Abstract
Introduction:
Mesenchymal stem cells (MSCs) transplantation for the treatment of acute hindlimb ischemia is recently attracting the attention of many scientists. Identifying the role of donor cells in the host is a crucial factor for improving the efficiency of treatment. This study evaluated the injury repair role of xenogeneic adipose-derived stem cell (ADSC) transplantation in acute hindlimb ischemia mouse model.
Methods:
Human ADSCs were transplanted into the limb of ischemic mouse. The survival rate of grafted cells and expression of human VEGF-R2 and CD31 positive cells were assessed in the mouse. In addition, the morphological and functional recovery of ischemic hindlimb was also assessed.
Results:
The results showed that one-day post cell transplantation, the survival percentage of grafted cells was 3.62% ± 2.06% at the injection site and 15.71% ± 12.29% around the injection site. The rate of VEGFR2-positive cells had highest expression at 4 days post transplantation, 5.46% ± 2.13% at the injection site; 9.12% ± 7.17% at the opposite of injection site, and 7.22% ± 4.59% at the lateral gastrocnemius. The percentage of CD31 positive cells increased on day 4 at the injection site to 0.8% ± 1.60%, and further increased on day 8 at the lateral gastrocnemius site and the opposite injection site to 1.56% ± 0.44% and 1.17% ± 1.69%, respectively. After 14 days, the cell presentation and the angiogenesis marker expression were decreased to zero, except for CD31 expression at the opposite of injection site (0.72% ± 1.03%). Histological structure of the cell-injected muscle tissue remained stable as that of the normal muscle. New small blood vessels were found growing in hindlimb. On the other hand, approximately 66.67% of mice were fully recovered from ischemic hindlimb at grade 0 and I after cell injection.
Conclusion:
Thus, xenotransplantation of human ADSCs might play a significant role in the formation of new blood vessel and can assist in the treatment of mouse with acute hindlimb ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose-derived mesenchymal stem cells (ADSCs) are popularly used for the treatment of several diseases. ADSCs possess the ability to proliferate and differentiate into several types of functional cells such as adipocyte, osteocyte, chondrocyte, and muscle cell (Halvorsen et al., 2000; Strem et al., 2005). They also play an important role in repairing damaged tissues.
The role of ADSCs was demonstrated in the same way as that of bone marrow-derived mesenchymal stem cell (BM-MSC) for disease treatment (Halvorsen et al., 2000; Strem et al., 2005).
ADSCs are also used in xenogeneic transplantation because of their immunosuppressive ability (Puissant et al., 2005). The low expression of human leukocyte antigen (HLA), co-stimulatory molecules, B7 and CD40 ligand, and overexpression of MHC class II and Fas ligand are the specific immunological characteristics of ADSCs. Besides, ADSCs can inhibit the secretion of INF-α, TNF-γ, TH1, TH2, and IL-10, associated with the activation of natural killer cells and the maturation of dendritic cells. ADSCs also increase the rate of synthesis of regulatory T cell associated with the modulation of the immune system (Aggarwal and Pittenger, 2005). Thus, ADSCs are considered as a superior source of cell therapy applications for the treatment of autoimmune diseases and controlling the graft versus host disease (Aggarwal and Pittenger, 2005; Polchert et al., 2008; Yanez et al., 2006).
ADSCs transplanted into mice with acute hindlimb ischemia can differentiate into endothelial cells, mobilize vascular precursor cells, enhance the secretion of vascular growth factors to repair ischemic tissue, and prevent tissue damage from apoptosis. ADSCs could also associate with local cells and stimulate the formation of new blood vessel (Tongers et al., 2011). In hypoxia condition, ADSCs are mobilized to damaged tissues via interaction between surface receptors and ligands (Honczarenko et al., 2006; Von Luttichau et al., 2005). Here, they secrete some vascular growth factors such as VEGF, HGF, and TGF (Lee et al., 2009; Nakagami et al., 2006). These growth factors express active signals to attract precursor cells and enhance the cell survival by stimulating the proliferation of endothelial cells and new blood vessel formation. The role of ADSCs is demonstrated by Jalees Rehman et al. (2004), who showed that ADSCs were able to secrete VEGF five times more as compared to normal stem cells, enhance proliferation, and decrease the apoptosis of endothelial cells in hypoxia culture conditions. As a result, the treatment efficiency was increased significantly (Rehman et al., 2004). ADSCs can also differentiate into endothelial cells, when cultured in a medium containing VEGF to take part in angiogenesis. They contribute in new blood vessel formation in hindlimb ischemia mouse models by stimulating the PI3K pathway of endothelial cells (Cao et al., 2005). The capacity to form new blood vessel was demonstrated by a significant increase in capillary density at the ADSC-injected ischemic tissue (Lu et al., 2009).
In this study, we focus on the evaluation of the secretion and the differentiation of human adiposederived stem cells (hADSCs) in angiogenesis after acute hindlimb ischemia in mice.
Methods
Establishment of acute hindlimb ischemia mouse model
An acute hindlimb ischemia mice model was established according to published protocols of Ngoc Bich Vu et al. (2012) using 3-5-month-old immunosuppressed mice (Pham et al., 2014a; Vu, 2013). All procedures involving animals were approved by the Animal Welfare Committee of the Stem Cell Research and Application Laboratory, University of Science, VNUHCM, VN. Briefly, mice were anesthetized by ketamine-xylazine, and were fixed to trays. Hairy limb was shaved and thigh skin was cut along approximately 1 cm. Femoral artery and vein were separated from muscle, and then ligated at 2 sites, one at the femoral triangle and the other at the popliteal artery. An incision was performed between the 2 ligations. Damaged tissue recuperation was evaluated using graded morphological scales at the area of muscle necrosis, following the guidelines of Takako Goto et al. (2006) (Goto et al., 2006) and our previous studies (Pham et al., 2014b; Vu et al., 2015). The damage of limb was classified as Grade 0 (G0), if no change; GI, if necrosis in nail and toes; GII, if necrosis in feet; GIII, if necrosis in knee; and GIV, if total leg necrosis.
Cell culture
hADSCs were isolated according to our previous study (Van Pham et al., 2013), with the following 3 criteria: (1) hADSCs maintained the differentiation potential to form chondrocyte and adipocyte (2) possessed plastic adherent ability and fibroblastic-like appearance and (3) expressed CD44, CD73, and CD90 and did not express CD14, CD34, and CD45. hADSCs were cultured in MSCcult medium containing DMEM/F12 supplemented with 10% fetal bovine serum, 1% antibiotic, 100× antimycotic, 10 ng/mL EGF, and 10 ng/mL bFGF (Sigma, USA) in a humidified incubator with 5% atmospheric CO2 at 37°C. On reaching 70-80% confluence, hADSCs were detached by treating with 0.25% trypsin/EDTA and subcultured in fresh medium.
Transduction of hADSCs with green fluorescent protein (GFP)-lentivirus
GFP lentivirus-transduced hADSCs were used for labeling the cells to assess the role of the transplanted cell in the host. copGFP control lentiviral particles (Santacruz, USA) are lentiviral particles containing a copGFP coding construct for copGFP expression in mammalian cells after transduction. The transduction of lentiviral-activated particles was carried out according to the manufacturer’s instructions. Briefly, 1.5 × 105 – 2.5 × 105 cells were seeded in a 6-well tissue culture flask. Polybrene (8 μg/mL) (Sigma, USA) was added after approximately 24 h. After one day, fresh medium without polybrene was replaced and copGFP lentiviral particles were supplemented into the medium. GFP lentivirus-transduced cells were cultured for 7 days. The cells were further subcultured and medium replenished, if needed.
Cells stably expressing copGFP were isolated from MSCcult medium, supplemented with puromycin (8 μg/mL) (Sigma), and observed under fluorescence microscopy to ensure that gene transduction was successful.
The role of transplanted cells in the host
Six-to-twenty-week-old acute hind limb ischemia mice were injected with GFP-transduced hADSCs (GFPhADSCs) with a dose of 106 cells/100 μL phosphate buffer saline (PBS) at the ligature blood vessel.
To evaluate the transplanted cell presentation at ischemic hindlimb, the mice were anesthetized and scanned by iBox Explorer Imaging Microscope system. The GFP-fluorescence signals in the ischemic hindlimb were imaged under UV light until 8 days after cell transplantation. The images were recorded and analyzed by Vision WorksLS Image Acquisition and Analysis Software.
The survival rate of the transplanted cells at the ischemic hindlimb was assessed by flow cytometry. Thigh muscle tissue of GFP-hADSC transplanted mice was collected. Muscle tissue was then separated to 3 parts: the cell injection site (IS), the opposite of the injection site (OIS), and the lateral gastrocnemius site (LGS) (Fig. 5C ). The muscle tissue was finely cut and trypsinized using 0.5% Trypsin/EDTA to detach single cells. The rate of GFP-positive cells was analyzed by CellQuest Pro software (BD Biosciences). These single cells were also evaluated by analyzing the expression of the human angiogenic marker in the mouse by labeling with anti VEGFR2-PE and CD31-PE (BD Biosciences), and incubated at room temperature for 15 min. Finally, labeled cell population was analyzed by flow cytometer and CellQuest Pro software.
H&E stain
Muscle tissues were fixed in 4% paraformaldehyde for 24 h. Then, the muscle tissues were transferred to 30% sucrose until they sink to the bottom. Tissue sections were frozen, then cut into 10-γm-thick section and mounted on a slide. Slides were stained with hematoxylin and eosin. Tissue structure was assessed under the microscope.
Evaluating recuperation of acute hindlimb ischemia mouse
Damaged tissue recuperation was evaluated by using graded morphological scales representing an area of muscle necrosis following the guidelines of Takako Goto et al. (2006) (Goto et al., 2006). Briefly, the damage of limb was classified as Grade 0 (G0), if no change; GI, if necrosis in nail and toes; GII, if necrosis in feet; GIII, if necrosis in knee; and GIV, if total leg necrosis.
Statistical analysis
All the results were analyzed by using the GraphPad Prism 6.0 software and Microsoft Office 2011. Differences were considered significant at p ≤ 0.05.
Results
Characteristics of transplanted cells
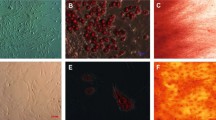
The morphology of GFP-hADSCs was similar to that of fibroblasts (Fig. 1A ). GFP-hADSCs were bright green under the fluorescence microscope (Fig. 1B ) and the percentage of GFP-positive hADSCs was over 97% (Fig. 1C ).
On the other hand, expression analysis of specific factors on MSC surface showed that ADSC was positive to VEGFR2 (100%) (Fig. 1E ), but negative to CD31 (1.48% ± 0.11% positive) (Fig. 1F ).
Transplanted cells presentation in the host
At 90 min after a GFP-hADSCs injection into the ischemic hindlimb, 100% of transplanted cells in the mouse exhibited green fluorescence at the injected location, when exposed to fluorescent light. Beyond that time, the percentage decreased from 93.75% on day 1 to 6.25% on day 7 (Fig. 2A ). On the other hand, both the intensity and area of fluorescence emission decreased. Stem cell-transplanted site exhibited strong luminous intensity, which was displayed in red (Fig. 2B ) over a large area, immediately after transplantation. After that, luminous intensity became weaker and was displayed by the yellow (Fig. 2C ) and green area (Fig. 2D ). Fluorescent signal was reduced and not found on day 8 (Fig. 2E ).
Transplanted cells presentation in the host. The rate of GFP expressed mouse was recoded for 8 days (A). Fluorescent light was displayed strong at injected site (B) as soon as cell injection, decreased at the third day (C) and the seventh day (D), no fluorescent signal was detected at the eighth day (E). (JS: the injection site, OJS: the opposite of injection site) (n=16).
Survival rate of transplanted hADSCs in the mouse
The survival rate of transplanted cell at the IS was 3.62% ± 2.06% (n=90 after 1 day and reduced to 3.03 ± 0.92% (n=11) on day 4. On the eighth day, GFP-hADSCs survival was significantly decreased by 13-fold, compared to day 4 (n=13; p>0.05). Interestingly, the presentation of GFP-hADSCs at the OIS was significantly higher than that at the IS (p<0.05), approximately 15.71% ± 12.29% (n=5) on the first day. However, this rate significantly reduced to 0.18% ± 0.09% on the eighth day (n=14; Fig. 3).
ADSC positive to VEGFR2 but negative to CD31
The rate of VEGFR2 positive cells was 3.57% ± 1.79% at IS and 6.69% ± 3.44% at LGS on the first day post transplantation, but not found at OIS (n=6). Then, it had the highest expression at 4 days post transplantation, approximately 5.46% ± 2.13% (n=10) at the IS; 9.12% ± 7.17% at the OIS (n=9), and 7.22% ± 4.59% at the LGS (n=9). After 14 days, it significantly decreased (p<0,05) (Fig. 4A-C ) to 2.07% ± 1.49% (n=9), 1.39% ± 0.67% (n=9), and 0.98% ± 0.94% (n=7) at the IS, OIS, and LGS, respectively. After 14 days, VEGFR2 expression was reduced to zero. This showed that hADSC could assist in angiogenesis via the VEGF signaling pathway.
CD31 is a specific marker of differentiation of hADSC to endothelial cell. This study investigated endothelial differentiation of GFP-hADSCs by estimating the percentage of human CD31-positive cells in mouse. When GFP-hADSCs were transplanted in the mouse with acute hindlimb ischemia, the percentage of CD31-positive cells increased to the highest on day 4 at IS, approximately 0.8% ± 1.60% (n=13). However, it increased to the highest on day 8 at LGS and OIS, approximately 1.56% ± 0.44% (n=9) and 1.17% ± 1.69% (n=1), respectively. On the other hand, it was highest at LGS on day 1 after transplantation, and reached 1.33% ± 2.05% (n=8), but not significantly different, when compared to IS and OIS. Cells in the hindlimb expressed CD31 at all sites on day 8, and reached 0.66% ± 0.57% (n=13) at IS, 1.17% ± 1.68% (n=10) at OIS, and 1.56% ± 1.44% (n=9) at LGS. On day 14, the rate of CD31 positive cells was decreased, compared to day 8 after transplantation (Fig. 4D-F ). Therefore, GFP-hADSCs could take part in endothelial differentiation of angiogenesis in mouse.
GFP-hADSCs stimulated the new blood vessel formation
New blood vessels were observed in GFP-hADSCsinjected acute hindlimb ischemic mice. The tiny blood vessels could be observed visually. New blood vessels had appeared in all the three areas (Fig. 5C ). However, the density of new blood vessels at the LGS was higher than that at the OIS and IS. On the other hand, the density of blood vessels was higher in the GFP-hADSCs group as compared with the PBS group (Fig. 5B ) and normal mice (Fig. 5A ). Thus, GFPhADSCs contributed in the formation of new blood vessels in mouse with acute hindlimb ischemia.
New blood vessel formation in acute hindlimb ischemic mouse after GFP-hADSCs transplantation. Distribution of blood vessel in the normal hindlimb compare to in the hADSC transplanted limb and PBS- injected limb at the fourth day. A high density of small blood vessels was presented at the lateral gastrocnemius site (LGS). A lower density was observed at the opposite of injection site (OIS) and injection site (IS).
GFP-hADSCs participated in restoring tissue structure better than no treatment
In a normal tissue, the skeletal muscle cells are arranged into bundles, and blood vessels (Yellow narrow) are scattered in the bundles (Fig. 6 ). In this study, the muscle bundles had broken structures, and muscle cells were incoherently arranged on day 3 in both the PBS and GFP-hADSCs-injected ischemic tissue. However, adipocyte formation was observed in the PBS group (Black narrow) from day 15 to 30, but new muscle cell (Green narrow) was found growing in the GFP-hADSCs group. On the other hand, there was new blood vessel formation in both the groups; however, the high density of small vessels was identified in the GFP-hADSCs group. This showed that GFP-hADSCs played an important role in remodeling damaged tissue, as in angiogenesis.
Recuperation of acute ischemic hindlimb
One day post transplantation, approximately 60% of mice had signs of tenderness, swelling, and skin crimson. The mice’s rate of recovery from limb ischemia and necrosis with GI was 57.78% (n=18). However, 22.23% of mice had serious injury with GII, GIII, and GIV. There were approximately 66.66% of mice without any damage or necrosis with GI after day 14 in the PBS injected-ischemic mice and about 33.34% of mice from GII to GIV (n=18). Thus, the recovery of GFP-hADSCs - transplanted acute ischemic hindlimb was better than non-treated limb.
Discussion
The disruption of blood flow leads to the lack of oxygen and nutrient supply to the tissue. This is established as hypoxia microenvironment at ischemic locations. In hypoxic condition, several inflammatory chemokines such as IL-1, TNF-α, TGF-β, and PDGF (Fox et al., 2007) are secreted to attract several cell types, which are able to repair the wound tissue (Overall et al., 1991; Ries and Petrides, 1995). hADSCs are one of the MSC sources possessing ideal woundhealing properties. In the host, hADSCs would be able to migrate and homing to damaged tissue, and stimulated to express receptors such as CXCR4 and CX3CR1, which play an important role in the homing of hADSCs (Togel et al., 2005; Zhuang et al., 2009) via Akt, ERK and p38 signal transduction pathways (Ryu et al., 2010). In other studies, hADSCs also exhibit several receptors associated with the ability of migration, such as CCR1, CCR4, CXCR5, CXCR6, CCR7, CCR9, and CCR10 (Honczarenko et al., 2006; Von Luttichau et al., 2005). In addition, hADSCs also express adhesion molecules such as integrin ligands, integrins, and selectins (Rüster et al., 2006). These molecules bind to ligands on the surface of the endothelial cells after hADSCs are stimulated by TNF- α from the damaged tissue (Rüster et al., 2006). Previous reports demonstrated that the migration involved the binding of VLA-4 on MSCs to VCAM-1 on the endothelial cells (Rüster et al., 2006). Furthermore, inflammatory markers stimulate hADSCs to produce matrix metalloproteinases (MMPs), which assist hADSCs to migrate across endothelial cells, lining the blood vessels into the injured tissue. Wenhui Jiang et al. (2006) showed that MSCs injected into the myocardium were homing to ischemic sites (Jiang et al., 2006). These studies have shown clear evidence of the migration patterns of hADSCs to damaged tissues. In our study, hADSCs were present not only at the local IS, but also were present surrounding the IS such as the OIS and LGS. This showed that transplanted cell migrated to other areas as well.
The cell migration was evaluated by measuring the fluorescence intensity reduction at the IS. However, the decrease in fluorescent intensity at the IS may also be because of the graft cell apoptosis or/and necrosis in the host. Transplanted cells can encounter with the lack of nutrients in the host (Forte et al., 2011). In ischemic condition, several processes such as the accumulation of metabolic wastes, oxidative stress, and the lack of nutrients and oxygen usually occurs, (Menon et al., 2014) leading to danger in the transplanted cells. D. Majumdar’s research also showed that the survival of human mesenchymal stromal cells is affected in ischemic microenvironment (Majumdar et al., 2013).
In hypoxia condition, ADSCs are stimulated to proliferate and exhibit wound-healing function (Lee et al., 2009; Nakagami et al., 2006). ADSCs are able to differentiate into endothelial cell, and secrete cytokines and angiogenesis growth factors such as VEGF, HGF, and FGF (Tongers et al., 2011). Some of the VEGF forms bind to its receptor such as VEGF-R2 (Flk-1/KDR), which is expressed almost exclusively in the endothelial cells (Neufeld et al., 1999). By the interaction of VEGF and VEGF-R2, vascular permeability was induced (Clauss, 2000; Henry et al., 2003; Hershey et al., 2003). VEGF binding stimulated proliferation and decreased apoptosis of endothelial cells, leading to the increased efficiency of ischemic hindlimb treatment. In addition, VEGF prevents apoptosis through phosphatidylinositol (PI)-3-kinase Akt pathway or through the stimulation of antiapoptosis Bcl-2 and A1 factors production in endothelial cells (Karar and Maity, 2011; Xiao et al., 2014). PI-3-kinase Akt pathway activates vascular growth factors such as eNOS and HIF-1α. In normal conditions, HIF-1α subunit is degraded by the hydroxylation of proline residues 402 and 564(Bruick and McKnight, 2001). In contrast, HIF-1 α dimerizes with HIF-1β into functional heterodimer that can activate transcription of target genes such as VEGF in a hypoxia microenvironment (Wang et al., 1995). eNOS is phosphorylated through HSP90-, which functions to express VEGF and activates PI3K/Akt pathway to produce nitric oxide (NO). The overexpression of HIF-1α leads to exhibit expression of VEGF (Semenza, 2003). The binding of VEGF to VEGFR2 not only phosphorylates proteins associated with the proliferation and survival of endothelial cells, but also forms blood vessels and increases the permeability of microvascular (Clauss, 2000; Flamme et al., 1995).
While hADSCs survived in the mouse with acute hindlimb ischemia, microenvironment signals assisted in accelerating angiogenesis pathways via the interaction of VEGF and VEGFR2 on the surface (Koch and Claesson-Welsh, 2012). The presence of VEGFR2 in the microenvironment showed that VEGF expression was stimulated. However, VEGF-R2 expression in vascular precursor cells depends on the stage of angiogenesis. VEGF expression was significantly increased in damaged tissues and assisted in all angiogenic processes, such as proliferation, tube formation, vascular branching, and remodeling. Jalees Rehman et al. (2004) demonstrated that hADSCs could secrete VEGF 5 times more in hypoxic condition as compared to normal conditions (Rehman et al., 2004).
Since hADSCs were differentiated into endothelial cells, they expressed marker CD31 (Bekhite et al., 2014). CD31 is a molecular marker, which has certain roles like adherence and transfer signal molecules, not only between endothelial and nearby cells, but also between endothelial cells and circulatory blood factors (Bekhite et al., 2014; Cao et al., 2005). Lauren J. Ficher et al. (2009) suggested that grafted cells begin to express CD31 marker after 2 days post transplantation and continue expressing until the eighth day. Our study showed that there was no expression of CD31 on the first day, but significantly increased on the eighth day.
This study demonstrated that hADSCs can survive and migrate to wound tissues in mouse. Besides, they also express CD31 and VEGF-R2, which imply their ability to differentiate into endothelial cells during angiogenesis in acute hindlimb ischemia mouse.
Conclusion
This study suggests that hADSCs play an important role in the angiogenesis of acute hindlimb ischemic mouse model. They can migrate, support angiogenesis, and differentiate into endothelial cells. The role of hADSCs is also demonstrated by assessing the formation of new blood vessels and the recuperation of acute hindlimb ischemic mouse. This shows promising potential to be used as an effective therapy in the treatment of vascular diseases.
References
Aggarwal, S., and Pittenger, M.F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822.
Bekhite, M.M., Finkensieper, A., Rebhan, J., Huse, S., Schultze- Mosgau, S., Figulla, H.R., Sauer, H., and Wartenberg, M. (2014). Hypoxia, leptin, and vascular endothelial growth factor stimulate vascular endothelial cell differentiation of human adipose tissue-derived stem cells. Stem Cells Dev 23, 333–351.
Bruick, R.K., and McKnight, S.L. (2001). A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340.
Cao, Y., Sun, Z., Liao, L., Meng, Y., Han, Q., and Zhao, R.C. (2005). Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332, 370–379.
Clauss, M. (2000). Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost 26, 561–569.
Flamme, I., Breier, G., and Risau, W. (1995). Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol 169, 699–712.
Forte, G., Pietronave, S., Nardone, G., Zamperone, A., Magnani, E., Pagliari, S., Pagliari, F., Giacinti, C., Nicoletti, C., Musaró, A., et al. (2011). Human Cardiac Progenitor Cell Grafts as Unrestricted Source of Supernumerary Cardiac Cells in Healthy Murine Hearts. STEM CELLS 29, 2051–2061.
Fox, J.M., Chamberlain, G., Ashton, B.A., and Middleton, J. (2007). Recent advances into the understanding of mesenchymal stem cell trafficking. British journal of haematology 137, 491–502.
Goto, T., Fukuyama, N., Aki, A., Kanabuchi, K., Kimura, K., Taira, H., Tanaka, E., Wakana, N., Mori, H., and Inoue, H. (2006). Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J Exp Clin Med 31, 128–132.
Halvorsen, Y.C., Wilkison, W.O., and Gimble, J.M. (2000). Adipose-derived stromal cells–their utility and potential in bone formation. Int J Obes Relat Metab Disord 24 Suppl 4, S41–44.
Henry, T.D., Annex, B.H., McKendall, G.R., Azrin, M.A., Lopez, J.J., Giordano, F.J., Shah, P.K., Willerson, J.T., Benza, R.L., Berman, D.S., et al. (2003). The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107, 1359–1365.
Hershey, J.C., Baskin, E.P., Corcoran, H.A., Bett, A., Dougherty, N.M., Gilberto, D.B., Mao, X., Thomas, K.A., and Cook, J.J. (2003). Vascular endothelial growth factor stimulates angiogenesis without improving collateral blood flow following hindlimb ischemia in rabbits. Heart Vessels 18, 142–149.
Honczarenko, M., Le, Y., Swierkowski, M., Ghiran, I., Glodek, A.M., and Silberstein, L.E. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24, 1030–1041.
Jiang, W., Ma, A., Wang, T., Han, K., Liu, Y., Zhang, Y., Dong, A., Du, Y., Huang, X., Wang, J., et al. (2006). Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch 453, 43–52.
Karar, J., and Maity, A. (2011). PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci 4, 5–1.
Koch, S., and Claesson-Welsh, L. (2012). Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2, a006502.
Lee, E.Y., Xia, Y., Kim, W.S., Kim, M.H., Kim, T.H., Kim, K.J., Park, B.S., and Sung, J.H. (2009). Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17, 540–547.
Lu, F., Li, J., Gao, J., Ogawa, R., Ou, C., Yang, B., and Fu, B. (2009). Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg 124, 1437–1446.
Majumdar, D., Bhonde, R., and Datta, I. (2013). Influence of ischemic microenvironment on human Wharton's Jelly mesenchymal stromal cells. Placenta 34, 642–649.
Menon, A., Napp, L.C., Galuppo, P., Bauersachs, J., and Tongers, J. (2014). Development of a Novel Model to Mimic Ischemic Microenvironments. Circulation 130, A19496–A19496.
Nakagami, H., Morishita, R., Maeda, K., Kikuchi, Y., Ogihara, T., and Kaneda, Y. (2006). Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb 13, 77–81.
Neufeld, G., Cohen, T., Gengrinovitch, S., and Poltorak, Z. (1999). Vascular endothelial growth factor (VEGF) and its receptors. Faseb j 13, 9–22.
Overall, C.M., Wrana, J.L., and Sodek, J. (1991). Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem 266, 14064–14071.
Pham, P., Bui, N.-T.A., Trinh, N.-L., Phi, T.L., Phan, K.N., and Vu, B.N. (2014a). A comparison of umbilical cord blood-derived endothelial progenitor and mononuclear cell transplantation for the treatment of acute hindlimb ischemia. Biomedical Research and Therapy 1, 1–12.
Pham, P.V., Bui, A.N.-T., Trinh, N.-L., Phi, L.T., Phan, N.K., and Vu, N.B. (2014b). A comparison of umbilical cord bloodderived endothelial progenitor and mononuclear cell transplantation for the treatment of acute hindlimb ischemia. Biomed Res Ther 1, 9–20.
Polchert, D., Sobinsky, J., Douglas, G., Kidd, M., Moadsiri, A., Reina, E., Genrich, K., Mehrotra, S., Setty, S., Smith, B., et al. (2008). IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 38, 1745–1755.
Puissant, B., Barreau, C., Bourin, P., Clavel, C., Corre, J., Bousquet, C., Taureau, C., Cousin, B., Abbal, M., Laharrague, P., et al. (2005). Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 129, 118–129.
Rehman, J., Traktuev, D., Li, J., Merfeld-Clauss, S., Temm-Grove, C.J., Bovenkerk, J.E., Pell, C.L., Johnstone, B.H., Considine, R.V., and March, K.L. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298.
Ries, C., and Petrides, P.E. (1995). Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler 376, 345–355.
Rüster, B., Göttig, S., Ludwig, R.J., Bistrian, R., Müller, S., Seifried, E., Gille, J., and Henschler, R. (2006). Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108, 3938–3944.
Ryu, C.H., Park, S.A., Kim, S.M., Lim, J.Y., Jeong, C.H., Jun, J.A., Oh, J.H., Park, S.H., Oh, W.I., and Jeun, S.S. (2010). Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun 398, 105–110.
Semenza, G.L. (2003). Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54, 17–28.
Strem, B.M., Hicok, K.C., Zhu, M., Wulur, I., Alfonso, Z., Schreiber, R.E., Fraser, J.K., and Hedrick, M.H. (2005). Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54, 132–141.
Togel, F., Isaac, J., Hu, Z., Weiss, K., and Westenfelder, C. (2005). Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67, 1772–1784.
Tongers, J., Losordo, D.W., and Landmesser, U. (2011). Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J 32, 1197–1206.
Van Pham, P., Bui, K.H.-T., Ngo, D.Q., Vu, N.B., Truong, N.H., Phan, N.L.-C., Le, D.M., Duong, T.D., Nguyen, T.D., Le, V.T., et al. (2013). Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Research & Therapy 4, 91–91.
Von Luttichau, I., Notohamiprodjo, M., Wechselberger, A., Peters, C., Henger, A., Seliger, C., Djafarzadeh, R., Huss, R., and Nelson, P.J. (2005). Human adult CD34-progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev 14, 329–336.
Vu, N.B., Trinh, V.N.-L., Phi, L.T., Phan, N.K., and Van Pham, P. (2015). Human Menstrual Blood-Derived Stem Cell Transplantation for Acute Hind Limb Ischemia Treatment in Mouse Models. In Regenerative Medicine: Using Non-Fetal Sources of Stem Cells, N. Bhattacharya, and G.P. Stubblefield, eds. (London: Springer London), pp. 205–215.
Vu, N.B., Trinh, V.N.-L., Phi, L.T., Bui, A.N-,T, Ta, V.T., Phan, N.K., and Van Pham, P. (2013). Optimizing the procedure for preparing immune-deficient hindlimb ischemia mouse model. Vietnam Joural of physiology 17, 27–36.
Wang, G.L., Jiang, B.H., Rue, E.A., and Semenza, G.L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92, 5510–5514.
Xiao, N., Qi, X.Y., Tang, L.N., Tan, L.L., Chen, Y.Q., and Zhao, H.M. (2014). VEGF promotes cardiac stem cells differentiation into vascular endothelial cells via the PI3K/Akt signaling pathway. Artif Cells Nanomed Biotechnol 42, 400–405.
Yanez, R., Lamana, M.L., Garcia-Castro, J., Colmenero, I., Ramirez, M., and Bueren, J.A. (2006). Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24, 2582–2591.
Zhuang, Y., Chen, X., Xu, M., Zhang, L.Y., and Xiang, F. (2009). Chemokine stromal cell-derived factor 1/CXCL12 increases homing of mesenchymal stem cells to injured myocardium and neovascularization following myocardial infarction. Chin Med J (Engl) 122, 183–187.
Cite this article as:
Dao, T., Vu, N., Phi, L., Le, H., Phan, N., Ta, V., & Pham, P. (2016). Human adipose-derived mesenchymal stem cell could participate in angiogenesis in a mouse model of acute hindlimb ischemia. Biomedical Research and Therapy, 3(8), 770–779.
Acknowledgements
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.06-2013.37.
Author information
Authors and Affiliations
Corresponding author
Additional information
1Laboratory of Stem Cell Research and Application, University of Science, Vietnam National University, Ho Chi Minh city, Viet Nam
2Department of Animal Physiology and Biotechnology, Biology Faculty, University of Science, Viet Nam National University, Ho Chi Minh city, Viet Nam
3Ha Noi Medical University, Ha Noi city, Viet Nam
*Corresponding author:vbngoc@hcmus.edu.vn
§These authors contributed equally to this work
©The Author(s) 2016. This article is published with open access by BioMedPress (BMP)
Competing Interests
The authors declare they have no competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dao, TT., Bich Vu, N., Phi, L. et al. Human adipose-derived mesenchymal stem cell could participate in angiogenesis in a mouse model of acute hindlimb ischemia. Biomed Res Ther 3, 37 (2016). https://doi.org/10.7603/s40730-016-0037-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40730-016-0037-1