Abstract
Pentacyclic triterpenic compounds including asiatic, betulinic, maslinic, oleanolic and ursolic acid occur naturally in many herbs and plant foods. It is well known that these triterpenoids possess anti-oxidative and anti-inflammatory activities. Furthermore, recent in vitro and in vivo researches indicated that these compounds could inhibit the production of advanced glycation end-products (AGEs). The impact of these triterpenes upon the activity and protein expression of enzymes involved in polyol pathway including aldose reductase and sorbitol dehydrogenase has been examined, and positive results are reported. These studies suggest that certain triterpenes are potent anti-glycative agents, and may benefit the prevention and/or therapy of glycation-related diseases such as diabetes mellitus and Alzheimer’s disease. In this review article, the anti-glycative activity and action mode of certain triterpenes are highlighted. These information may promote the anti-glycative application of these natural compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
Glycative stress from excessive production of advanced glycation end-products (AGEs) enhances the pathogenic progression of several chronic diseases such as diabetes, aging and Alzheimer’s disease. Several enzymes such as aldose reductase and glyoxalase I are involved in AGEs formation and accumulation. Thus, any agent(s) with the capability to limit AGE generation, promote AGE degradation, and/or reduce the protein expression and activity of associated enzyme(s) may potentially alleviate glycative stress and delay the development of glycation associated diseases.
2. Pentacyclic triterpenes
Triterpenes consist of six isoprene units, and are abundant in the plants in the form of free acids or aglycones [1]. There are more than 80 distinct types of triterpenes have been identified, according to structure and chemical characteristics. Triterpenes are grouped into euphanes, taraxanes, oleananes, lupaneps, ursanes and baccharanes, in which ursanes and oleananes are the major triterpene skeletons in higher plants. In Asian countries including Taiwan, China and Japan, many herbs used for formula or folk medicine are rich in triterpenes and provide medical effects to prevent or treat a variety of diseases [2]. Furthermore, many triterpenes are widely used as edible flavors, pigments, polymers, fibers and glues for food industry or pharmaceuticals. Pentacyclic triterpenes could be further classified into lupane, ursane and oleanane groups. Recently, the bioactivities of certain pentacyclic triterpenoids such as asiatic acid, betulinic acid, corosolic acid, glycyrrhizic acid, maslinic acid, oleanolic acid, ursolic acid, uvaol and erythrodiol have attracted more attention, and been considered as important sources of nutriceuticals and complementary medicines. These pentacyclic triterpenes are present in herbs such as ground ivy (Glechoma hederacea), plantain (Plantago major L.), thyme (Thymus vulgaris), glossy privet (Ligustrum lucidum Fructus), and hawthorn fruit (Crataegi Pinnatifidae Fructus); fresh fruits such as apple (Malus domestica Borkh), carambola (Averrhoa carambola), blueberry (Vaccinum dunalianum), guava (Psidium guajava), calamondin (Citrus microcarpa Bonge), persimmon (Diospyros kaki L.), and loquat (Eriobotrya japonica); and fresh vegetables such as olive (Olea europaea L.), gynura (Gynura bicolor DC), daylily (Hemerocallis fulva L.), basil (Ocimum basilicum), water convoevueus (Ipomoea aquatic), spinach (Spinacia oleracea L.), mahogany (Toona sinensis) and leaf mustard (Brassica juncea). The content of each pentacyclic triterpene in these edible plant foods is dependent on the species, season, and conditions of cultivation [3-5]. Since the consumption of fresh vegetables and fruits is encouraged for healthy enhancement, more interest has been raised to understand the benefits of these special plant food component(s) upon health and/or disease prevention. Therefore, exploring the bioactivities and action modes of certain pentacyclic triterpenes merits our attention.
3. Bioactivities of pentacyclic triterpenes
It has been documented that some pentacyclic triterpenes possess anti-oxidative, anti-inflammatory, anti-cancer and vasodilatory activities [6, 7]. These findings suggest that these pentacyclic triterpenes are potent agents for diseases prevention and/or alleviation.
3.1. Anti-oxidative activities
Excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) including superoxide anion, hydrogen peroxide, hydroxyl radical, or nitric oxide has been highly linked to the development and progression of many chronic diseases including aging, diabetes mellitus, cancer, atherosclerosis, Alzheimer’s disease, cirrhosis and Parkinson’s disease [8-10]. It is known that these ROS and RNS, via their free radical property, could directly attack cell apparatus such as mitochondria and impair functions or induce cell death. Furthermore, these ROS and RNS, via acting as signal transduction mediators, could mediate the protein expression of genes encoded for enzymes or factors which are responsible for cell differentiation, growth, migration, invasion and/or apoptosis. These events, in turn, harm the antioxidative defense system, enhance oxidative stress and impair organ functions [11, 12]. In addition, these free radicals could affect both local and systemic immune systems, which consequently promotes inflammatory reactions, and even causes irreversible inflammatory injury [13, 14]. Many cell lines, animals and even human studies highlight the evidence that pentacyclic triterpenes could provide anti-oxidative activities via scavenging free radicals; sparing non-enzymatic antioxidants such as reduced glutathione, ascorbic acid and alpha-tocopherol; increasing the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase and glutathione S-transferase; as well as suppressing NADPH oxidase, [15, 16]. By executing these above actions, certain pentacyclic triterpenes are able to diminish oxidative stress, which might contribute to delay the development and/or retard the progression of chronic diseases. Meanwhile, more human and even clinical studies are required to further confirm the anti-oxidative protection and application of these triterpenes.
3.2. Anti-inflammatory activities
Inflammation is a protective process involving host cells, blood vessels, associated proteins and other mediators which are intended to eliminate the invaders and necrotic cells, as well as to repair tissues. Many T-helper cell type 1 and 2 inflammatory cytokines and chemokines including interleukin (IL)-1, IL-6, c-reactive protein (CRP), tumor necrosis factor (TNF)-alpha, cyclooxygenase (COX)-2, monocyte chemoattractant protein (MCP)-1 and prostaglandin E2 (PGE2), are mediators involved in the host immune and defensive system against stimuli such as pathogens and chemicals [17, 18]. However, under pathological situations such as diabetes mellitus, cardiovascular disease, autoimmune disease and cancer, the overproduction of inflammatory factors causes cytokine imbalance, evokes inflammatory injury, or even leads to tissue destruction. Furthermore, these cytokines and chemokines activate macrophages and/or modulate crucial mediators responsible for pathological processes, which in turn favor the development of acute and chronic diseases. For instance, IL-8 promotes the formation of transforming growth factor (TGF)-beta, which enhances angiogenesis and fibrosis in solid tumors and facilitate cancer metastasis [19, 20]. Thus, the use of appropriate agent(s) with anti-inflammatory effects could decrease the generation of inflammatory stimuli and delay disease progression. The anti-inflammatory effects and possible action modes of several pentacyclic triterpenes in cell lines, animals, and even humans have been reported [21-23]. It is reported that asiatic acid and betulinic acid could regulate nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPK), two important signaling pathways, to lower the production of ROS and inflammatory cytokines [24, 25]. Taraxasterol could reduce TNF-alpha, NO and PGE2 levels in sera from mice with endotoxic shock [26]. These studies also indicated that these triterpenes were able to regulate both up-stream and down-stream inflammatory factors including COX-2, and inhibit the protein expression of cytokines and/or chemokines. These evidence support that these pentacyclic triterpenes could alleviate inflammatory stress, and decline the progression of chronic diseases.
3.3. Other bio-activities
Anti-tumor. Betulinic acid induces mitochondrial membrane permeabilization and causes apoptosis in prostate cancer cells [27]. Maslinic acid executes its cytotoxic activities toward lung cancer A549 cells through mediating mitochondrial intrinsic apoptotic and hypoxia-inducible factor (HIF)-1alpha pathways [28]. Ursolic and oleanolic acid cause apoptosis in liver cancer cells via disturbing mitochondrial membrane ion homeostasis [29]. These findings suggest that these triterpenes are potent anti-cancer agents.
Anti-microorganisms. Asiatic acid and corosolic acid could enhance susceptibility of P. aeruginosa biofilms to tobramycin [30]. Ursolic acid, glycyrrhizic acid and their derivatives may protect liver cells against injury induced by hepatitis B or C virus [31, 32]. Ursolic acid and its derivatives could inhibit the growth of several bacteria including E. coli, S. aureus, P. aeruginosa and K. pneumonia [33]. Apparently, these compounds could be applied for infection prevention or therapy.
Anti-obesity. Ursolic acid may stimulate lipolysis by translocating hormone-sensitive lipase, lowering perilipin A expression, and up-regulating adipose triglyceride lipase in primary culture adipocytes [34]. 18β-Glycyrrhetinic acid inhibits adipogenic differentiation and stimulates lipolysis in matured adipocytes [35].
Based on the above reported bioactivities, these pentacyclic triterpenes are potent medicinal agents and could be considered as candidates for new drug development. So far, more information is also available regarding their activities against glycation, another important pathological factor involved in the progression of many chronic diseases.
4. Glycation and chronic diseases
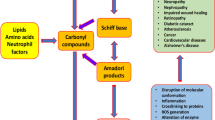
Non-enzymatic glycation with the formation of Maillard reaction products, also known as AGEs, plays an important role in the pathogenesis of many chronic diseases, e.g., diabetes mellitus, Alzheimer’s disease, atherosclerosis, osteoarthritis, inflammatory arthritis and cataracts [36-38]. Any agent(s) with the capability to inhibit the formation of AGEs may potentially diminish glycative stress and delay or retard the progression of glycation-related diseases. It is known that hyperglycemia, a common pathological characteristic of diabetes, promotes glucose metabolism through the polyol pathway [39]. Aldose reductase (AR), the first and rate-limiting enzyme in this pathway, reduces glucose to sorbitol, which could be further converted to fructose by sorbitol dehydrogenase (SDH), the second enzyme in this pathway [40, 41]. This flux through SDH increases fructose formation, and in turn enhances AGE production and promotes microvascular abnormalities [42, 43]. On the other hand, glyoxalase (GLO)-1, part of the glyoxalase system existed in cytosol of cells, metabolizes reactive alpha-carbonyl compounds such as glyoxal and methylglyoxal, and consequently reduces the available precursors of AGEs [44]. Because AR, SDH and GLO-1 are key enzymes involved in endogenous glycative reactions, and responsible for the generation or degradation of AGEs, the development of new drugs to mediate these pathways and mitigate glycative stress should pay more attention to these enzymes. That is, any agent with the ability to suppress the activity and protein expression of AR and SDH, as well as enhance GLO-1 activity and protein expression, may suppress glycative reactions and decrease AGEs production.
5. AGEs
AGEs are mainly formed by the reactions between reducing sugars including glucose, ribose and ascorbate, and the amino groups of amino acids from proteins or other moieties from lipids or nucleic acids. Amadori rearrangement is involved in AGEs synthesis and leads to various forms and characteristics. That is, AGEs are formed via a complex cascade of reactions including dehydration, condensation, fragmentation, oxidation, and cyclization. In addition, reactive dicarbonyl compounds such as methylglyoxal are precursors, also endogenous source, for the extracellular generation of AGEs [45], because methylglyoxal could easily react with lysine or arginine residues to produce imidazolone adducts, Nε-(carboxyethyl)lysine (CEL) and other compounds [46]. Thus, AGEs are protein-bound mixtures consist of nitrogen- or oxygen-containing heterocyclic compounds. Besides pentosidine and Nε-(carboxymethyl)lysine (CML), the structures and chemical properties of many AGEs need further studies to be characterized. Although lowering AGEs in circulation and tissues could attenuate systemic glycative stress, both decreasing AGE formation and increasing AGE degradation and excretion are difficult challenges because AGEs possess the properties of irreversibly cross-linked, heterogeneous and insoluble protein aggregates. However, the development of anti-AGEs drug is definitely necessary and important in order to control the high incidence and morbidity of glycation associate diseases. Glycated hemoglobin, CML, glycated albumin, and pentosidine may serve as markers of disease progression [47, 48] because they are common AGEs present in the circulation or organs of patients with diabetes mellitus or Alzheimer’s disease. Circulating AGEs are consisted of two sources: exogenous and endogenous. The former is from the dietary intake; the latter are synthesized in the organs such as kidney or heart under normal and pathological conditions. The intake of foods rich in glycative products enriches the circulating AGE pool and also increases local and systemic glycative stress [49]. Obviously, persons with glycation associated diseases should limit their dietary intake of AGE containing foods in order to avoid the deterioration of existed diseases. Endogenous AGEs may be formed during natural aging for most people. Under normal physical condition, the tissue content of AGEs depends on the kinds and rates of AGE formation and degradation. Usually, AGEs deposit in circulation or organs during natural aging is ascribed to the time-dependent property because advanced glycation is always coupled with oxidative stress. That is, AGEs content under this condition could be decreased as long as oxidative stress has been neutralized [50]. However, the progression of diabetes, renal failure, atherosclerosis and neurodegenerative disease markedly promotes endogenous AGE production. It is reported that hyperglycemia or other pathological conditions such as renal failure accelerate the production and accumulation of AGEs locally or systemically, in which oxidative stress plays an important role to boost AGE generation via glycoxidation and lipid peroxidation [51, 52]. On the other hand, AGE degradation is dependent on its ligation to macrophage scavenger receptors, protein turnover rate and renal capability for clearance [53]. Apparently, glycation is regulated by multiple factors and conditions. The management of glycation associate diseases is complicated and attracts more challenge.
6. AGEs in diabetes mellitus
AGEs are effective contributors toward the pathogenesis of diabetes related macro- and microvascular complications, and the circulating AGEs level are positively correlated to the clinical stage of patients with diabetic complications such as nephropathy or cardiomyopathy [54, 55]. Renal tubular and interstitial cells are the most vulnerable targets for increased glycative stress because hyperglycemia stimulates the tubular cells to secrete vasoactive hormones or factors including angiotensin II, transforming growth factor (TGF)-beta and extracellular matrix proteins such as collagen, which in turn facilitate the synthesis of cross-link components and thicken the basement membrane [56]. These events not only increase AGEs formation in the target cells, but also activate intracellular signal transduction pathways including MAPK and NF-κB, and accelerate the generation of ROS, RNS, inflammatory cytokines, fibrotic factor like fibronectin and angiogenic factors like vascular endothelial growth factor [57, 58]. Finally, an organ or a system is malfunctioned and even failure. In addition, circulating AGEs could be reabsorbed and further metabolized by the proximal tubular epithelial cells. Massive AGEs in renal tissue through declining protein breakdown evoke renal cellular hypertrophy, and cause diabetic nephropathy [59]. After stimulated by AGEs, the organs like kidney could further secrete various intracellular second messengers such as nitric oxide synthase, and subsequently induce the expression of adhesion molecules to benefit the progression of fibrosis and angiogenesis [60, 61]. These studies strongly link AGEs to oxidative, inflammatory and fibrotic injury in diabetes. Therefore, decreasing the level of AGEs in circulation and organs is helpful in order to attenuate or delay the occurrence of these complications.
7. AGEs in Alzheimer’s disease
The brain of patients with Alzheimer’s disease has two major neuro-pathological hallmarks, extracellular senile plaques and intracellular neurofibrillary tangles. Senile plaques contain beta-amyloid (Aβ) peptide, and neurofibrillary tangles contain hyper-phosphorylated microtubule associated protein tau [62]. AGEs could be found in both neurofibrillary tangles and senile plaques. Furthermore, CML is mainly localized in the cytoplasm of neurons, astrocytes and microglia in the brains of elder people or patients with Alzheimer’s disease [63, 64]. So far, it is still unknown that these AGEs deposited in brain tissue are endogenously synthesized or exogenously from dietary intake, or may be both. However, it is documented that the accumulation of CML and pentosidine, two major glycative products, are highly associated with the progression of Alzheimer’s disease and other aging associated diseases [65, 66]. These literatures strongly suggest that AGEs play crucial roles for the pathogenesis of brain degradation. AGEs are able to induce neuronal cell apoptosis by acting as neurotoxins or inflammatory mediators to stimulate inflammatory cytokines production and enhance inflammatory stress [67, 68]. Thus, AGEs accumulation in brain neurovascular wall not only changes brain functions, but also speeds up the deterioration of dementia including Alzheimer’s disease. The level of CML and its glycation-specific precursor hexitollysine is markedly increased in neurons of patients with Alzheimer’s disease, especially those with intracellular neurofibrillary pathology [69]. It is commonly considered and accepted that oxidative stress destabilizes many macromolecules including sugars, lipids, proteins and DNA, which promotes glycation reactions and causes an early response in many chronic neurodegenerative diseases such as normal aging process and Alzheimer’s disease. Furthermore, the increased hexitollysine or CML in brain could be partially ascribed to lipid peroxidation in this tissue because of the rich lipid and oxygen content [70], which inevitably leads to neuronal dysfunctions. This oxidative-stress hypothesis regarding the pathogenesis of Alzheimer’s disease suggests that AGEs overproduction in brain tissue is accompanied by excessive formation of oxygen-derived free radicals in cerebrovascular disorders [71].
8. Receptors for AGEs
The receptor for AGE, also called as RAGE, is expressed in diverse types of cells including endothelial and tubular epithelial cells. RAGEs could engage many ligands associated with distinct pathological processes, which in turn promote the progression of these diseases. AGE is one class of RAGE ligand, and the engagement of AGE with RAGE as occurred in diabetic complications, renal failure or amyloidosis is responsible for glycative stress in these illnesses. So far, enhanced RAGE expression has been reported in various cells under diabetic conditions [72]. It is commonly explained as that hyperglycemia stimulates the production, protein expression, of RAGE ligands, which subsequently interact with AGEs in the circulation and tissues and forms AGERAGE complex. Furthermore, AGEs, from either dietary source or endogenous source, are able to up-regulate RAGE expression [73]. On the other hand, RAGE acts as a signal transduction receptor for Aβ peptide accumulated in the affected brain parenchyma and cerebral vasculature to enhance the development of Alzheimer’s disease. Thus, the increased AGE-RAGE complex activates signaling pathways, aggravates neuro-inflammation, and finally impairs memory and learning [74]. Meanwhile, RAGE’s ligands are also consisted of S100/calgranulins, high-mobility group box 1 and beta-sheet fibrils, and the reactions between RGAE and these ligands generate pro-inflammatory and prothrombotic molecules and ROS. These events eventually augment oxidative and inflammatory injury, and thrombotic risk in the target tissues, such as atherosclerotic plaques and cardiac infarction [75, 76]. Besides endothelial and epithelial cells, RAGE and its ligands are also presented in tumor cells, neurons cells, podocytes, and smooth muscle cells. The engagement of RAGE and its ligands may trigger diverse signaling cascades including p21ras, ERK1/2, p38 JNK, and Jak/STAT in these targets [77, 78]. Although RAGEs and their ligands play independent roles in the pathology of several illnesses, AGE-RAGE interaction still attracts more attention because it directly activates many crucial signaling mechanisms. It has been indicated that AGE-RAGE interaction stimulates O2- production and raises oxidative stress [79], induces vascular inflammation and thrombosis via activating NF-κB [80, 81], and up-regulates the protein expression of adhesion molecules, chemokines, pro-inflammatory cytokines, matrix metalloproteinases and RAGE itself [82, 83]. Consequently, NF-κB elicits the expression of downstream genes encoded for TNF-alpha, IL-6 and MCP-1, which promote inflammatory reactions and cause irreversible impairment in the target tissues [84, 85]. Glycative stress from AGEs, RAGEs and their interaction is a contributor toward the progression of diabetes mellitus and Alzheimer’s disease; however, the impact of this AGE-RAGE axis upon other diseases like cancer could not be ignored. For instance, ROS and cytokines generated from this axis lead to DNA oxidative and inflammatory damage, and may initialize carcinogenesis. Besides AGEs and Aβ, RAGE can bind to other ligands such as low-density lipoprotein and calgranulins. Apparently, the impact of AGEs, RAGEs, RAGE ligands and their interactions upon human health is not limited to glycative stress. Therefore, any strategy against glycation-associated chronic diseases must consider: (1) lowering the exogenous and endogenous levels of AGEs in the circulation; (2) decreasing the available other ligands of RAGE in the circulation; and (3) interfering the interaction of RAGE and its ligands. That is, any possible inhibitor(s) with the capability to block AGE formation, decline RAGE expression, or interrupt the AGE-RAGE interaction could be considered as a potent candidate for treating diabetes mellitus, Alzheimer’s disease, and other glycation-related diseases.
9. Anti-glycative potential of pentacyclic triterpenes
Circulating AGEs could be from the dietary intake and from endogenous generation. Many foods are rich in pentosidine and/or furosine, and some of them are processed by sugar, heat or certain sauces [86, 87]. It is reported that foods cooked by baking or deep frying also contain high AGE levels, in which high temperature, lipids and proteins participate Maillard reactions and lead to the synthesis of various forms of AGEs [88, 89]. Apparently, in order to decrease circulating levels of AGEs from exogenous sources, the consumption of foods rich in glycative products should be limited, especially for patients with diabetes or Alzheimer’s disease. On the other hand, endogenous AGEs could be formed between reducing sugars and amino acids present in the circulation and tissues. The pool of reducing sugars and amino acids in the human body is large and unlimited, unfortunately. Thus, lowering the intake of reducing sugars or amino acids may not make any sense for improving health because this limitation definitely impairs nutritional status. The other alternative is to ingest other natural compound(s) with anti-glycative effects to counteract endogenous AGE formation and/or mediate AGE metabolism. Recently, in vitro inhibitory effects of some pentacyclic triterpenes such as astragalosides, boswellic acid and corosolic acid upon the formation of AGEs or their precursors like methylglyoxal and CML have been reported [90-92]. The results suggest that these pentacyclic triterpenes may halt the interactions between reducing sugars and amino acids, decreasing AGE generation and attenuating glycative stress via non-enzymatic actions. Both oxidative and inflammatory reactions benefit glycative processes, and the anti-oxidative and anti-inflammatory activities of several pentacyclic triterpenes such as ursolic acid, oleanolic acid, corosolic acid, maslinic acid, glycyrrhizic acid and erythrodiol have been already demonstrated in rodents [93-96]. Thus, it is highly possible that these pentacyclic triterpenes directly decrease oxidative and inflammatory stress, which in turn and indirectly mitigates glycative stress. These findings, at least, agreed that these compounds were potent agents and could be considered for anti-glycative protection. The other possibility is to explore the agent(s) with the ability to mediate AR, SDH or GLI, which subsequently reduces the production of AGEs or increases the degradation of AGEs. It is reported that oleanolic and ursolic acid could inhibit the activity and/or protein expression of AR and SDH, two major enzymes in polyol pathway, which consequently lowered AGEs levels in kidney, liver or brain [97-99]. Furthermore, AGEs metabolism could be facilitated by up-regulating GLO-1. Other studies revealed that protocatechuic acid and glycyrrhizic acid could enhance the expression of GLO-1, and lowered the level of fructose, methylglyoxal and CML in brain of aging mice or kidney of diabetic mice [99, 100]. These studies suggest that endogenous AGE generation could be suppressed by certain pentacyclic triterpenes, which finally contributes to diminish glycative stress under those pathological conditions. The third possibility is to discover the anti-RAGE agent(s). It is strongly convincible that suppressing RAGE expression and/or interrupting the AGERAGE interaction could more efficiently block glycative reactions and retard pathological progression in glycation associated diseases. A cell line study indicated that glycyrrhizic acid was able to down-regulate RAGE expression [101]. Ursolic acid could decline RAGE expression in brain of aging mice [102]. The decreased RAGE expression could definitely lower AGERAGE complex and diminish glycative stress. Although the support from in vivo studies regarding the anti-RAGE effects of pentacyclic triterpenes might not be sufficient, the effort toward this direction should be encouraged because the anti-RAGE agent(s) may provide multiple medical benefits. In addition, human studies and clinical trial for these pentacyclic triterpenes regarding their anti-glycative effects and action modes will be highly beneficial to demonstrate their effects and elucidate the actions. Certainly, the exploration of other non-triterpenoic antiglycative agents is also warranted to fight glycation-associated disease.
10. Blood-brain barrier
One crucial challenge of researches regarding pentacyclic triterpenes is their bioavailability. It is a common and important sense that the development of any pharmacological agent against Alzheimer’s disease has to consider whether this substance could pass through the blood-brain barrier, tightly packed layers of endothelial cells, which surrounds the brain to block high-molecular- weight molecules from penetrating it. This blood-brain barrier mainly acts to block the influx of intravascular substances from the circulation to the brain, and also mediates the transport of substances from brain to circulation via several transport systems such as carrier-mediated transport, active efflux transport and receptor-mediated transport [103, 104]. Furthermore, blood-brain barrier is essential for maintaining brain Aβ homeostasis and regulating Aβ transport [105]. Obviously, it is definitely essential to examine whether pentacyclic triterpenes with the capability to pass through the blood-brain barrier.
11. Conclusion
Pentacyclic triterpenes are compounds naturally occurring in many plant foods. Based on their anti-oxidative and anti-inflammatory activities, regulation upon AR, SDH and GLO-1, these agents may improve glycative stress. Future studies should probe the effects and action modes of these pentacyclic triterpenes upon RAGEs, AGE-RAGE interaction and blood-brain barrier penetration. These information could enhance the application of these agents for prevention and attenuation of glycation-associated diseases including, but not limiting, diabetes mellitus and Alzheimer’s disease.
References
McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell 1997; 7: 1015-26.
Xu R, Fazio GC, Matsuda SPT. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004; 65: 261-91.
Yin MC, Chan KC. Non-enzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J Agric Food Chem 2007; 55: 7177-81.
Dennehy C. Botanicals in cardiovascular health. Clin Obstet Gynecol 2001; 44: 814-23.
Tolstikova TG, Khvostov MV, Bryzgalov AO. The complexes of drugs with carbohydrate-containing plant metabolites as pharmacologically promising agents. Mini Rev Med Chem 2009; 9: 1317-28.
Rodríguez-Rodríguez R, Herrera MD, Perona JS, Ruiz-Gutiérrez V. Potential vaso-relaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br J Nutr 2004; 92: 635-42.
Channa S, Dar A, Yaqoob M, Anjum S, Sultani Z. Bronchovasodilatory activity of fractions and pure constituents isolated from Bacopa monniera. J Ethnopharmacol 2003; 86: 27-35.
Eleuteri E, Magno F, Gnemmi I, Carbone M, Colombo M, La Rocca G, et al. Role of oxidative and nitrosative stress biomarkers in chronic heart failure. Front Biosci 2009; 14: 2230-7.
Calabrese V, Guagliano E, Sapienza M, Mancuso C, Butterfield DA, Stella AM. Redox regulation of cellular stress response in neurodegenerative disorders. Ital J Biochem 2006; 55: 263-82.
Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol 2004; 24: 354-65.
Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Curr Med Chem 2009; 16: 4654-67.
Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem 2008; 104: 657-67.
Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, et al. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem Soc Trans 2007; 35: 1151-5.
Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int 2005; 9: 37-46.
Sudhahar V, Kumar SA, Varalakshmi P. Role of lupeol and lupeol linoleate on lipemic-oxidative stress in experimental hypercholesterolemia. Life Sci 2006; 78: 1329-35.
Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 2009; 75: 1549-60.
Bayón Y, Alonso A, Herna´ndez M, Nieto ML, Sánchez Crespo M. Mechanisms of cell signaling in immune mediated inflammation. Cytokines Cell Mol Ther 1998; 4: 275-86.
Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc 2001; 22: 133-7.
Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14: 6735-41.
Prud’homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 2007; 87: 1077-91.
Safayhi H, Sailer ER. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med 1997; 63: 487-93.
Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med 2007; 39: 715-21.
Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, et al. Anti-inflammatory and antitumor promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull 2005; 28: 1995-9.
Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszeń M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011; 280: 152-63.
Yan SL, Yang HT, Lee YJ, Lin CC, Chang MH, Yin MC. Asiatic acid ameliorates hepatic lipid accumulation and insulin resistance in mice consuming a high-fat diet. J Agric Food Chem 2014; 62: 4625-31.
Zhang X, Xiong H, Li H, Cheng Y. Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol Immunotoxicol 2014; 36: 11-6.
Ganguly A, Das B, Roy A, Sen N, Dasgupta SB, Mukhopadhayay S, et al. Betulinic acid, a catalytic inhibitor of topoisomerase I, inhibits reactive oxygen species mediated apoptotic topoisomerase I-DNA cleavable complex formation in prostate cancer cells but does not affect the process of cell death. Cancer Res 2007; 67: 11848-58.
Hsia TC, Liu WH, Qiu WW, Luo J, Yin MC. Maslinic acid induces mitochondrial apoptosis and suppresses HIF-1α expression in A549 lung cancer cells under normoxic and hypoxic conditions. Molecules 2014; 19: 19892-906.
Yan SL, Huang CY, Wu ST, Yin MC. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol Vitro 2010; 24: 842-8.
Garo E, Eldridge GR, Goering MG, DeLancey Pulcini E, Hamilton MA, Costerton JW, et al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob Agents Chemother 2007; 51: 1813-7.
Wu HY, Chang CI, Lin BW, Yu FL, Lin PY, Hsu JL, et al. Suppression of hepatitis B virus x protein-mediated tumorigenic effects by ursolic acid. J Agric Food Chem 2011; 59: 1713-22.
Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem J 2005; 392: 191-9.
do Nascimento PG, Lemos, Santiago GM, et al. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014; 19: 1317-27.
Kim J, Jang DS, Kim H, Kim JS. Anti-lipase and lipolytic activities of ursolic acid isolated from the roots of Actinidia arguta. Arch Pharm Res 2009; 32: 983-7.
Moon MH, Jeong JK, Lee YJ, Seol JW, Ahn DC, Kim IS, et al. 18β- Glycyrrhetinic acid inhibits adipogenic differentiation and stimulates lipolysis. Biochem Biophys Res Commun 2012; 420: 805-10.
Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 2007; 62: 427-33.
Kalousová M, Zima T, Tesar V, Dusilová-Sulková S, Skrha J. Advanced glycoxidation end products in chronic diseasesclinical chemistry and genetic background. Mutat Res 2005; 579: 37-46.
Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest 1997; 99: 457-68.
Toth E, Racz A, Toth J, Kaminski PM, Wolin MS, Bagi Z, et al. Contribution of polyol pathway to arteriolar dysfunction in hyperglycemia. Role of oxidative stress, reduced NO, and enhanced PGH(2)/ TXA(2) mediation. Am J Physiol Heart Circ Physiol 2007; 293: 3096-104.
Madhu SV, Verma NP, Goel A, Kapoor VK. Polyol pathway in the pathogenesis of diabetic complications and aldose reductase inhibitors. J Assoc Physicians India 1992; 40: 679-81.
Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int 2000; 58: 3-12.
Sato T, Iwaki M, Shimogaito N, Wu X, Yamagishi S, Takeuchi M. TAGE (toxic AGEs) theory in diabetic complications. Curr Mol Med 2006; 6: 351-8.
Takeuchi M, Yamagishi S. Alternative routes for the formation of glyceraldehyde-derived AGEs (TAGE) in vivo. Med Hypotheses 2004; 63: 453-5.
Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest 1998; 101: 1142-7.
Degenhardt TP, Thorpe SR, Baynes JW. Chemical modification of proteins by methylglyoxal. Cell Mol Biol (Noisy-le-grand) 1998; 44: 1139-45.
Nemet I, Varga-Defterdarovi_c L, Turk Z. Methylglyoxal in food and living organisms. Mol Nutr Food Res 2006; 50: 1105-17.
Mostafa AA, Randell EW, Vasdev SC, Gill VD, Han Y, Gadag V, et al. Plasma protein advanced glycation end products, carboxymethyl cysteine, and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol Cell Biochem 2007; 302: 35-42.
Takeuchi M, Sato T, Takino J, Kobayashi Y, Furuno S, Kikuchi S, et al. Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer’s disease. Med Hypotheses 2007; 69: 1358-66.
Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci 2010; 65: 963-75.
Peppa M, Uribarri J, Vlassara H. Aging and glycoxidant stress. Hormones (Athens) 2008; 7: 123-32.
Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal 2009; 11: 3071-109.
Tuttle KR, Anderberg RJ, Cooney SK, Meek RL. Oxidative stress mediates protein kinase C activation and advanced glycation end product formation in a mesangial cell model of diabetes and high protein diet. Am J Nephrol 2009; 29: 171-80.
Chandra KP, Shiwalkar A, Kotecha J, Thakkar P, Srivastava A, Chauthaiwale V, et al. Phase I clinical studies of the advanced glycation end-product (AGE)-breaker TRC4186: safety, tolerability and pharmacokinetics in healthy subjects. Clin Drug Invest 2009; 29: 559-75.
Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991; 325: 836-42.
Meerwaldt R, Links T, Zeebregts C, Tio R, Hillebrands JL, Smit A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc Diabetol 2008; 7: 2-9.
Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes 2002; 51: 3532-44.
Khan ZA, Chakrabarti S. Cellular signaling and potential new treatment targets in diabetic retinopathy. Exp Diabetes Res 2007; 2007: 3186-7.
Berrou J, Tostivint I, Verrecchia F, Berthier C, Boulanger E, Mauviel A, et al. Advanced glycation end products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med 2009; 23: 513-20.
Ozdemir AM, Hopfer U, Rosca MV, Fan XJ, Monnier VM, Weiss MF. Effects of advanced glycation end product modification on proximal tubule epithelial cell processing of albumin. Am J Nephrol 2008; 28: 14-24.
Jandeleit-Dahm K, Cooper ME. The role of AGEs in cardiovascular disease. Curr Pharm Des 2008; 14: 979e86.
Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des 2008; 14: 962-8.
Armstrong RA. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol 2009; 47: 289-99.
Reddy VP, Obrenovich ME, Atwood CS, Perry G, Smith MA. Involvement of Maillard reactions in Alzheimer disease. Neurotox Res 2002; 4: 191-209.
Takeda A, Wakai M, Niwa H, Dei R, Yamamoto M, Li M, et al. Neuronal and glial advanced glycation end product [Nepsilon- (carboxymethyl)lysine] in Alzheimer’s disease brains. Acta Neuropathol 2001; 101: 27-35.
Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol 2001; 171: 29-45.
Di Loreto S, Caracciolo V, Colafarina S, Sebastiani P, Gasbarri A, Amicarelli F. Methylglyoxal induces oxidative stress-dependent cell injury and up-regulation of interleukin-1beta and nerve growth factor in cultured hippocampal neuronal cells. Brain Res 2004; 1006: 157-67.
Seidl R, Schuller E, Cairns N, Lubec G. Evidence against increased glycoxidation in patients with Alzheimer’s disease. Neurosci Lett 1997; 232: 49-52.
Bär KJ, Franke S, Wenda B, Müller S, Kientsch-Engel R, Stein G, et al. Pentosidine and N(epsilon)-(carboxymethyl)-lysine in Alzheimer’s disease and vascular dementia. Neurobiol Aging 2003; 24: 333-8.
Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, et al. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med 2001; 31: 175-80.
Perez-Gracia E, Blanco R, Carmona M, Carro E, Ferrer I. Oxidative stress damage and oxidative stress responses in the choroid plexus in Alzheimer’s disease. Acta Neuropathol 2009; 118: 497-504.
Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 1997; 23: 134-47.
D’Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol 2010; 6: 352-60.
Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Dietderived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci 2005; 1043: 461-6.
Yan SD, Bierhaus A, Nawroth PP, Stern DM. RAGE and Alzheimer’s disease: a progression factor for amyloid-beta induced cellular perturbation? J Alzheimers Dis 2009; 16: 833-43.
Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 2000; 1498: 99-111.
Yan SF, Barile GR, D’Agati V, Du Yan S, Ramasamy R, Schmidt AM. The biology of RAGE and its ligands: uncovering mechanisms at the heart of diabetes and its complications. Curr Diab Rep 2007; 7: 146-53.
Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem 2003; 278: 34834-44.
Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med 2007; 7: 711-24.
Yamagishi S. Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial 2009; 13: 534-9.
Lin L, Park S, Lakatta EG. RAGE signaling in inflammation and arterial aging. Front Biosci 2009; 14: 1403-13.
Gawlowski T, Stratmann B, Ruetter R, Buenting CE, Menart B, Weiss J, et al. Advanced glycation end products strongly activate platelets. Eur J Nutr 2009; 48: 475-81.
Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett 2007; 581: 1928-32.
Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)- induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem Biophys Res Commun 2010; 391: 1405-8.
Liu Y, Liang C, Liu X, Liao B, Pan X, Ren Y, et al. AGEs increased migration and inflammatory responses of adventitial fibroblasts via RAGE, MAPK and NF-kappaB pathways. Atherosclerosis 2010; 208: 34-42.
Tan AL, Sourris KC, Harcourt BE, Thallas-Bonke V, Penfold S, Andrikopoulos S, et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am J Physiol Renal Physiol 2010; 298: 763-70.
Goldberg T, Cai W, Melpomeni P, Dardaine V, Baliga BS, Uribarri J, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Ass 2004; 104: 1287-91.
Chao PC, Hsu CC, Yin MC. Analysis of glycative products in sauces and sauce-treated foods. Food Chem 2009; 113: 262-6.
Erbersdobler HF, Somoza V. Forty years of furosine-forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol Nutr Food Res 2007; 51: 423-30.
Motomura K, Fujiwara Y, Kiyota N, Tsurushima K, Takeya M, Nohara T, et al. Astragalosides isolated from the root of astragalus radix inhibit the formation of advanced glycation end products. J Agric Food Chem 2009; 57: 7666-72.
Rao AR, Veeresham C, Asres K. In vitro and in vivo inhibitory activities of four Indian medicinal plant extracts and their major components on rat aldose reductase and generation of advanced glycation endproducts. Phytother Res 2013; 27: 753-60.
Maramaldi G, Togni S, Franceschi F, Lati E. Anti-inflammaging and antiglycation activity of a novel botanical ingredient from African biodiversity (Centevita™). Clin Cosmet Investig Dermatol 2013; 7: 1-9.
Kim JM, Jang DS, Lee YM, Yoo JL, Kim YS, Kim JH, et al. Aldosereductase- and protein-glycation-inhibitory principles from the whole plant of Duchesnea chrysantha. Chem Biodivers 2008; 5: 352-6.
Liu Y, Hartley DP, Liu J. Protection against carbon tetrachloride hepatotoxicity by oleanolic acid is not mediated through metallothionein. Toxicol Lett 1998; 95: 77-85.
Nguemfo EL, Dimo T, Dongmo AB, Azebaze AG, Alaoui K, Asongalem AE, et al. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L.C (Guttiferae). Inflammopharmacology 2009; 17: 37-41.
Yamaguchi Y, Yamada K, Yoshikawa N, Nakamura K, Haginaka J, Kunitomo M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci 2006; 9: 2474-9.
Yan SL, Yang HT, Lee HL, Yin MC. Protective effects of maslinic acid against alcohol-induced acute liver injury in mice. Food Chem Toxicol 2014; 74: 149-55.
Wang ZH, Hsu CC, Huang CN, Yin MC. Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur J Pharmacol 2010; 628: 255-60.
Jang SM, Kim MJ, Choi MS, Kwon EY, Lee MK. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism 2010; 59: 512-9.
Wang ZH, Hsieh CH, Liu WH, Yin MC. Glycyrrhizic acid attenuated glycative stress in kidney of diabetic mice through enhancing glyoxalase pathway. Mol Nutr Food Res 2014; 58: 1426-35.
Tsai SJ, Yin MC. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by D-galactose. Food Chem Toxicol 2012; 50: 3198-205.
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB, Song J, et al. Protection of glycyrrhizic acid against AGEs-induced endothelial dysfunction through inhibiting RAGE/NF-κB pathway activation in human umbilical vein endothelial cells. J Ethnopharmacol 2013; 148: 27-36.
Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, et al. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb Cortex 2010; 20: 2540-8.
Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, et al. Transporters in the brain endothelial barrier. Curr Med Chem 2010; 17: 1125-38.
Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul 2009; 3: 71-89.
Chen X, Walker DG, Schmidt AM, Arancio O, Lue LF, Yan SD. RAGE: a potential target for Abeta-mediated cellular perturbation in Alzheimer’s disease. Curr Mol Med 2007; 7: 735-42.
Author information
Authors and Affiliations
Additional information
© Author(s) 2015. This article is published with open access by China Medical University
# Equal contribution as the first author.
* Corresponding author. Department of Nutrition, China Medical University, 16th Floor, No. 91, Hsueh-Shih Road, Taichung 404, Taiwan
E-mail address: mcyin@mail.cmu.edu.tw (M.-C. Yin)
Open Access This article is distributed under terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided original author(s) and source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, MC. Inhibitory effects and actions of pentacyclic triterpenes upon glycation. BioMed 5, 13 (2015). https://doi.org/10.7603/s40681-015-0013-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40681-015-0013-x