Abstract
The ecological plaque hypothesis for the aetiopathogenesis of caries implies a microbial shift towards a more acidogenic and aciduric dental plaque microflora, due to a frequent carbohydrate intake. Certain plaque bacteria exhibit metabolic activity, at a low pH. A correlation exists between the increased numbers of some aciduric bacterial species, e.g. mutans streptococci and lactobacilli, and caries activity. The aim of this study was to study the acidogenic (lactate production / mg plaque x min., at pH 7.0) and aciduric potential (lactate production at pH 5.5 / lactate production at pH 7.0) of dental plaque in relation to the caries activity. Samples of dental plaque were collected from fifteen caries free and fifteen caries active children. Plaque suspensions in Ringer’s solution containing 1% sucrose and buffered with 0.5 M MOPS (pH 7.0) or MES (pH 5.5) were incubated aerobically at 37 ºC for 10-20 min. The production of lactic acid in the suspensions was determined by an enzymatic assay. In caries free children, significantly lower acidogenic potential at both pHs were recorded than in caries active children. The highest difference between the groups was in the acidogenic activity at neutral pH. On the contrary, the aciduric potential was lower in the caries active group than in the caries free. Caries activity correlated with the acidogenic potentials of dental plaque at both pH 7.0 and 5.5. A new perspective of the ecological plaque hypothesis based on the increased catabolic ability of plaque is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most recently suggested aetiopathogenetic mechanism of caries, the ecological plaque hypothesis, proposes that the disease is the result of a shift in the ecological balance of dental plaque towards a more cariogenic flora [1]. The shift is usually caused by an exogenous factor, the frequent intake of fermentable carbohydrates, which leads to changes in the plaque environment, i.e. increased duration of acidic conditions. The environmental changes favor acid tolerant cariogenic bacteria that continue their catabolic activity even at low pH. These conditions affect the mineralization / demineralization equilibrium for the tooth hard tissues and can lead to the formation of caries lesions.
The ecological plaque hypothesis considers the caries disease as an ecological catastrophe characterized by a proportional increase of aciduric bacteria in the oral cavity, especially in dental plaque [2]. In its extended form, the hypothesis rsefers to dynamic changes in the plaque ecosystem, the changes including expression of aciduric characteristics by the bacteria and the concomitant establishment of new aciduric species as well [3], [4]. Several studies have correlated the caries activity with increased numbers of certain aciduric bacteria, such as mutans streptococci and lactobacilli [5], [6], [7], [8], [9]. However, data also indicate the involvement of several other aciduric and acidogenic bacteria in the caries process, these bacteria outnumbering the mutans streptococci and lactobacilli in dental plaque [10], [11], [12], [13], [14].
Lactate is the main acidic end product found in dental plaque during its exposure to excess of fermentable sugars, irrespective of the subject’s caries activity [15], [16], [17]. Upon sugar challenge, the concentration of lactate generated is higher in plaque from caries active than inactive subjects [16], [17], [18]. Furthermore, a faster pH drop and a lower minimal pH are associated with caries activity [10], [19].
The frequent acidification of dental plaque due to sugar exposure may induce adaptive microbial mechanisms to enhance acidogenicity and acidurance and it may also enable the proportional increase of aciduric bacterial species or strains in this environment [4]. The extent of these ecological changes depends on the intensity of plaque acidification that includes the extent of pH drop, the frequency and the duration of the acidic periods. Besides the sugar exposure, plaque acidification can be modified by several factors such as salivary flow and buffering, diffusion phenomena etc.
The results in most of the studies published are in line with the ecological plaque hypothesis in terms of association between caries activity and numbers of aciduric bacteria and also increased acid production and pH lowering in situ, as mentioned above. However, there is no direct evidence on the acid production rate of dental plaque in relation to environmental pH and the caries activity of the subject. In an attempt to find further support for the ecological changes occurring in the oral cavity upon development of caries disease, the present study was conducted with the aim to determine the capacity of dental plaque to produce lactate at neutral or acidic conditions and also to examine the relation of the acidogenic capacity to the caries activity of the subjects.
Materials and methods
Subjects
Thirty children 5-16 year-old participated (Table 1). All subjects declared being in good general health and receiving no medication. They visited the School of Dentistry in Thessaloniki, Greece for routine examination and dental treatment if needed. None of them was using orthodontic appliances and all children exhibited cooperative behaviour. The subjects were randomly selected as they were visiting the clinic. After having been examined, the subjects were asked for participating in the study and informed written consent was obtained from all subjects and / or children’s parent. The study was approved by the Ethics Committee of the Dental School of the University of Thessaloniki.
Dental examination was performed by the same dentist (GA), to determine the dmft/DMFT, using a dental mirror and an explorer under the dental chair’s light. Dental radiographs were taken if necessary. All the teeth with caries lesions of C2 (dentin lesion), C3 (pulp perforation), or C4 (only roots) counted as dt or DT. Half of the subjects recruited (15 children) were caries free and had no filling therapy during at least the last year while the remaining 15 children had active caries lesions that required treatment.
Sample preparation
The subjects refrained from tooth brushing for 2-3 days to allow plaque formation. On the 3rd day, they visited the dental clinic without having consumed any food or drink except water, during the 3 hours prior to plaque collection. Dental plaque was carefully scrapped off with a carver from all accessible tooth surfaces and placed in a pre-weighed plastic tube. The plaque sample was weighed to the nearest mg and suspended in modified Ringer solution containing (per liter) 0.6 g CaCl2, 2.1 g KCl, 5.0 g NaCl, and 0.05 g MgSO4 x 7H2O to yield approximately 15 mg plaque/ml. The suspension was homogenized with a glass mortar.
Determination of acidogenic and aciduric potentials
To an aliquot of plaque suspension, an equal volume of modified Ringer solution containing 1 M buffer and 2% sucrose was added. The buffer compound used in the neutral reaction mixtures was 3-[N-Morpholino]propanesulfonic acid (MOPS), pH 7.0 and in the acidic mixtures 3-[N-Morpholino]ethanesulfonic acid (MES), pH 5.5. The suspensions were incubated aerobically, at 37 ºC, for 10 minutes (neutral mixtures) or 20 minutes (acidic mixtures). At the end of the incubation, the mixtures were placed in an ice-water bath for 10 minutes to stop the metabolic activity and then centrifuged (10,000 x g, 5 min). The supernatants were aspirated and stored at -75 ºC until analyzed. The whole procedure was completed within 1 h from the sample collection.
The concentration of L-lactic acid in the supernatants was determined with an enzymatic method using a commercially available reagent kit (Sentinel Diagnostics, Sentinel CH, Milan, Italy)
The acidogenic potential of dental plaque was defined as the concentration (in mg) of lactic acid per mg plaque and min of incubation, at either neutral or acidic pH. The ratio of acidogenic potential at acidic pH by acidogenic potential at neutral pH is defined as the aciduric potential.
Statistical analysis
The data were analyzed with the ANOVA test (analysis of variance, software PASW Statistics 18) to show the correlation of the dental plaque/saliva potentials between the caries active and caries free groups. Pearson's correlation 2-tailed test was used to examine the correlation between caries activity and acidogenic or aciduric potential.
Results
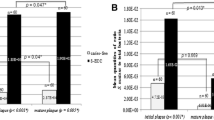
Statistically significant differences were observed between caries active and caries free children in the acidogenic and aciduric potentials of their dental plaque (Fig. 1). In caries free children, the acidogenic potential, at pH 7,0 ranged within 0.11 and 0.28 (mean 0.19) mg lactic acid / mg plaque x min. At pH 5.5, the mean value recorded (0.10) was approximately half of the one at pH 7.0. The acidogenic potential at pH 5.5 ranged from 0.04 to 0.18. In caries active children (mean dmft=6), the corresponding values were 0.62 (range 0.12-1.51) and 0.23 (range 0.05-0.72) at pH 7.0 and 5.5, respectively.
The aciduric potential varied from 0.2 to 0.8 (mean 0.52) for the caries free children and from 0.12 to 1.51 (mean 0.4) for the caries active subjects. The difference between the groups was –even barely- statistically significant (Fig. 1).
The correlation between the caries activity (number of decayed teeth) of the subjects and the acidogenic or aciduric potential of their plaque is shown in figure 2 (Fig 2). The acidogenic potentials, i.e. the lactate production, at pH 7 and at pH of 5.5 correlated (p<0.05) with the number of cavities and the highest correlation coefficient was observed with the potential at pH 5.5. On the contrary, no correlation was found between the caries activity and the aciduric potential.
Discussion
In accordance with earlier findings [16], [17], [18], [20], [21] the dental plaque from caries active children was found to produce more lactate from sucrose than the plaque from caries free children. The increased lactate-producing capacity was evident both at neutral and at acidic pH, indicating a significantly increased acidogenic and aciduric potential of the plaque flora in the caries active subjects. Furthermore, the increase in the acidogenic potentials correlates with the caries activity in terms of number of decayed teeth. These results are in line with previous observations showing that dental plaque covering enamel carious lesions exhibits higher pH-lowering capacity than plaque from healthy surfaces [22].
The increased acidogenic potential of the plaque flora from the caries active subjects was more prevalent at pH 7.0 than at pH 5.5. Comparing with the caries free subjects, the acidogenic potential of the caries active plaque was about three times higher at neutral pH but only twice as high at acidic pH. Thus, the aciduric potential, as defined in this paper, appears lower in the caries active subjects. The terms aciduricity and acid tolerance have often been used for oral bacteria to indicate their capacity of being catabolically active in an acidic environment. To be able to distinguish between an increased catabolic capacity in an acidic environment from an overall increased acidogenic capacity, we expressed aciduricity in relation to the catabolic capacity of the bacteria at the neutral pH. In this context, the present results show that the plaque flora of caries active subjects is rather characterized by a totally increased catabolic capacity, being stronger at neutral than at acidic pH, than by an increased aciduricity.
The pattern of a totally increased acidogenic capacity observed for the plaque of caries active subjects is similar to the one found for caries associated bacteria such as Streptococcus mutans and Streptococcus sobrinus, while the acidogenic pattern of plaque from caries free subjects resembles those of non-caries associated streptococcal species [23], [24]. Caries associated streptococci account for a minor part of plaque flora even in caries active patients [25], [26], being thus partly responsible for the total lactic acid production. Moreover, the acid production activity of these bacteria cannot be related alone to caries activity of the patients [27]. Strains of other oral microbial species were also found to possess acidogenic and aciduric capacities of similar magnitude [26], [28], which possibly implies their considerable contribution in the acid production in vivo. The present results support the aspect of an overall increased catabolic activity, irrespective of the environmental pH, that characterizes the plaque flora of caries active people.
Upon a sugar challenge, plaque with an increased acidogenic potential will produce a higher amount of acids and will cause a more rapid pH drop on the tooth surface than in the case of plaque with a lower acid production velocity. From a cariological perspective, this increased acidogenicity is decisive for tooth demineralization to occur, especially if it cannot be efficiently counteracted by the salivary flow. The good correlation presently found between the acidogenic potentials and the caries activity of the children supports the suggestion.
Acid tolerance has been suggested as an important property of the caries associated bacteria and several in vitro studies with mixed cultures indicated that a low pH rather than carbohydrate availability is responsible for microflora shifts associated with development of dental caries [29], [30], [31]. According to the ecological plaque hypothesis, aciduric bacteria have an ecological advantage that allows them to increase in numbers in sugar exposed dental plaque, while the growth of the non-aciduric species is suppressed.
It might be argued whether the increased aciduricity of the plaque flora is the main characteristic of the ecological shift induced by the frequent sugar intake in caries active people. Certain plaque bacteria besides mutans streptococci and lactobacilli can adaptively increase their acidogenicity when exposed to an acidic environment for 1 hour [32]. This in vitro finding indicates that a rapid adaptation of the plaque flora is possible if shortly exposed in vivo to an acidic environment, without a concomitant frequent exposure to fermentable carbohydrates. Thus, the shift of the microflora in terms of increased numbers of specific aciduric bacterial species in plaque from caries active subjects is not fully supported by the low pH of the plaque environment. The frequent exposure to excess fermentable carbohydrate, mainly sucrose, is also a prerequisite for this shift in the microflora [2].
Based on the present results and those earlier published and mentioned above, we suggest the following modification of the ecological plaque hypothesis and its extended form. The exogenous factor, i.e. the frequent sugar intake, increases the concentration of the carbohydrate in the plaque environment to levels and for certain time periods that favours bacteria with an increased catabolic capacity, i.e. a rapid acid production velocity. Together with a possible suppression of the non aciduric flora due to the low pH caused by the sugar fermentation, the increased carbohydrate concentration allows strongly acidogenic and aciduric bacterial species to colonize the habitat of dental plaque at higher numbers. The plaque microflora that undergoes such an ecological shift may contain high numbers of species with these characteristics, such as mutans streptococci and lactobacilli, but also high numbers of strains belonging to other microbial species and gained these acidogenic properties [33].
Figures
References
PD. Marsh Microbial Ecology of Dental Plaque and its Significance in Health and Disease ADR vol. 8 no. 2 263–271 July 1994
PD Marsh: Are dental diseases examples of ecological catastrophes? Microbiology; 149: 279–294, 2003
N Takahashi, B Nyvad. Caries ecology revisited: microbial dynamics and the caries process. Caries Res; 42: 409–418, 2008
N Takahashi, B Nyvad. The role of bacteria in the caries process ecological perspectives. J Dent Res; 90: 294–303, 2010
S Hamada, HD Slade. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev; 44: 331–384, 1980
JW Loesche. Role of Streptococcus mutans in human dental decay. Microbiol Rev; 50: 353–380, 1986
Stecksén-Blicks. Lactobacilli and Streptococcus mutans in saliva, diet and caries increment in 8- and 13-year-old children. Scand J Dent Res; 95: 18–26, 1987
D Beighton, PH Hellyer, EJ Lynch, MR Heath. Salivary levels of mutans streptococci, lactobacilli, yeasts, and root caries prevalence in non-institutionalized elderly dental patients. Community Dent Oral Epidemiol; 19: 302–307, 1991
JM Tanzer, J Livingston, AM Thompson. The microbiology of primary dental caries in humans. J Dent Educ; 65: 1028-1037, 2001
J van Houte, C Sansone, K Joshipura, R Kent. In vitro acidogenic potential and mutans streptococci of human smooth-surface plaque associated with initial caries lesions and sound enamel. J Dent Res; 70: 1497-1502, 1991
J van Houte. Role of micro-organisms in caries etiology. J Dent Res; 73: 672-681, 1994
J van Houte, J Lopman, R Kent. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res; 75: 1008–1014, 1996
C Sansone, J van Houte, K Joshipura, R Kent, HC Margolis. The association of mutans streptococci and non-mutans streptococci capable of acidogenesis at a low pH with dental caries on enamel and root surfaces. J Dent Res; 72: 508–516, 1993
D Beighton. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol; 33: 248–255, 2005
DAM Geddes. Acids produced by human dental plaque metabolism in situ. Caries Res; 9: 98–109, 1974
GE Minah, WJ Loesche. Sucrose metabolism in resting-cell suspensions of caries-associated and non-caries-associated dental plaque. Infect Immun; 17: 43–54, 1977
XJ Gao, Y Fan, RL Jr Kent, J Van Houte, HC Margolis. Association of caries activity with the composition of dental plaque fluid. J Dent Res; 80: 1834–1839, 2001
HC Margolis, EC Moreno. Composition and cariogenic potential of dental plaque fluid. Crit Rev Oral Biol Med; 5: 1–25, 1994
K Igarashi, Y Hamada, H Nishimaki, S Sakurai, K Kamiyama. The acidogenic potential of plaque from sound enamel, white spot lesions, and cavities in childen. Pediatr Dent; 9: 212-215, 1987
K Shimizu, K Igarashi, N Takahashi. Chairside evaluation of pH-lowering activity and lactic acid production of dental plaque: correlation with caries experience and incidence in preschool children. Quintessence Int.; 39: 151–158, 2008
HR Englander, WJ Carter, LS Fosdick. The formation of lactic acid in dental plaques J Dent Res; 35: 792–799, 1956
P Lingström, FO van Ruyven, J van Houte, R Kent. The pH of dental plaque in its relation to early enamel caries and dental plaque flora in humans. J Dent Res; 79: 770-777, 2000
de Soet JJ, FA Toors, de Graaff J. Strain-related acid production by oral streptococci. Caries Res;34 (6):486-90. 2000 Nov-Dec
de Soet JJ, B Nyvad, M Kilian. Strain-related acid production by oral streptococci. Caries Res; 34: 486–490, 2000
B Nyvad, M Kilian. Microflora associated with experimental root surface caries in humans. Infect Immun;58 (6):1628-33, 1990 Jun
FO van Ruyven, P Lingström, J van Houte, R Kent. Relationship among mutans streptococci, "low-pH" bacteria, and iodophilic polysaccharide-producing bacteria in dental plaque and early enamel caries in humans. J Dent Res; 79(2): 778-784, 2000 Feb
B kohler, D Birkhed, S Olsson. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res; 29(5): 402–6, 1995
S Kneist, H Kubieziel, B Willershausen, H Kupper, A Callaway. Modeling of S Nutans and A naeslundi acid production in vitro with caries incidence of low- and high-risk children Quintessence Int; 43(5):413-20. 2012 May
PD Marsh, DJ Bradshaw The effect of fluoride on the stability of oral bacterial communities in vitro. J Dent Res; 69: 668-71, 1990 Feb
DJ Bradshaw, PD Marsh Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res; 32(6):456–62. 1998
DJ Bradshaw, PD Marsh, RJ Hodgson, JM Visser. Effects of glucose and fluoride on competition and metabolism within in vitro dental bacterial communities and biofilms. Caries Res; 36(2):81-6. 2002 Mar-Apr
N Takahashi, T Yamada. Acid-induced acid tolerance and acidogenicity of non-mutans streptococci Oral Microbiol Immunol.;14(1):43-8. 1999 Feb
B Nyvad, M Kilian. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res; 24(4): 267–72, 1990
Author information
Authors and Affiliations
Additional information

George Andreadis was born in Thessaloniki, Greece, in 1971. He received the D.D.S. degree from the Dental School of the University of Thessaloniki. He then received his M.Sc. degree in pediatric dentistry from Leeds Dental Institute, UK, in 2002, and he is now finishing his Ph.D. degree in the University of Thessaloniki on the topic of acidogenicity and aciduricity of the dental plaque and saliva.
His research in Leeds was on the slow-release fluoride devices and has published his work. At the moment he works in his own dental practice and has lectured in international conferences.

Sotos Kalfas received his D.D.S. (in 1982) and Odont. Dr. (in 1989) degrees from the School of Dentistry in Malmö, University of Lund, Sweden, where he became Associate Professor in Oral Microbiology. In 1991, he was granted a postdoctoral fellowship stayed as a guest researcher at the Department of Oral Biochemistry, Dental School of Tohoku University in Japan. He worked as a senior dental officer and director of Clinical Oral Microbiology in School of Dentistry, Umeå University, Sweden. Currently, he is Professor of Preventive Dentistry in the School of Dentistry, Aristotle University of Thessaloniki, Greece. His research is mainly focused on microbial-host interactions in relation to caries and periodontal diseases.
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andreadis, G., Kalfas, S. Correlation of Dental Plaque Acidogenicity and Acidurance with Caries Activity – Perspectives of the Ecological Plaque Hypothesis. GSTF J Adv Med Res 1, 9 (2014). https://doi.org/10.7603/s40782-014-0009-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40782-014-0009-6