IV) dithiocarbamate complex, the (N=C) chromophore and sulphur atom that contain lone pair are the two important bands in order to prove the formation of the complex. The addition of another band also can appear in the spectrum because of metal-ligand charge transfer [16].
Dithiocarbamate usually will show three bands in UV region which are π-π* transition of N-C=S system, π-π* transition of S-C=S and also n-π* transition of lone pair at sulphur atom [17]. As shown in the Table III, complex 1 show the absorption of the (N=C) chromophore at 260 nm, complex 2 at 259 nm, complex 3 at 260 nm and complex 4 at 260 nm.
The absorption band of (N=C) chromophore due to the intramolecular π - π* transitions in dithiocarbamate ligand. With this chromophore existence, it shows the involvement of NCS2 group in the complexes. Atom sulphur that contain lone pair shows the bands exist which arising from nonbonding electron of sulphur due to n- π* transitions. Only complex 2 show the bands of sulphur.
The third absorption band in the spectra belongs to the absorption of change transition from metal to ligand (M-L). The transition of electron form dπ-pπ bond between p-orbital of sulphur and empty 5d orbital of tin. The absorption band for the transition can be observed clearly as shoulder due to the extended conjugation of the complexes. The absorption band for complex 1 at 281 nm, complex 2 at 301 nm, complex 3 at 279 nm and complex 4 at 296 nm.
G. Thermogravimetric Analysis(TGA)
Thermogravimetric analysis is analytical technique that used to study changes in physical properties with temperature. Thermogravimetric analysis measures mass of sample in a specified atmosphere as the temperature of sample is programmed [18]. This analysis was carried out from room temperature to the temperature of 800 °C with 10 °C/minutes in nitrogen gas.
Based on the data obtained, all complexes start to degrade with temperature above 150 ºC. For complex 1 and 3, it produces two degradation processes in this thermal analysis. For complex 1, the first degradation occurs at temperature around 250 ºC and the second degradation occurs at temperature around 345 ºC. Besides that, complex 3 also produces two degradation processes in this thermal analysis. The first degradation occurs at temperature around 245 ºC and the second degradation occurs at around 350 ºC. The presence of two degradation curve shows that complex 1 and 3 are more stable.
On the other hands, for complex 2 and 4, they produced only one degradation processes in this thermal analysis. For complex 2, the degradation curve occurred at temperature around 150 ºC. For complex 4, this complex started to degrade and produced only one degradation curve at temperature around 260 ºC. The presence of only one degradation curve shows that complex 2 and 4 are thermally less stable compared to the complex 1 and 3 that produced two degradation curves.
H. Antibacterial Test
The result for antibacterial test is shown in Table IV. According to the table, all complexes exhibit antibacterial behavior against selected Gram-positive and Gram-negative bacteria test strains. The positive and negative controls used were penicillin and streptomycin. Streptomycin did not produce inhibition zone for all bacterial strains which will guarantee the result produced is accurate. By referring to the Table IV, every synthesized complex can act as antibacterial agent against almost all selected bacterial strains.
All of the synthesized complexes produced antibacterial activity against all selected bacterial strains. Most of organotin(IV) dithiocarbamate complexes can inhibit bacteria growth due to the biological activity of organotin and dithiocarbamate compounds [19]. The result for inhibition zones can be classified according to their size.
According to the data collected, all compounds showed broad spectrum activities toward Gram-positive and Gram-negative bacteria. However, the biological activities indicated only moderate to weak activities. Complex 1 shows weak inhibition zone against two bacterial strains which are B. cereus and S. typhi. For complex 2, it also shows weak inhibition zone against selected bacterial strains which are S. aureus and E. coli. Besides that, complex 3 shows active interaction against S. aureus and E. coli but weak inhibition zone and no inhibition zone against other two bacterial strains. For complex 4, it shows active interaction against S. aureus, B. cereus and S. typhi and no inhibition zone against E. coli.
The result for complex 4 is better than complex 1, complex 2 and complex 3 against Gram-positive bacteria. This is because triorganotin compounds are more biologically active than diorganotin compounds. Besides, type of organic ligand bonded to central tin also plays major role in its biological activity. Presence of phenyl groups at central tin like in triphenyltin(IV) N-methylbutyldithiocarbamate (complex 4) will produce higher toxicity for the complex against bacteria [20].
Conclusion
Recently, studies on synthesis, characterization and biological activity test towards organotin(IV) dithiocarbamate have attracted attention. In this study, ligands which are di-2-ethylhexyldithiocarbamate and N-methylbutyldithiocarbamate were reacted to two organotin(IV) complexes which are dibutyltin(IV) and triphenyltin(IV) complexes.
Four complexes of organotin(IV) dithiocarbamate were successfully synthesized and characterized by using melting point, infra-red spectroscopy (FTIR), ultraviolet visible spectroscopy (UV-vis), CHNS elemental analysis and thermogravimetric analysis (TGA). All of the synthesized complexes were characterized using basic spectroscopy to check the bonding characteristics for all products.
The four new organotin(IV) dithiocarbamate complexes that were successfully synthesized are dibutyltin(IV) di-2-ethylhexyldithiocarbamate, Bu2Sn[C16H34NCS2]2, triphenyltin(IV) di-2-ethylhexyldithiocarbamate, Ph3SnC16H34NCS2, dibutyltin(IV) N-methylbutyldithiocarbamate, Bu2Sn[S2CNC5H12]2 and triphenyltin(IV) N-methylbutyldithiocarbamate, Ph3SnS2CNC5H1.
Melting point analysis was used on all of the complexes to check the purity of a compound. A compound can be assumed as pure if it has melting point range less than 3 ºC. The small melting range showed that all the complexes almost pure. Besides that, elemental analysis has been done to all four organotin(IV) dithiocarbamate complexes. CHNS elemental analysis is done to determine the weight percentage of carbon (C), hydrogen (H), nitrogen (N) and sulphur (S) in molecule. Based on the analysis, all values are within the acceptable range and the percentage of the elements for experimental value agreed with the theoretical values
There are three important peaks which can be observed in IR spectra. They are the vibration of (C=N+), (C-S) and (Sn-S). The presence of these peaks in the spectra will ensure that dithiocarbamate ligand is coordinated to central tin. Based on the result from the analysis of FT-IR, the formation of ligands were confirmed by the presence of v(C=N+) at 1464 - 1497 cm-1. The stretching of v(C-S) at 963 - 1025 cm-1 can determine the symmetrical or unsymmetrical bonding of dithiocarbamate ligand whether as bidentate, anisobidentate or monodentate ligand. The stretching of v(Sn-S) at 412 - 445 cm-1 can conclude that the dithiocarbamate ligand has been coordinated with metal tin to form a complex.
Another analysis that was used in this study is ultraviolet visible (UV-vis) spectroscopy analysis. This analysis was done to observe the electronic transition present in synthesized complexes. The UV-vis analysis showed chromophore of (N=C) within range 259 -260 nm and also showed the absorption band for nonbonding electron on sulphur and charges transferred between the tin metal and ligand (M-L). The presence of (N=C) chromophore proved the availability of NCS2 group in all complexes. The second chromophore present in these complexes can be referred as free electron in atom sulphur. Sulphur atom posses a lone electron which increased the length of π system through resonance. This caused more shift happens and gives the n- π* transfer. For the third chromophore which metal to ligand (M-L), it represent the ligand to metal charge transfer and this band exhibit in low intensity due to the charge transfer n- π*.
The analytical technique that used to study changes in physical properties with temperature was done by TGA analysis. Complex 1 and 3 show two degradation curve but complex 2 and 4 shows only one degradation curves. It can be said that complex 1 and 3 are more stable compare to the complex 2 and 4.
These complexes also were further tested in biological application which was antibacterial test. In the test, these complexes were tested against four selected bacterial strains. The antibacterial test also was done towards selected bacterial strains such as B. cereus, B. cereus, E. coli and S. thypi. All synthesized complexes inhibited the growth of these bacterial strains. All compounds show broad-spectrum activities toward Gram-positive and Gram-negative bacteria. However, the biological activities indicated only moderate to weak activities. The result for complex 4 is better than complex 1, complex 2 and complex 3 against Gram-positive bacteria.
References
J. M. Batt,“The World of Organotin Chemicals: Applications, Substitutes and the Environment”. 1-10, 199–9.
A. Alama, B. Tasso, F. Novelli, and F. Sparatore, “Organometallic compounds in oncology: implications of novel organotins as antitumor agents,” Drug Discov. Today, vol. 14, no. 9-10, pp. 500–508, 2009.
N. Awang, I. Baba, B. M. Yamin, M. S. Othman, and N. F. Kamaludin, “Synthesis, characterization and biological activities of organotin (IV) methylcyclohexyldithiocarbamate compounds,” Am. J. Appl. Sci., vol. 8, no. 4, pp. 310–317, 2011.
H. Nabipour, S. Ghammamy, S. Ashuri, and S. Aghbolagh, “Synthesis of a New Dithiocarbamate Compound and Study of Its Biological Properties,” J. Org. Chem., pp. 75–80, 2010.
O. S. Jung and Y. S. Sohn, “Coordination chemistry of organotin(IV) dithiocarbamate complexes,” Bull Korean Che. Soc. 9(6): pp. 365-367, 1988.
M. H. Z. Rozaini, R. C. Ali and L. C. Rose, “Normal Micellar Value Determination in Single and Mixed Surfactant System Employing Fluorescence Technique,” International Journal of Technology, 2: pp. 93-99, 2012.
F. Yusoff, A. Aziz, N. Mohamed, and S. Ab Ghani, “Synthesis and characterizations of BSCF at different ph as future cathode materials for fuel cell,” Int. J. Electrochem. Sci., vol. 8, no. 8, pp. 10672–10687, 2013.
N. A. Harun, M. J. Benning, B. R. Horrocks, and D. A. Fulton, “Gold nanoparticle-enhanced luminescence of silicon quantum dots co-encapsulated in polymer nanoparticles.,” Nanoscale, vol. 5, no. 9, pp. 3817–27, 2013.
S. T. Anuar, Y. Y. Zhao, and S. M. Mugo, “The development of a capillary microreactor for transesterification reactions using lipase immobilized onto a silica monolith,” Journal of Molecular Catalysis B: Enzymatic. 92: pp. 62-70, 2013.
D. S. H. Ng, L. C. Rose, H. Suhaimi, H. Mohamad, M. Z. H. Rozaini, and M. Taib, “Preliminary evaluation on the antibacterial activities of citrus hystrix oil emulsions stabilized by tween 80 and span 80,” Int. J. Pharm. Pharm. Sci., vol. 3, no. SUPPL. 2, pp. 209–211, 2011.
A. Graisa, Y. Farina, E. Yousif, and M. Kassem, “Synthesis and characterization of some diorganotin(IV) complexes of n-tolyl-m-nitrobenzohydroxamic acid,” vol. 3, no. 6, pp. 2006–2009, 2008.
A. K. Mishra, N. Manav, and N. K. Kaushik, “Organotin(IV) complexes of thiohydrazones: synthesis, characterization and antifungal study”. Spectrochimica Acta Part A. 61: pp. 3097–3101, 2005.
S. S. Aishah, “Synthesis and characterization of thiourea derivatives with the reactivity towards the selected microbacteria,” Undergraduate Thesis, Universiti Malaysia Terengganu, 2009.
R. Sharma and N. K. Kaushik, “Thermal studies on some organotin(IV) complexes with piperidine and 2-aminopyridine dithiocarbamate,” Journal of Thermal Analysis and Calorimeter, vol. 78, pp. 953–964, 2004.
J. Cookson and P. D. Beer, “Exploiting the dithiocarbamate ligand in metal-directed self-assembly,” Dalton Transactions pp. 1459-1472, 2007.
R. Singh and N. K. Kaushik, “Spectral and thermal studies with anti-fungal aspects of some organotin(IV) complexes with nitrogen and sulphur donor ligands derived from 2-phenylethylamine,” Spectrochim. Acta - Part A Mol. Biomol. Spectrosc., vol. 71, no. 2, pp. 669–675, 2008.
S. M. Mamba, “Synthesis, Characterization and Applications of Dithiocarbamate Transition Metal Complexes,” Inorganica Chim. Acta, pp. 7–36, 2009.
J. W. Robinson, “Undergraduate Instrumental Analysis. 6th Ed. New York. Marcel Dekker”: pp. 576-585, 2005.
N. Rehman, M. K. Baloch, B. Muhammad, A. Badshah, and K. M. Khan, “Characteristic spectral studies and in vitro antifungal activity of some Schiff bases and their organotin(IV) complexes,” Chinese Science Bulletin No 2: pp. 119-222, 2004.
N. Awang, I. Baba, N. S. A. M. Yousof, and N. F. Kamaludin, “Synthesis and characterization of organotin(IV) n-Benzyl-n-isopropyldithiocarbamate compounds:cytotoxic assay on human hepatocarcinoma cells (HepG2),” American Journal of Applied Sciences 7 (8): pp. 1047-1052, 2010.
Acknowledgments
The authors would like to acknowledge the School of Fundamental Sciences, Universiti Malaysia Terengganu for granting the fundamental research project grant scheme (FRGS) under research vote of FRGS59262 and RACE56006. Special thanks also dedicated to the supporting staff of Institute Marine of Biotechnology (IMB) and Horseshoe Crabs Laboratory, School of Fundamental Sciences, Universiti Malaysia Terengganu for research facilities, numerous aids, support and valuable contributions to this research.
Author information
Authors and Affiliations
Additional information
1 School of Fundamental Sciences, Universiti Malaysia Terengganu.
2 Institute of Marine Biotechnology, Universiti Malaysia Terengganu.
3 Horseshoe Crabs Laboratory, School of Fundamental Sciences, Universiti Malaysia Terengganu.
4 Chemical Nanoscience Laboratory, School of Chemistry, Newcastle University, Newcastle upon Tyne NE1 7RU, United Kingdom.
5 Lipid Chemistry Group (LCG), Department of Agricultural Food and Nutritional Sciences (AFNS), University of Alberta, Edmonton AB T6G 2P5, Canada.
Author’s Profile








This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sainorudin, M., Sidek, N., Ismail, N. et al. Synthesis, Characterization and Biological Activity of Organotin(IV) Complexes featuring di-2-ethylhexyldithiocarbamate and N-methylbutyldithiocarbamate as Ligands. GSTF J Chem Sci 2, 2 (2015). https://doi.org/10.7603/s40837-015-0002-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40837-015-0002-3