Abstract
Pharmacovigilance is a method of quick detection and reporting, of adverse drug reactions and adverse drug events after drug is in clinical use, thus preventing major drug events. Ignorance of physicians in developing world, about reporting adverse drug reaction is a big roadblock to pharmacovigilance. This cascades into many problems e.g.; increased lab to clinic interval, increased premarketing expense for newer drugs. In quickly changing genomes scenario it leads to almost fatal therapeutic failures. The huge population of India and lack of appropriate post marketing surveillance contribute towards disasters due to adverse drug reactions. With evolution of pharmaceutical industry, the Indian doctors have gained wide knowledge of drugs but the area of adverse drug reactions still remains neglected. Indian Government launched National Pharmacovigilance Programme in 2004 to inculcate the culture of Adverse Drug Reaction reporting among Indian health professionals. Medical Council of India has also made Pharmacovigilance Programme mandatory in every medical college. Still the picture is disheartening. Motivated to improve Adverse Drug Event reporting in Chattrapati Shivaji Subharti Hospital, the present survey was conducted to find Knowledge, Attitude, Practices (KAP) of physicians, surgeons & nurses regarding Adverse Drug Reaction reporting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse drug reactions are a major cause of morbidity and mortality. Pharmacovigilance is a method of quick detection, of adverse drug reactions and adverse drug events after drug is in clinical use, thus preventing major drug events.
The true essence of Pharmacovigilance would be achieved only when physicians, nurses and other health professionals inculcate the habit of voluntary adverse drug reaction reporting.
India is emerging as a major hub of pharmaceutical industry. The Indian pharmaceutical market has flourished with the introduction of new drugs with the help of clinical research. But, in the realm of this tremendous boom of pharmaceuticals, the hazards due to adverse drug reactions appear to have been ignored. Indian companies are among the world leaders in the production of generics and vaccines – now producing more than 20% of the world’s generics. Since Indian firms will be marketing products globally they will have to fulfill the regulatory requirements of respective countries as well, post marketing surveillance of new drugs being one. But unfortunately ADR reporting is very low in India. This ugly situation can be improved by implementing adequate laws such as insurance against adverse drug reactions for general public, inclusion of pharmacovigilance under corporate social responsibility, encouraging regulatory authorities to take educative and publicizing role and by identifying each and every component of a drug including the excipients and preservatives while assessing the root cause of particular adverse drug event.
Pharmacovigilance in India
The National Pharmacovigilance Programme of India is largely based on the recommendations made in the WHO document titled “Safety Monitoring of Medicinal Products- Guidelines for Setting Up and Running a Pharmacovigilance Centre”. The Programme aims to foster the culture of Adverse Drug Event notification and aims to generate broad based adverse drug reaction database on the Indian population and share the information with others, and to ensure optimum safety of drug products in the Indian market. Despite the efforts of Drug Controller General Of India (DCGI) and Indian Council of Medical Research (ICMR) in establishing adverse drug reaction monitoring centres in many hospitals in the major cities of India and the presence of a large number of tertiary care facilities , pharmacovigilance is still in its infancy in India. Gross under reporting of adverse drug reactions is a cause of concern, the reasons for which may be many. Realizing the importance of the task, Medical Council of India has made it mandatory for all medical colleges to have an adverse drug reaction training, monitoring and reporting program. There is still a lack of understanding on this topic like how it functions, what are the benefits of sharing Adverse Drug Reaction knowledge and its purpose and importance. To fulfill the above aim it is important to find out the perception of doctors about adverse drug reaction reporting and identify steps which will improve adverse drug reaction reporting and fulfill the goals of Medical Council Of India. Keeping these facts in mind, this study was conducted to assess awareness of pharmacovigilance among physicians and nurses in an Indian peripheral care teaching hospital.
Role of training programme in adverse drug reaction reporting
Many studies show that active interventions, training programme, sensitization and continuous follow up, significantly increase number of spontaneous adverse drug reaction reporting.
A study conducted in Portugal included in its training program a workshop with sessions giving a brief introduction about the problem of Adverse Drug Reactions and its impact on public health, This was followed by an approach to spontaneous reporting of Adverse Drug Reactions. It also conducted telephonic interviews. Educational interventions conducted in workshops or telephone interviews significantly increased the number and relevance of spontaneous Adverse Drug Reactions reports by pharmacists of Northern Portugal.1
A study conducted in Manipal, India also included an educational intervention that was divided into a theoretical and a practical part. The theoretical part consisted of a presentation on how to report a suspected adverse drug reaction, economic and epidemiological importance of reporting the Adverse Drug Reactions and its effect on patient safety, the definition of pharmacovigilance, classification of ADRs (various tools for assessing ADRs in terms of causality, preventability, severity, Adverse Drug Reactions reporting cards from various countries, ADR alert cards, WHO online database for reporting adverse drug reactions). This study also utilized Knowledge, Attitude, Practices questionnaire to assess the knowledge of health care professionals. The result of this study showed increased awareness of pharmacovigilance among health care professionals.2
Mala Kharkhar, et al conducted a study in Mumbai on Knowledge, Attitude and Perception of doctors on Adverse Drug Reaction monitoring by designing a right questionnaire based study. The study revealed that practitioners were aware of Adverse Drug Reactions reporting. Their perception toward Adverse Drug Reactions reporting was right but it did not reflect in their attitude when it came to the act of reporting of Adverse Drug Reactions.3
Mrin Moy Chakrabarty,et.al in their study found that ensuring proper education and frequent updating of health care professionals by training them in data collection, filtration, verification, interpretation and coding of Adverse Drug Reactions, medicines coding, causality assessment, signal detection, risk management, and action in case of serious/fatal adverse drug events (ADE) had improved reporting of Adverse Drug Reactions.4
Chetna K. Desai and co-workers in her study used a pretested Knowledge, Attitude, Practices questionnaire comprising of 15 questions. The study showed that physicians were aware of the Adverse Drug Reactions and the importance of their reporting. However, under reporting and lack of knowledge about the reporting system were clearly evident.5
Roald Gerritsen and co workers compared the lecture-based pharmacovigilance training methods with the practice based method by analyzing the number and quality of reports sent in by graduate general practitioners who had been offered one of both approaches during their vocational training. The practice based methods resulted in significantly more and better documented reports and more often concerned unlabelled events than the lecture-based method.6
Peter Mansellet. al opined that pharmacovigilance had tended to be a backroom function in the pharmaceutical industry, and a largely reactive one too. Patient safety and public health should sit at the heart of corporate social responsibility.7
Patrick K.J Crowley and co-workers discovered that Drugs may interact directly with excipients or with residues or impurities therein. Such interactions may be modest, slow and take time to be detected in conventional stability studies. This can complicate and even delay project progression. When designing dosage forms therefore it is important to be aware of the potential for excipients to adversely affect quality and may lead to ADRs.8
Our own experience at subharti
a) Adverse Drug Reaction reporting is very poor in our 500 bedded, well equipped and fully staffed Chattrapati Shivaji Subharti Hospital, Meerut, Meerut is an important medical care centre of western U.P, located about 70 km from national capital, New Delhi. It boasts of about 250 practising doctors of various specialities, super specialities, with large number of hospitals and nursing homes. Sadly, spontaneous adverse drug reaction reporting is negligible despite continuous effort of our department which is a peripheral centre under National Pharmacovigilance Programme launched by Government of India in 2004. Worried by poor reporting of adverse drug events from our hospital, I was given thesis dissertation topic on Pharmacovigilance. The present study, a pilot study was conducted on 100 health care professionals of Chattrapati Shivaji Subharti Hospital (CSSH) Meerut to ascertain their Knowledge, Attitude, Practices (KAP) with the help of a questionnaire.
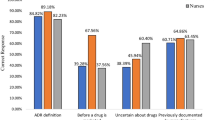
Observations & results:
All the data were tabulated and analyzed and the results were expressed in percentages. Based on the response received from the concerned Healthcare professionals, the following conclusions were derived:
The reasons for underreporting of ADRs were:
-
Non-availability of forms (97%)
-
Absence of stringent laws pertaining to adverse drug events reporting (89%)
-
Lack of awareness of Adverse Drug Reaction reporting form of Central Drug Safety Control Organisation (CDSCO) 68%
-
Non-communication about Adverse Drug Reactions of a new product by medical representatives (78%)
-
Non-communication about Adverse Drug Reactions of a new product by medical representatives (78%)
-
Lack of training as well as paucity of time (73%) (Table 1)
Suggested measures to increase reporting were:
-
Continuous Medical education (96%)
-
Compulsory reporting of adverse drug reactions (89%)
-
Information to consumer (80%)
-
Postal/online service for filling forms (76%) (Table 2)
Concluding remarks:
Based on the study, the following measures have been undertaken to improve ADR reporting in Subharti Medical College:
-
1)
Designing of simple and easy to fill ADR reporting forms separately for outpatient departments (OPDs) and internal patient departments (IPDs) in order to collect vital information essential for filling standard CDSCO forms;
-
2)
Regular training sessions for interns, junior residents and nurses about filling up of ADR forms designed by our department and CDSCO;
-
3)
Regular visits to various OPDs to sensitize and remind doctors about ADR reporting;
-
4)
Training of junior residents on various tools for assessing ADRs in terms of causality, severity and preventability;
-
5)
Based on the information received from various departments about suspected ADRs, CDSCO forms are filled up centrally and sent to zonal office of pharmacovigilance.
The study is still ongoing by undertaking above mentioned measures and it is our belief that with these measures in place, the receipt of information about adverse drug events would improve substantially.
References
I. Ribiero-Vaz, M. Teresa Herdiero, J.Polonia, & A. Figueiras. “Strategies to increase the sensitivity of pharmacovigilance in Portugal”. Rev SaudePublica 2011; 45(1)
R. Rajesh, S.Vidyasagar, & D. MuralidharVarma. “An educational intervention to assess knowledge, attitude, practice of pharmacovigilance among health care professionals in an Indian tertiary care teaching hospital”. International Journal of Pharm Tech Research 2011.
M. Kharkhar, & S. Bowalekar . “Knowledge, attitude and perception/practices (KAP) of medical practitioners in India towards adverse drug reaction (ADR) reporting”. PerspectClin Res 2012;3:90–4
M. Chakrabarty, & V. Thawani “Starting a pharmacovigilancecenter: Actions for implementation”. J PharmacolPharmacother 2011;2:295–9
CK Desai, G. Iyer, J. Panchal, S. Shah, & R.K Dixit. “An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital”. PerspectClin Res 2011; 2: 129–36.
R.Gerritsen, H.Faddegon, F.Dijkers, K.Grootheest & E. Puijenbroek.” Effectiveness of pharmacovigilance training of General Practitioners”. Drug Safety 2011-Volume34-Issue 9.
P.Mansell “Patient safety “Part of the DNA””. Available from http://www.pharmatimes.com/corporate profile 2012 Nov
P. J. Crowly, & L.G. Martini Effects of excipients on the stability of medicinal products. Chemistry Today Vol28 Sep/Oct 2010
Author information
Authors and Affiliations
Additional information

Dr. Neha Bhati, is currently pursuing M.D. (Post Graduate) degree in Pharmacology from Subharti Medical College, Meerut City (India). She did her High School Diploma from Memorial High School, Houston, Texas (USA). She returned to India for further studies and passed MBBS (Bachelor of Medicine, Bachelor of Surgery) degree from Gandhi Medical College, Bhopal (India). She presented the research paper titled “The value of Pharmacovigilance and post marketing surveillance in a developing country with severe Health resource crunch” in the First Annual International Conference of Pharmacology and Pharmaceutical Science held in Singapore from November 18-19, 2013 and won “Best Student Paper Award” in the conference.
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhati, N., Gupta, S. & Khosla, P. Assessment of Knowledge, Attitude and Practices (KAP) of Health Professionals Towards Adverse Drug Reactions (ADRs) and Pharmacovigilance in a Tertiary Hospital of North India. GSTF J Adv Med Res 1, 15 (2014). https://doi.org/10.7603/s40782-014-0015-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40782-014-0015-8